Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Adamtaylor.info
11402 • The Journal of Neuroscience, December 10, 2003 • 23(36):11402–11410
Imaging Reveals Synaptic Targets of a Swim-Terminating
Neuron in the Leech CNS
Adam L. Taylor,1,2
Garrison W. Cottrell,1
David Kleinfeld,3
and William B. Kristan Jr2
1Department of Computer Science and Engineering, 2Neurobiology Section, Division of Biological Sciences, and 3Department of Physics, University of
California, San Diego, La Jolla, California 92093
In the leech, the command-like neuron called cell Tr2 is known to stop swimming, but the connections from cell Tr2 to the swim central
pattern generator have not been identified. We used fluorescence resonance energy transfer voltage-sensitive dyes to identify three
neurons that are synaptic targets of cell Tr2. We then used electrophysiological techniques to show that these connections are monosyn-
aptic, chemical, and excitatory. Two of the novel targets, cell 256 and cell 54, terminate swimming when stimulated. These neurons are
likely to mediate swim cessation caused by cell Tr2 activity, and thus play the role of intermediate control cells in the leech CNS.
Key words: connectivity; leech; swimming; swim termination; voltage-sensitive dye; fluorescence resonance energy transfer; coherence;
command neuron
stimulated (Brodfuehrer and Friesen 1986a; Brodfuehrer and
Hierarchical control is an organizational pattern found in both
Burns, 1995; O'Gara and Friesen 1995). One of these, cell Tr2, is
invertebrate and vertebrate nervous systems. In vertebrate ner-
found in the head brain of the leech (Brodfuehrer and Friesen
vous systems, the forebrain and brainstem are involved in the
1986a; O'Gara and Friesen, 1995). Stimulation of this cell termi-
initiation, termination, and coordination of complex rhythmic
nates an ongoing swim bout. It is not known, however, how cell
behaviors, but the cycle-to-cycle control of these behaviors is
Tr2 connects to the swim central pattern generator (CPG), and
mediated by pattern-generating networks in the spinal cord
thus it is not known how it causes swim termination. One possi-
(Grillner et al., 1997). Similar hierarchical control is found in
bility is that cell Tr2 connects directly to the swim CPG cells. For
invertebrates, with higher-order neurons activating pattern gen-
instance, cell Tr2 could terminate swimming by inhibiting all or
erators, which then synapse on motor neurons (Orlovsky et al.,
most of the swim CPG or by exciting part of the swim CPG in
such a way as to disrupt rhythm generation. Such a scheme is used
Single neurons that evoke or terminate whole behaviors
in the
Xenopus tadpole, with mid-hindbrain reticulospinal neu-
(command-like neurons) have been found in many invertebrate
rons directly inhibiting the motor neurons involved in swimming
preparations, whereas they seem to be rare in vertebrate prepara-
(Perrins et al., 2002). A second possibility is that cell Tr2 inhibits
tions, presumably because of the larger number of neurons in
the known swim-gating cells, thereby stopping swimming
these systems (Kupfermann and Weiss, 1978; Pearson, 1993; but
through these intermediaries. Because the swim-gating cells are
see Roberts et al., 1997; Perrins et al., 2002). For instance, stimu-
necessary for the maintenance of swimming, this should be suf-
lating a single command-like neuron in the leech (Weeks and
ficient to stop an ongoing swim (Weeks, 1981; Nusbaum and
Kristan, 1978; Brodfuehrer and Friesen, 1986a) or in
Tritonia
Kristan, 1986). A third possibility is that cell Tr2 activates an
(Frost and Katz, 1996) can elicit full-blown bouts of swimming,
intermediate network that then projects to the swim CPG cells
whereas it is necessary to stimulate many neurons in the brain-
and swim-gating cells. This intermediate network might be part
stem of the lamprey (Orlovsky et al., 1999) to achieve a similar
of command-to-motor pathways for multiple behaviors, and to-
gether with the swim-gating cells could provide a neuronal locus
In the leech, in addition to swim-initiating neurons, there are
in which pro-swimming and anti-swimming inputs are resolved.
command-like neurons that terminate an ongoing swim when
A precedent for this organization is provided by studies in whichconflicting sensory inputs lead to a single, defined behavioraloutput (Kristan and Shaw, 1997).
Received Sept. 1, 2003; revised Oct. 8, 2003; accepted Oct. 8, 2003.
As a first step toward resolving which of these schemes (if any)
This work was supported by a La Jolla Interfaces in Science Predoctoral Fellowship, funded by the Burroughs
is used in the leech, we used fluorescence resonance energy trans-
Wellcome Fund (A.L.T.); National Institutes of Health (NIH) Training Grant GM08107 (A.L.T.); and NIH ResearchGrants MH43396 (G.W.C., D.K., W.B.K.), RR13419 (D.K.), and NS35336 (W.B.K.). We thank J. E. Gonzalez and R. Y.
fer (FRET) voltage-sensitive dye imaging to search for synaptic
Tsien for assistance with the FRET voltage-sensitive dyes; Panvera LLC for supplying the dyes
gratis; K. Briggman,
targets of cell Tr2 (Gonza´lez and Tsien, 1995, 1997; Cacciatore et
S. B. Mehta, T. M. Esch, the Kristan Laboratory, and GURU (Gary's Unbelievable Research Unit) for valuable discus-
al., 1999; Zochowski et al., 2000). We found three neurons in the
sions; and the two anonymous reviewers.
midbody ganglia that receive monosynaptic input from cell Tr2
Correspondence should be addressed to Adam L. Taylor, Volen Center, Mail Stop 013, Brandeis University, 415
and determined that two of these synaptic targets were able to
South Street, Waltham, MA 02454-9110. E-mail:
[email protected].
Copyright 2003 Society for Neuroscience 0270-6474/03/2311402-09$15.00/0
terminate swimming when stimulated. None of these cells are
Taylor et al. • Imaging Reveals Synaptic Targets in Leech
J. Neurosci., December 10, 2003 • 23(36):11402–11410
• 11403
either swim CPG cells or swim-gating cells, suggesting that they
Figure 3 were edited manually to remove background fluorescence using
are part of an intermediate network by which Tr2 activity termi-
Corel Photo-Paint (Corel Corporation, Ottawa, Ontario, Canada).
nates swimming.
