Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Integrative oncology (weil integrative medicine library)


DONALD I. ABRAMS AND MANUEL GUZMAN
Key Concepts
■ Cannabis has been used in medicine for thousands of years prior to achieving its current status
as an illicit substance
■ Cannabinoids, the active components of Cannabis sativa, mimic the effects of the endogenous
cannabinoids (the so-called endocannabinoids), activating specific cannabinoid receptors,particularly CB1 found predominantly in the central nervous system and CB2 found in cellsinvolved with immune function.
■ Delta-9-tetrahydrocannabinol, the main bioactive cannabinoid in the plant, has been available
as a prescription medication approved for chemotherapy-induced nausea and vomiting andtreatment of anorexia associated with the AIDS wasting syndrome.
■ In addition to treatment of nausea and anorexia, cannabinoids may be of benefit in the
treatment of cancer-related pain, possibly in a synergistic fashion with opioid analgesics.
■ Cannabinoids have been shown to be of benefit in the treatment of HIV-related peripheral
neuropathy suggesting that they may be worthy of study in patients with chemotherapy-relatedneuropathic symptoms.
■ Cannabinoids have a favorable drug safety profile, medical use predominantly limited by their
psychoactive effects and their limited bioavailability.
■ There is no conclusive evidence that chronic cannabis use leads to the development of any
malignancies; some preclinical studies actually suggest a protective effect.
■ Cannabinoids inhibit tumor growth in laboratory animal models by modulation of key cell-
signaling pathways, inducing direct growth arrest and tumor cell death, as well as byinhibiting tumor angiogenesis and metastasis.
■ Cannabinoids appear to be selective antitumor compounds because they kill tumor cells
without affecting their nontransformed counterparts
■ More basic and clinical research is needed to ascertain not only the