Optical recording. We acquired fluorescence images using an upright
microscope (Axioskop 2FS; Zeiss). We usually used a 40⫻, 0.8 numericalaperture (NA) water-immersion objective (Acroplan; Zeiss), but occa-
Materials and Methods
sionally used a 20⫻, 0.5 NA water-immersion objective (Acroplan; Zeiss)
Preparation. Subjects were adult
Hirudo medicinalis (4 – 8 gm), the Euro-
when a wider field of view was desired. For epi-illumination we used a
pean medicinal leech, obtained from Leeches USA (Westbury, NY) and
tungsten halogen lamp (64625 HLX; Osram Sylvania, Danvers, MA) in a
maintained in artificial pond water at 15°C. We dissected out the full
standard housing (HAL 100; Zeiss), powered by a low-ripple power sup-
nerve cord, including the head brain, all 21 midbody ganglia, and the tail
ply (JQE 15-12M; Kepco, Flushing, NY). For all imaging, we used only
brain. We removed the connective tissue sheath from ganglia in which we
the coumarin emission, because it provided brighter fluorescence and
planned to impale cells or image activity. Usually we desheathed the
higher voltage sensitivity. The filter set consisted of a 405 ⫾ 15 nm
ventral surface of the head brain (subesophageal ganglion only) and the
bandpass excitation filter, a 430 nm dichroic mirror, and a 460 ⫾ 25 nm
ventral side of one of the midbody ganglia from segments 10 –13. We
bandpass emission filter (Chroma Technology Corporation, Brattleboro,
prepared at least one dorsal posterior (DP) nerve, usually chosen from
VT). We used a water-cooled CCD camera (MicroMax 512 BFT; Roper
ganglia 14 through 17, for extracellular recording. A motor neuron in this
Scientific, Tucson, AZ) operated in frame-transfer mode to acquire the
nerve bursts during the dorsal contractile phase of swimming, so the
optical data, at a frame rate of 20 Hz. The CCD chip in this camera has
nerve serves as a monitor of swimming activity (Ort et al., 1974). In some
512 ⫻ 512 pixels, but we normally binned at 4 ⫻ 4 pixels, yielding a 128 ⫻
experiments, we stabilized the imaged ganglion by pinning small strips of
128 image. The quantum efficiency of the camera at the coumarin emis-
sausage casing across them to minimize motion artifact (Cacciatore et al.,
sion peak was 80%. The CCD chip was maintained at ⫺25°C during
1999). The preparation was maintained in a chamber filled with ⬃5 ml of
imaging. Imaging data were acquired using the software package Win-
leech saline, consisting of (in m
View/32 (Roper Scientific, Trenton, NJ). We synchronized the optical
M): 115 NaCl, 4 KCl, 1.8 CaCl , 1.5 MgCl ,
10 dextrose, 4.6 Tris maleate, and 5.4 Tris base, pH 7.4.
and electrical recordings by feeding the frame timing signals emitted by
Staining with FRET dyes. We first stained the ganglion to be imaged
the camera into the A-to-D board, along with all the electrophysiology
with the FRET donor,
N-(6-chloro-7-hydroxycoumarin-3-carbonyl)-
dimyristoylphosphatidylethanolamine (CC2-DMPE) (Panvera LLC,
The combination of CC2-DMPE and DiSBAC (3) yielded sensitivities
Madison, WI), a coumarin-labeled phospholipid (Gonza´lez et al., 1999).
in the range of 2– 8%/100 mV for 1 Hz sinusoidal voltage signals with a 10
mV amplitude, centered around a baseline voltage of ⫺50 mV. Much of
M staining solution from 3 l of 5 mM CC2-DMPE in
DMSO, 1 l of 20 mg/ml pluronic F-127 in DMSO (Molecular Probes,
the variation in sensitivity seemed to be attributable to differences in
Eugene OR), and 500 l of saline. We pinned out the nerve cord in a
soma size, with larger somata giving higher sensitivity.
Sylgard-coated dish, placed a small plastic cylinder over it, and then
Analysis. After acquiring the data, we analyzed them using Matlab
sealed it with petroleum jelly to make a watertight chamber. We then
(The Mathworks, Natick, MA). We outlined the images of individual
replaced the saline in the chamber with the staining solution and stained
somata manually using a custom-made graphic user interface. All pixels
the ganglion for 30 min. During staining, we constantly recirculated the
within each cellular outline were then averaged in each frame, yielding a
staining solution using a peristaltic pump (model RP-1; Rainin, Oakland,
raw fluorescence signal for each cell, which we denote by
F(
t). The noise
CA). After 30 min, the staining solution was taken off, and the tissue was
in these single-cell signals was normally shot-noise dominated. Raw flu-
washed several times with fresh saline.
orescence signals were usually corrupted by a slow downward drift at-
We next stained the whole nerve cord with the FRET acceptor, bis(1,3-
tributable to bleaching, but this was eliminated by fitting it with a math-
diethyl-thiobarbiturate)-trimethine oxonol [DiSBAC (3)] (Panvera
ematical function and dividing it out of the signal. For each cell outline,
LLC), an oxonol dye (Tsien, 1976; Gonza´lez and Tsien, 1995). We made
bleaching was fit by computing a moving average of
F(
t) with a Gaussian
window ( ⫽ 500 msec). This slow signal we denote by
F
(
t). Because
M staining solution from 8 l of 12 mM DiSBAC (3) in DMSO and
12 ml saline. This solution was then bath-sonicated for at least 1 min. We
this slow signal also includes the DC component of
F(
t), we have:
replaced the bathing solution with this solution and left it on for at least
30 min. In many experiments, we left the oxonol solution on for as long
共
t兲 ⫽
共
t兲 ⫺ 1.
as imaging was done, replacing it with fresh staining solution every few
hours. After taking off the staining solution, we replaced it with fresh
We estimated coherence using multi-taper methods (Thomson, 1982;
saline that was sometimes continuously perfused using a gravity perfu-
Percival and Walden, 1993; Cacciatore et al., 1999). Only the differences
sion system. Leaving the oxonol in the bath did not increase background
from the procedures used in Cacciatore et al. (1999) will be described.
fluorescence because its extinction coefficient is small at 405 nm, the
For our coherence estimates, we fixed the frequency resolution, ⌬
f, de-
wavelength of the excitation light, and oxonol has 20-fold less fluorescent
fined as the half-width of the spectral bands, at ⌬
f ⫽ 2/3 Hz, and the
yield in aqueous solution than in the membrane (Rink et al., 1980).
number of tapers was adjusted according to the duration of the data set.
Electrophysiology and cell fills. We recorded intracellularly from cells
For the data presented here, the trial duration was always
T ⫽ 9.5 sec;
using 40 – 60 M⍀ glass microelectrodes filled with 1 M potassium acetate,
thus the number of tapers with good leakage properties was:
using an Axoclamp 2A amplifier (Axon Instruments, Foster City, CA).
K ⫽ 2
T⌬
f ⫺ 1 ⫽ 11.
We recorded extracellularly using suction electrodes and a four-channeldifferential amplifier (model 1600; A-M Systems, Sequim, WA). We dig-
To calculate standard errors for the coherence estimates, we used the
itized all electrical data at 1–2 kHz using a 12-bit analog-to-digital (A-
jackknife (Thomson and Chave, 1991). For the error in the coherence
to-D) board (PCI-MIO-16E-4; National Instruments, Austin, TX) and
phase, we used the same procedure as described by Cacciatore et al.
custom LabVIEW (National Instruments) software.
(1999). For the coherence magnitude, we used a slightly different proce-
We filled individual neurons using either tetramethylrhodamine dex-
dure. After calculating the coherence magnitude estimate,
C , and the
tran [3000 molecular weight (MW); Molecular Probes] or Alexa 488
take-away-one coherence magnitude estimates,
C , the estimate and the
dextran (10,000 MW; Molecular Probes). Microelectrodes were back-
take-away-one estimates were replaced with the transformed values
filled using solutions with concentrations of 50 mg/ml (rhodamine) or 25
given by
y ⫽
f(
C ) ⫽ ln[
C 2/(1 ⫺
C 2)], as suggested in Thomson and
mg/ml (Alexa). Cells were then impaled, and dye was iontophoretically
Chave (1991). The SE of the transformed estimate is given by the jack-
injected. Dye was allowed to diffuse for ⬃1 hr, and tissue was fixed
knife expression:
overnight, dehydrated, cleared, and mounted on a slide for viewing. Fillswere imaged using a confocal microscope (1024ES; Bio-Rad, Hercules,
CA). Dyes were excited by the 488 nm (Alexa) or 568 nm (rhodamine)
⫽ 冑
N 冘 共 ⫺
emission line of a KrAr laser (60-WL-DZ; Bio-Rad). Images shown in
11404 • J. Neurosci., December 10, 2003 • 23(36):11402–11410
Taylor et al. • Imaging Reveals Synaptic Targets in Leech
one cell Tr2 was impaled and stimulated via current injection
yi. Thus a one SE bar on
y would be the interval (
y ⫺
(Fig. 1
A). In each trial, we injected a series of pulse trains into cell
,
y ⫹ ). The error bar used for
C is the interval (
f⫺1(
y – ),
f⫺1(
y
Tr2, using a level of current sufficient to elicit one spike per pulse.
Each train was 500 msec long, with an intertrain interval of 500
msec, yielding a train frequency of 1 Hz. Within each train, we
used a pulse frequency of 20 Hz. During stimulation, we imaged
1 ⫺
e⫺共
y⫺
y兲 , 冑
1 ⫺
e⫺共
y⫹
y兲
a region of the stained ganglion using a CCD camera, producinga time series of fluorescence images (Fig. 1
B). We typically viewed
Because the distribution of
y is roughly Gaussian, this interval provides
approximately one-third of the ganglionic surface (20 – 80 cells) at a
an ⬃68% confidence interval. This interval also has the advantage that it
time. For each trial, we manually drew ellipses around each of the
is guaranteed to be a subset of [0,1], which is not the case if the above
visible cell bodies (Fig. 1
C) and assigned an alphanumeric label to
transformation is not used.
To test whether the coherence magnitude of a given cell was signifi-
each ellipse. We then averaged the pixel values inside the ellipse of
cantly greater than zero, i.e., larger than would be expected by chance
each cell to provide a single time-varying optical signal for that cell (a
from a signal with zero coherence, we compared the estimate of
C with
subset of these signals is shown in Fig. 1
D).
the null distribution for coherence magnitude. It can be shown that for
We quantified how strongly each cell responded to cell Tr2
the null distribution the coherence magnitude will exceed the value
activity by estimating the coherence between each optical signal
公1⫺␣1/(
K⫺1) only in 100␣% of trials (Hannan, 1970; Jarvis and Mitra,
and the electrical recording of the cell Tr2 membrane potential
2001). We used a low ␣ level of 0.001 in all experiments, to avoid false
(Cacciatore et al., 1999) (see Materials and Methods). The coher-
positives. We also calculated the multiple comparisons ␣ level for each
ence magnitude gives a measure of how well two signals correlate
trial, given by ␣
⫽ 1 ⫺ (1 ⫺ ␣)
n, where
n is the number of cells, and
with one another at each frequency, with a value of 1 implying
verified that it did not exceed 0.05 on any trial.
When plotting the coherence in polar plots (see Figs. 1
E,
H, 2
B), we
perfect correlation and a value of zero implying no correlation.
corrected for the phase shift caused by the slow response of the dye signal
The coherence phase gives the phase difference between the two
by shifting the coherence phase by ⫹54°. This is the phase angle by which
signals at each frequency. We focused on the coherence at 1 Hz,
the fluorescence signal lags the voltage when the voltage trajectory is a 1
the stimulation frequency, where most of the power in the cell
Hz sinusoid. It is close to tan ⫺1(2f) ⫽ 66°, the theoretical phase lag for
Tr2 voltage signal is concentrated.
f ⫽ 1 Hz, given the reported time constant of the dye, ⫽ 360 msec
We used the coherence magnitude at 1 Hz to rank the optical
(Cacciatore et al., 1999). (The difference arises because the reported time
signals in order of how strongly they responded to Tr2 stimula-
constant was based on data for a range of frequencies, and the ⫹54° shift
tion. The six optical signals shown in Figure 1
D are those that had
was based solely on data for 1 Hz signals.) The phase shift was not ad-justed in any way on a per-trial basis to achieve a better match between
the highest coherence magnitude at 1 Hz. They are shown in
coherence estimates for optical versus electrical signals.
descending order of magnitude. Because the coherence estimate
Cell identification. We identified the novel neurons described here
is a statistic, we could calculate the coherence magnitude required
(cells 252, 256, and the pair of cells 54) on the basis of their soma position,
to reach statistical significance. Only the top three signals in Fig-
soma size, amplitude of action potential as recorded in the soma, and
ure 1
D (shown in color) responded strongly enough to conclude
presence of one-for-one EPSPs from cell Tr2. All three cells are found on
that their coherence with cell Tr2 was not attributable to chance.
the ventral aspect of the ganglion. Cells 256 and 252 are found in the
We refer to these cells as "followers" of cell Tr2, after Peterlin et al.
posterior medial packet, and cells 54 are found in the posterior lateral
(2000). These cells were considered to be good candidates for
packets. In a few cases, the identity of a cell was established purely on thebasis of soma position, size, and strong optical coherence with cell Tr2 at
being synaptic targets of cell Tr2. The coherence phase at the
a phase lag between 60° and 120°. This was done only after a cell had been
stimulation frequency served as a measure of the latency of the
singled out optically and verified electrically in many animals and only in
response of a neuron to cell Tr2 stimulation (Fig. 1
E). The phase
unambiguous cases. Cells that were reliably identified in many animals
can also distinguish excitatory from inhibitory effects (Cacciatore
were assigned numeric "names" (e.g., cell 252) by matching up their
et al., 1999).
usual soma position and size with those on the canonical map of the leech
Using optical recording, we identified three followers of cell
ganglion (Muller et al., 1981).
Tr2 that appeared in similar positions across several prepara-
Cell Tr2 was identified on the basis of the description given in Brod-
tions. The most coherent of these was an unpaired cell in the
fuehrer and Friesen (1986a). Cell Tr1 was typically found just medial to
posterior medial packet, cell 256. A cell in the approximate posi-
the Retzius cell in segment R1, and cell Tr2 was typically just medial tocell Tr1. The soma of cell Tr2 was typically slightly larger than that of Tr1
tion of cell 256, which had strong coherence with cell Tr2, was
(⬃60 vs ⬃50 m), and both cells displayed large (20 – 40 mV) action
seen in 45 preparations (Figs. 1
C–
E). The optical signal of cell 256
potentials that rose without prepotential. In all preparations in which we
often displayed a near-sinusoidal modulation at the stimulation
could elicit swimming (67 of 96), we verified that cell Tr2 stopped or
frequency. A second strongly coherent cell, cell 252, was unpaired
strongly slowed swimming when stimulated. In all such cases but one, the
and located in the same packet. A highly coherent cell in the
cell initially identified as cell Tr2 on the basis of position, soma size, and
approximate position of cell 252 was seen in 21 preparations
spike shape was found to stop or strongly slow swimming. In seven
(Figs. 1
C–
E). The most coherent cell in the posterior medial
preparations, we filled cell Tr2 with fluorescent tracer and verified that
packet was usually in cell 256 position, with a cell in cell 252
the morphology of the cell was consistent with that described in Brod-fuehrer and Friesen (1986a).
position the next most coherent. A third strongly coherent cellwas also identified, cell 54 (Figs. 1
F–
H ). Unlike the others, it was
a paired cell, with one homolog in each of the posterior lateral
Using imaging, we identified three putative synaptic targets of
packets. We observed a strongly coherent cell 30 times, in 22
cell Tr2 in the segmental ganglia
preparations, in the approximate position of cell 54. In addition
To identify candidate synaptic targets of cell Tr2, we imaged
to displaying large coherence magnitude, all four neurons typi-
many neurons simultaneously while stimulating cell Tr2. A mid-
cally had coherence phase lags between 60° and 120° (after cor-
body ganglion (usually ganglion 10) was stained with both com-
recting for the dye time constant; see Materials and Methods).
ponents of the FRET system (see Materials and Methods), and
Because the coherence is evaluated at 1 Hz, this corresponds to a
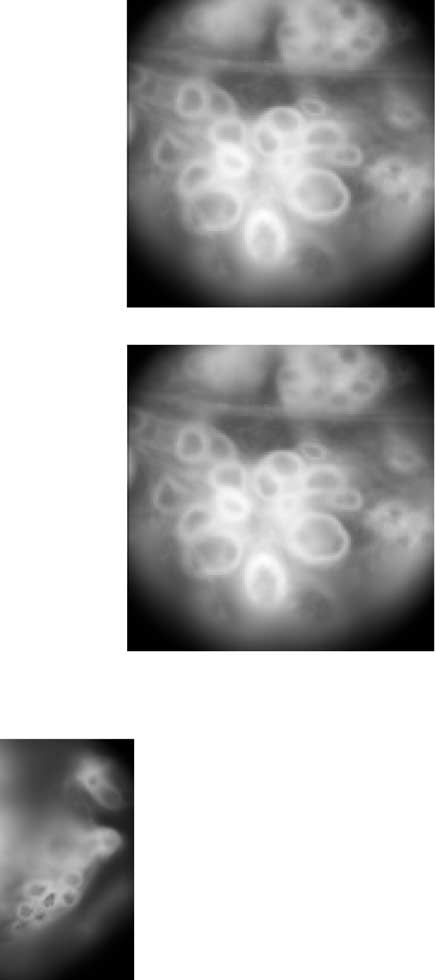
Taylor et al. • Imaging Reveals Synaptic Targets in Leech
J. Neurosci., December 10, 2003 • 23(36):11402–11410 • 11405
signal-to-noise ratio and a lower samplingrate. The strong agreement between theoptical and electrical recordings produceda similarly strong agreement between thecoherence estimates derived from the tworecordings (Fig. 2 B): the two estimates arewithin the error bars of one another.
When individual cell Tr2 spikes were
elicited, they produced single EPSPs in allthree cell types, at a constant latency, typ-ically between 125 and 175 msec (Fig. 2C).
The latency seen is consistent with theconduction delay for the cell Tr2 spikes totravel from the head brain to ganglion 10(Brodfuehrer and Friesen, 1986a). Wechecked for EPSPs in 30 of the 45 opticallyidentified cells 256, in 4 of the 21 cells 252,and in 6 of the 30 cells 54. In every case, wefound that single cell Tr2 spikes causedone-for-one EPSPs in the observed fol-lower (Fig. 3A–C). To test whether theseconnections
changed the concentration of divalent cat-ions in the saline and examined the re-sponses of the three cell Tr2 followers
Figure 1.
Optical discovery of cell Tr2 targets. A, A schematic of the preparation used to find cell Tr2 targets. The drawing
(Figs. 3A–C) (Nicholls and Purves, 1970;
represents the isolated leech nerve cord, consisting of the head brain (HB), 21 midbody ganglia (circles), and the tail brain (TB). Cell
Berry and Pentreath 1976; Byrne et al.,
Tr2 was recorded intracellularly in the head brain, and the voltage-sensitive dye components were applied to a midbody ganglion,
1978; Liao and Walters, 2002). In all cases,
in this case ganglion 10. Ant, Anterior. B, The raw fluorescence image of ganglion 10, obtained by averaging all frames from a
the EPSP was preserved in 20 mM Ca 2⫹/20
movie of cellular fluorescence over time. The frame includes the posterior medial packet and parts of the right and left posterior
mM Mg 2⫹ saline, which blocks polysynap-
lateral packets, all of which are on the ventral side of the animal. Scale bar, 50 m. C, Ellipses that were drawn by hand and usedto average the pixels for each cell in the field. Each cell was given an arbitrary alphanumeric label unless it was impaled and
tic PSPs, and disappeared in 0 mM
definitively identified in that preparation. Cell p54(L) is likely to be cell 54(L) (p is for putative), but we did not impale it and verify
Ca 2⫹/20 mM Mg 2⫹ saline, which blocks
its identity in this preparation. Colored cells were significantly coherent with the cell Tr2(L) electrical recording, at the ␣ ⫽ 0.001
chemical synaptic transmission. Thus the
level (see Materials and Methods). Cells in black were not significantly coherent. Colors were chosen only to match up cells in C with
connections from cell Tr2 to all three neu-
traces in D and points in E; they are otherwise arbitrary. D, Simultaneous electrical recording of cell Tr2(L) and optical recordings
rons appear to be monosynaptic and
from six of the cells shown in C. An increase in fluorescence reflects a depolarization of the cell. The optical traces are ordered by the
magnitude of their coherence with the cell Tr2(L) electrical recording at 1 Hz. The coherence magnitude values are given to the
For all cells, the latency of the EPSPs
right of each trace. The bars above the cell Tr2(L) voltage trace show when current was being passed into cell Tr2(L). E, Polar plot
increased when the preparation was in 20
of the coherence between each optical recording and the cell Tr2 electrical recording, at the 1 Hz drive frequency, for the 43 cells
mM Ca 2⫹/20 mM Mg 2⫹ saline. This was
circled in C. The distance from the center represents the coherence magnitude, and the angle represents the coherence phase. The
presumably caused by a reduction in the
outer circle represents a coherence magnitude of 1, the highest possible value. The dashed line indicates the ␣ ⫽ 0.001 thresholdfor significance. The error bars represent 1 SE. A positive–negative phase angle means the signal leads or lags the cell Tr2 signal by
conduction velocity of action potentials
that amount. All coherence estimates for fluorescence signals are shifted by ⫹54°, to correct for the phase shift caused by the time
attributable to elevated spike threshold.
constant of the dye response (see Materials and Methods). The data shown in A–E can also be presented as a movie, which is
We verified that conduction velocity was
available at www.jneurosci.org. F, Analogous to C, but for a preparation in which cell 54(L) was impaled to identify it definitively.
slower in this saline by recording en pas-
The circled cells are in the left posterior packet of the ventral side. Cells in the posterior medial packet cannot be seen clearly
sant from the connective between ganglia
because they are above the focal plane. Scale bar, 50 m. G, H, Analogous to D and E, but for the field shown in F.
8 and 9. We found that the spikes did in-deed take longer to propagate to the re-cording site in 20 mM Ca 2⫹/20 mM Mg 2⫹
time lag of between 167 and 333 msec. Given that a typical con-
saline and that the increased EPSP latency scaled with the in-
duction velocity for a Tr2 spike is ⬃15 msec per segment (Brod-
creased delay (data not shown).
fuehrer and Friesen, 1986a; our data not shown) and that theserecordings were done in ganglion 10, these latencies suggest a
Morphology and segmental extent of cell Tr2 targets
direct excitatory connection from cell Tr2 (Fig. 1 E, H ).
The three cell Tr2 targets had distinct morphologies, which wevisualized by filling the cells with fluorescent tracers (Fig. 3D–F ).
Cell Tr2 is monosynaptically connected to candidate targets
Cell 256 (Fig. 3D) is an unpaired cell, with a roughly symmetric
After we found a strong follower of cell Tr2 using optical record-
pattern of neuritic branching. It sends a single process into the
ing, we impaled the follower (in the same preparation) to deter-
anterior medial connective. Cell 252 (Fig. 3E) is also unpaired,
mine the nature of its connection to cell Tr2. For all three cell
with a roughly symmetric pattern of neuritic branches. Unlike
types described above, we found that the strong coherence with
cell 256, it sends two processes out of the ganglion, one in the
cell Tr2 activity was attributable to summating volleys of EPSPs in
anterior medial connective and one in the posterior medial con-
the target cell [Fig. 2 A (data for cell 252 is shown)]. As expected,
nective. Cell 54 (Fig. 3F ) is a paired cell. Each member of the pair
the optical signal was a low-pass-filtered version of the electrical
has a large tuft of neurites ipsilateral to the soma and sends its
signal (because of the time constant of the dye), with a lower
primary neurite contralateral. The primary neurite divides into
11406 • J. Neurosci., December 10, 2003 • 23(36):11402–11410
Taylor et al. • Imaging Reveals Synaptic Targets in Leech
Figure 3.
Monosynapticity and morphology of cell Tr2 targets. A, A series of spike-triggered
averages (STAs) of simultaneous recordings from cells Tr2(L) and 256(10). In each case, theblack trace shows the average of a number of individual sweeps, each of which is shown in gray.
Four STAs, each from a different condition, are shown. The cell Tr2 spike that we used to triggereach sweep is shown only in the first condition for clarity of presentation. The cell Tr2 spike innormal saline is shown in the top trace. The black bar under the cell Tr2 trace indicates whencurrent was passed. The second trace (Normal) is the simultaneously recorded activity in cell256. The third trace (20/20) is the cell 256 recording from an STA done in 20 mM Ca 2⫹/20 mMMg 2⫹ saline. The fourth trace (0/20) is in 0 mM Ca 2⫹/20 mM Mg 2⫹ saline. The bottom trace(Wash) is again in normal saline. The four different salines were applied in that order, in a singlepreparation. Number of sweeps per condition are as follows: normal, 19; 20/20, 11; 0/20, 11;Wash, 11. B, Analogous data to that shown in A but for cell 252. Number of sweeps per conditionare as follows: normal, 21; 20/20, 21; 0/20, 11; Wash, 21. C, Analogous data to that shown in A,but for cell 54. Number of sweeps per condition are as follows: normal, 6; 20/20, 20; 0/20, 20;Wash, 20. D, Confocal image of cell 256 filled with the fluorescent dye tetramethylrhodaminedextran. E, Confocal image of cell 252 filled with the fluorescent dye Alexa 488. F, Confocalimage of cell 54(R) and 54(L), filled with the fluorescent dyes tetramethylrhodamine dextranand Alexa 488, respectively.
two processes that then exit the ganglion, one via the contralateral(to the soma) anterior lateral connective and the other via the
Figure 2.
Simultaneous electrical and optical recordings from a cell Tr2 target, cell 252. A,
Simultaneously recordings of cell Tr2(R) voltage, cell 252(10) voltage, and cell 252(10) fluores-
contralateral posterior lateral connective.
cence. Note the clear 1 Hz component in the fluorescence signal, despite the small size of the
We found segmental homologs of cells 256, 252, and 54 in all
membrane potential fluctuations. The first negative deflection of the cell 252(10) optical re-cording (arrow) seems to precede the stimulus onset because of the filtering used to debleach
the optical signals (see Materials and Methods). B, Coherence of the optical and electrical re-cordings of cell 252(10), both with respect to the cell Tr2(R) membrane potential. As in Figure
Tr2 spikes. The black trace is a spike-triggered average of 21 individual sweeps, with the indi-
1 E, the coherence from the optical recording is shifted by ⫹54° to correct for the phase shift
vidual sweeps shown in gray. Each cell Tr2 spike is followed by an EPSP in cell 252, with a latency
caused by the time constant of the dye response (see Materials and Methods). The coherence for
of 147 ⫾ 3 msec (mean ⫾ SD). The black bar under the cell Tr2 trace indicates when current was
the electrical recording is not corrected. C, Activity in cells Tr2(R) and 252(10), triggered on cell
passed. opt, Optical; elec, electrical.
Taylor et al. • Imaging Reveals Synaptic Targets in Leech
J. Neurosci., December 10, 2003 • 23(36):11402–11410 • 11407
midbody ganglia investigated (segments 9 through 12). Usingimaging or electrical recordings, or both, we identified a total of36 cells 256(10) in as many animals, 20 cells 252(10) in as manyanimals, and 36 cells 54(10) in 24 animals. The homologs in othersegments were identified one to four times each per segmentexamined. Given the extensive segmental homology of the leechbody plan, it is likely that cells 256, 252, and 54 are present in all ormost midbody ganglia (Muller et al., 1981).
We found that both cell Tr2 homologs connected to all targets
within a given midbody ganglion. That is, cells Tr2(L) and Tr2(R)both connected to cells 256(n), 252(n), 54(L,n), and 54(R,n),where n is the segment number (data not shown).
Cells 256 and 54 terminate swimming
We tested the ability of cells 256, 252, and 54 to terminate swim-
ming by activating them individually during ongoing swimming
episodes. We found that cells 256 and 54 stopped swimming
episodes when driven strongly (Fig. 4) (data shown for cell 256
only). Consistent with this, cells 256 and 54 showed an increased
firing rate during swim stops caused by cell Tr2 stimulation.
We found that driving cell 256 stopped swimming at least
once in 67% of animals in which it was attempted (n ⫽ 9) (Fig.
4 A) and that such stimulation stopped swimming in 50% ofattempts (n ⫽ 26). We typically drove cell 256 at ⬃30 Hz for 3– 4sec to obtain effective swim termination. This rate is substantiallyhigher than the rate at which cell 256 spikes during cell Tr2-induced swim stops. In the example shown in Figure 4 B, cell 256spiked at 7.3 Hz during a Tr2-induced swim stop (n ⫽ 1).
To verify that the apparent effect of cell 256 was not attribut-
able to chance, we performed a more controlled experiment ontwo nerve cords. Each trial in this experiment consisted of a nerveshock, which initiated swimming, followed at a set latency bystimulation of cell 256 at a high rate for 4 sec. A trial was countedas a "success" if swimming stopped during the stimulus and a"failure" if it stopped after the stimulus. (We discarded trials inwhich swimming stopped before the onset of stimulation.) Wethen compared the success rate in this condition with the successrate in a control condition in which cell 256 was not stimulated. Acontrol trial was counted as a success if the swim stopped during
Figure 4.
Cell 256 stops swimming. A, Examples of a swim terminated by cell 256. Simulta-
neous recordings from cell 256(12), cell 208(11) (a swim CPG cell), and the DP nerves in seg-
the "stimulation window," although no stimulus was delivered
ments 12 and 16 are shown. Bursts of spikes in the DP nerves at ⬃1 Hz indicate an ongoing
during this time. Swimming stopped during the stimulation win-
swim episode (Ort et al., 1974). The bar indicates when current (⫹0.8 nA) was being passed.
dow in 100% of the six stimulated trials, as compared with 20% of
The inset shows the spiking activity of cell 256. Individual spikes are indicated by dots. The
the five control trials. This difference is significant ( p ⬍ 0.02;
average spike rate of cell 256 during current passage was 32 Hz in this trial. B, Cell 256 activity
one-sided Fisher–Irwin test).
during a cell Tr2-induced swim termination. Simultaneous recordings from cell Tr2(L), cell
A single cell 54 was also capable of stopping swimming when
256(10), and the DP nerve in segment 15 during a cell Tr2-induced swim stop are shown. The
stimulated strongly, although cells 54 stopped swimming more
bar shows when current was passed. Current was delivered in a 4 sec train of 25 msec, ⫹1.0 nA
effectively when both homologs were stimulated simultaneously
pulses at 20 Hz. The cell Tr2 recording saturated during current passage and is truncated in the
(data not shown). In some trials, swimming slowed (burst fre-
figure. The average spike rate of cell 256(10) during the stimulus was 7.3 Hz.
quency decreased) for the duration of cell 54 stimulation but didnot stop. Driving both cells 54 slowed or stopped swimming at
252 did depolarize when Tr2 stimulation stopped swimming
least once in 100% of animals (n ⫽ 5) and in 93% of trials overall
(n ⫽ 1), but driving cell 252 during swimming in three animals
(n ⫽ 14). Driving both cells 54 stopped swimming in 60% of
had weak and variable effects (data not shown).
individuals and in 29% of trials. Driving a single cell 54 slowed or
Finally, none of the identified cell Tr2 targets seemed to func-
stopped swimming at least once in 100% of animals (n ⫽ 4) and
tion as either a swim CPG cell or as a swim-initiating cell. None
in 73% of trials overall (n ⫽ 22). Driving a single cell 54 stopped
underwent membrane potential oscillations during swimming,
swimming in 50% of individuals in 9% of trials.
and none initiated swimming reliably when stimulated in the
As with cell 256, the spike rates needed to stop swimming by
quiescent animal (data not shown).
driving cells 54 were higher than the rates observed when westopped swimming by stimulating cell Tr2. The average spike raterequired for cell 54 to stop swimming was 42 ⫾ 11 Hz (n ⫽ 5; in
three animals), whereas its average spike rate when a cell Tr2
We identified three novel synaptic targets of cell Tr2 using FRET
stopped swimming was only 2.8 ⫾ 0.9 Hz (n ⫽ 3; in two animals).
voltage-sensitive dye imaging. Previously, no monosynaptic tar-
The role of cell 252 in terminating swimming is not clear. Cell
gets of cell Tr2 were known. All target neurons were found reliably
11408 • J. Neurosci., December 10, 2003 • 23(36):11402–11410
Taylor et al. • Imaging Reveals Synaptic Targets in Leech
abolically more efficient than having cell Tr2 make synapses ontoall 20n– 40n downstream cells and would effectively be a form ofamplification.
Integration of anti-swimming inputs
Another possibility is that cells 256 and 54 have a broader role in
the leech nervous system. It seems likely that their activity is not
simply a function of the activity of cell Tr2 but reflects inputs
from other neurons as well. For instance, cells 256 and 54 might
act as integrators of various anti-swimming inputs. Previous au-
thors have shown that there are parallel swim-activating and
swim-inactivating systems in the leech (Brodfuehrer and Burns,
1995), and it is possible that cells 256 and 54 serve as part of an
anti-swim-gating network, parallel to the swim-gating cells (cells
204, 21, and 61). These two networks would be expected to in-
hibit one another, thus forming a distributed "decision system"
for swimming. Various pro-swimming and anti-swimming in-
Figure 5.
Summary of cell Tr2 targets and their effects on swimming. Connections drawn
with bars indicate monosynaptic chemical excitatory connections. Arrows with negative signs
puts would converge on the swim-gating and anti-swim-gating
next to them indicate an observed inhibitory effect the neuronal basis of which has not been
systems, and whichever system received the greater net input
identified. Percentages next to arrows indicate the fraction of animals in which the given cell (or
would prevail. Such a scheme has been proposed for neurons in
cell pair, for cells 54) was effective in stopping swimming. The dashed arrow with a question
the medial temporal and lateral intraparietal areas of monkey
mark next to it indicates an unknown effect.
cortex that are thought to mediate judgments about motion di-rectionality (Shadlen and Newsome, 1996, 2001; Shadlen et al.,1996; Gold and Shadlen, 2000).
across animals, in multiple midbody ganglia. Two of the targets, cells
Some support for such an anti-swim-gating hypothesis comes
256 and 54, stopped swimming when driven to fire at relatively high
from the observation that the organization of the cell Tr2 swim-
rates. These results suggest that cells 256 and 54, and possibly cell
terminating circuitry seems to grossly parallel elements of the
252, provide a segmental neuronal layer that relays the activity of cell
swim-initiation circuitry. In part of the swim-initiation circuitry,
Tr2 to the swim-gating cells or to the swim CPG (Fig. 5). Thus we
cell Tr1, a head brain cell pair, synapses on segmental swim-
have identified what is likely to be the first step of the inhibitory
gating cells. These cells then connect to the swim CPG (Brodfue-
neuronal pathway from cell Tr2 to the swim CPG.
hrer and Friesen, 1986a,b; Nusbaum and Kristan, 1986). Simi-larly, cell Tr2, a head brain cell pair, synapses on cells 256 and 54,which then (directly or indirectly) connect to the swim CPG. In
What is the role of cells 256 and 54 in behavior?
both systems, however, it seems that the connections of the seg-
We have shown that stimulating individual cells 256 and 54 can
mental cells are not strictly intrasegmental: cell 204 makes all its
stop swimming but that the rates required are higher than those
known connections with CPG cells intersegmentally (although
observed in the cells when driving cell Tr2 to stop swimming (Fig.
4). This is not surprising. Cells 256 and 54 were present in all four
intrasegmental connections may exist), and the morphology of
segmental ganglia searched, and it is probable that homologs exist
cells 256 and 54 suggests that they too are likely to make many of
in most of the 21 midbody ganglia. When all of these homologs
their synapses intersegmentally. Thus the organization of the cell
are activated by cell Tr2 activity, the summed effect is likely to be
Tr2 swim-terminating circuitry seems roughly to parallel the cell
quite strong. In this light, it is perhaps surprising that stimulating
Tr1 circuitry for swim initiation.
a single cell 256 or cell 54 is able to stop swimming. It seemsreasonable that the combined activity of perhaps 20 cells 256 and40 cells 54, all firing at modest rates, would be sufficient to explain
the swim-terminating effects of cell Tr2.
In the anti-swim-gating scheme described above, all of the cells
If the entire functional role of cells 256 and 54 were to relay the
256 and 54 in the animal are collectively "representing" a single
activity of cell Tr2, it would seem simpler for cell Tr2 to synapse
thing: the extent to which the current situation of the animal
directly onto the swim CPG cells to stop swimming. In Xenopus
favors or disfavors swimming. Another possibility is that the cells
tadpoles, for instance, reticulospinal neurons that stop an ongo-
256 and 54 in different segments are representing different
ing swim when stimulated connect directly onto a class of spinal
things. For instance, cell 256 or 54 (or both) might play the role of
motor neurons that help generate the swim rhythm (Roberts et
a "segmental swim slowing" neuron, which is used by the leech
al., 1997; Perrins et al., 2002). What does having cells 256 and 54
CNS to slow down or phase delay the swim oscillation locally (in
in the circuit contribute that could not be achieved by having cell
the same segment and perhaps a few neighboring segments). This
Tr2 synapse directly onto the swim-gating cells or the swim CPG
might be useful as part of a sensory feedback loop acting to main-
tain the proper phase in each segment in the face of local pertur-
One possibility is that cells 256 and 54 act to distribute the
bations. Such local feedback, although not mediated by non-CPG
output of cell Tr2. That is, each cell Tr2 could synapse on some
interneurons, has been identified in the leech and lamprey swim
20 – 40 cells 256 or 54, or both, and each of these could then
circuits (Viana Di Prisco et al., 1990; Wallen, 1997; Cang and
synapse on some number of swim CPG cells, call it n. In this way,
Friesen, 2000; Cang et al., 2001). If cells 256 and 54 play such a
the activity of cell Tr2 would effect 20n– 40n downstream cells,
role, it would presumably complement the direct feedback iden-
although making only 20 – 40 synapses itself. This might be met-
tified previously (Cang et al., 2001).
Taylor et al. • Imaging Reveals Synaptic Targets in Leech
J. Neurosci., December 10, 2003 • 23(36):11402–11410 • 11409
A similar possibility is that cell 256 or 54 plays the role of a
interactions between ventral stretch receptors and swim-related neurons.
"segmental shortening" neuron. Such a neuron could be used
J Comp Physiol [A] 187:569 –579.
during crawling to generate the coordinated wave of local short-
Frost WN, Katz PS (1996) Single neuron control over a complex motor
ening that comprises the contraction phase of a crawl step. Such
pattern. Proc Natl Acad Sci USA 93:422– 426.
Gold JI, Shadlen MN (2000) Representation of a perceptual decision in de-
cells have been posited to exist in a model of the leech crawl CPG
veloping oculomotor commands. Nature 404:390 –394.
(Cacciatore et al., 2000). In both this and the segmental-swim-
Gonza´lez JE, Tsien RY (1995) Voltage sensing by fluorescence resonance
slowing scheme, cells 256 and 54 would effectively serve two re-
energy transfer in single cells. Biophys J 69:1272–1280.
lated but distinct roles: to mediate swim termination by Tr2 and
Gonza´lez JE, Tsien RY (1997) Improved indicators of cell membrane poten-
coordinate other behaviors.
tial that use fluorescence resonance energy transfer. Chem Biol4:269 –277.
Gonza´lez JE, Oades K, Leychkis Y, Harootunian A, Negulescu PA (1999)
Using imaging to find synaptic targets
Cell-based assays and instrumentation for screening ion-channel targets.
We found imaging to be an essential tool in identifying synaptic
Drug Discov Today 4:431– 439.
targets of cell Tr2. Even having established the morphology and
Grillner S, Georgopoulos AP, Jordan LM (1997) Selection and initiation of
motor behavior. In: Neurons, networks, and motor behavior (Stein PSG,
electrophysiological properties of cell 256, we relied on imaging
Grillner S, Selverston AI, Stuart DG, eds), pp 3–19. Cambridge, MA: MIT.
to find this cell in each preparation. The exact position of the
Hannan EJ (1970) Multiple time series. New York: Wiley.
soma of cell 256 varies and is in an area of the ganglion with many
Jarvis MR, Mitra PP (2001) Sampling properties of the spectrum and coher-
somata of similar size. Therefore, our primary means of identify-
ency of sequences of action potentials. Neural Comput 13:717–749.
ing the cell is to drive cell Tr2 and image all the neurons in its
Kozloski J, Hamzei-Sichani F, Yuste R (2001) Stereotyped position of local
general location. In contrast, we can reliably identify cells 252 and
synaptic targets in neocortex. Science 293:868 – 872.
54 solely on the basis of soma position and spike shape, although
Kristan Jr WB, Shaw BK (1997) Population coding and behavioral choice.
we used imaging for their initial discovery.
Curr Opin Neurobiol 7:826 – 831.
It should be noted that other targets of cell Tr2 may exist.
Kupfermann I, Weiss KR (1978) The command neuron concept. Behav
Brain Sci 1:3–39.
Although the optical method greatly facilitates the search for syn-
Liao X, Walters ET (2002) The use of elevated divalent cation solutions to
aptic targets, it does not guarantee that one will find all of them.
isolate monosynaptic components of sensorimotor connections in Aply-
In particular, the dorsal side of the midbody ganglia was not
sia. J Neurosci Methods 120:45–54.
examined as extensively as the ventral side, so there may be addi-
Muller KJ, Nicholls JG, Stent GS (1981) Neurobiology of the leech. Cold
tional Tr2 targets with their somata on the dorsal surface.
Spring Harbor, NY: Cold Spring Harbor Laboratory.
Our work is related to previous work using imaging to explore
Nicholls JG, Purves D (1970) Monosynaptic chemical and electrical con-
connectivity. Kozloski et al. (2001) recently used calcium imaging
nexions between sensory and motor cells in the central nervous system of
to explore cortical connectivity in the mouse, building on earlier
the leech. J Physiol (Lond) 209:647– 667.
work developing the technique (Peterlin et al., 2000). Those au-
Nusbaum MP, Kristan Jr WB (1986) Swim initiation in the leech by
serotonin-containing interneurones, cells 21 and 61. J Exp Biol
thors used imaging to find neurons in mouse cortex that are
targets of an impaled neuron. This enabled them to examine the
O'Gara BA, Friesen WO (1995) Termination of leech swimming activity by
cell-type specificity of cortical microcircuitry. The ability to
a previously identified swim trigger neuron. J Comp Physiol [A]
record the electrical activity of a number of individual neurons,
177:627– 636.
using various optical techniques, should open the way for estab-
Orlovsky GN, Deliagina TG, Grillner S (1999) Neuronal control of locomo-
lishing the connectivity of neuronal circuits in both simple and
tion: from mollusc to man. Oxford: Oxford UP.
complex brains.
Ort CA, Kristan Jr WB, Stent GS (1974) Neuronal control of swimming in
the medicinal leech. II. Identification and connections of motor neurons.
J Comp Physiol [A] 94:121–154.
Pearson KG (1993) Common principles of motor control in vertebrates and
invertebrates. Annu Rev Neurosci 16:265–297.
Berry MS, Pentreath VW (1976) Criteria for distinguishing between mono-
Percival DB, Walden AT (1993) Spectral analysis for physical applications.
synaptic and polysynaptic transmission. Brain Res 105:1–20.
Cambridge, UK: Cambridge UP.
Brodfuehrer PD, Burns A (1995) Neuronal factors influencing the decision
Perrins R, Walford A, Roberts A (2002) Sensory activation and role of in-
to swim in the medicinal leech. Neurobiol Learn Mem 63:192–199.
hibitory reticulospinal neurons that stop swimming in hatchling frog
Brodfuehrer PD, Friesen WO (1986a) Initiation of swimming activity by
tadpoles. J Neurosci 22:4229 – 4240.
trigger neurons in the leech subesophageal ganglion. I. Output connec-
Peterlin ZA, Kozloski J, Mao BQ, Tsiola A, Yuste R (2000) Optical probing
tions of Tr1 and Tr2. J Comp Physiol [A] 159:489 –502.
of neuronal circuits with calcium indicators. Proc Natl Acad Sci USA
Brodfuehrer PD, Friesen WO (1986b) Initiation of swimming activity by
97:3619 –3624.
trigger neurons in the leech subesophageal ganglion. II. Role of segmental
Rink TJ, Montecucco C, Hesketh TR, Tsien RY (1980) Lymphocyte mem-
swim-initiating interneurons. J Comp Physiol [A] 159:503–510.
brane potential assessed with fluorescent probes. Biochim Biophys Acta
Byrne JH, Castellucci VF, Kandel ER (1978) Contribution of individual
mechanoreceptor sensory neurons to defensive gill-withdrawal reflex in
Roberts A, Soffe SR, Perrins R (1997) Spinal networks controlling swim-
Aplysia. J Neurophysiol 41:418 – 431.
ming in hatchling Xenopus tadpoles. In: Neurons, networks, and motor
Cacciatore TW, Brodfuehrer PD, Gonza´lez JE, Jiang T, Adams SR, Tsien RY,
Kristan Jr WB, Kleinfeld D (1999) Identification of neural circuits by
behavior (Stein PSG, Grillner S, Selverston AI, Stuart DG, eds), pp 83–90.
imaging coherent electrical activity with FRET-based dyes. Neuron
Cambridge, MA: MIT.
23:449 – 459.
Shadlen MN, Newsome WT (1996) Motion perception: seeing and decid-
Cacciatore TW, Rozenshteyn R, Kristan WB Jr (2000) Kinematics and mod-
ing. Proc Natl Acad Sci USA 93:628 – 633.
eling of leech crawling: evidence for an oscillatory behavior produced by
Shadlen MN, Newsome WT (2001) Neural basis of a perceptual decision in
propagating waves of excitation. J Neurosci 20:1643–1655.
the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol
Cang J, Friesen WO (2000) Sensory modification of leech swimming: rhyth-
86:1916 –1936.
mic activity of ventral stretch receptors can change intersegmental phase
Shadlen MN, Britten KH, Newsome WT, Movshon JA (1996) A computa-
relationships. J Neurosci 20:7822–7829.
tional analysis of the relationship between neuronal and behavioral re-
Cang J, Yu X, Friesen WO (2001) Sensory modification of leech swimming:
sponses to visual motion. J Neurosci 16:1486 –1510.
11410 • J. Neurosci., December 10, 2003 • 23(36):11402–11410
Taylor et al. • Imaging Reveals Synaptic Targets in Leech
Thomson DJ (1982) Spectral estimation and harmonic analysis. Proc IEEE
undulatory swimming in lamprey. In: Neurons, networks, and motor
behavior (Stein PSG, Grillner S, Selverston AI, Stuart DG, eds), pp 75– 82.
Thomson DJ, Chave AD (1991) Jackknifed error estimates for spectra, co-
Cambridge, MA: MIT.
herences, and transfer functions. In: Advances in spectrum analysis and
Weeks JC (1981) Neuronal basis of leech swimming: separation of swim
array processing (Shykin S, ed), pp 58 –113. Englewood Cliffs, NJ: Pren-
initiation, pattern generation, and intersegmental coordination by selec-
tive lesions. J Neurophysiol 45:698 –723.
Tsien RY (1976) The design and use of organic chemical tools in cellular
Weeks JC, Kristan Jr WB (1978) Initiation, maintenance, and modulation
physiology. PhD thesis, University of Cambridge.
of swimming in the medicinal leech by the activity of a single neuron. J
Viana Di Prisco G, Wallen P, Grillner S (1990) Synaptic effects of intraspinal
Exp Biol 77:71– 88.
stretch receptor neurons mediating movement-related feedback during
Zochowski M, Wachowiak M, Falk CX, Cohen LB, Lam YW, Antic S, Zecevic
locomotion. Brain Res 530:161–166.
D (2000) Imaging membrane potential with voltage-sensitive dyes. Biol
Wallen P (1997) Spinal networks and sensory feedback in the control of
Bull 198:1–21. 0
Source: http://adamtaylor.info/taylor-et-al-2003.pdf
Fact sheet BT Wholesale American Share Fund ARSN: 087 594 509 About the Fund Performance The BT Wholesale American Share Fund (Fund) is an actively managed portfolio of North American shares. The Fund invests primarily in United States and Canadian shares. The Fund may also hold cash and may use derivatives.
Long-Term Use of Contraceptive Depot Medroxyprogesterone Acetate in Young Women Impairs Arterial Endothelial Function Assessed by Cardiovascular Morten B. Sorensen, PhD; Peter Collins, MD; Paul J.L. Ong, MA; Carolyn M. Webb, PhD; Christopher S. Hayward, MD; Elizabeth A. Asbury, MSc; Peter D. Gatehouse, PhD; Andrew G. Elkington, MB, BS; Guang Z. Yang, PhD; Ali Kubba, MB, ChB; Dudley J. Pennell, MD

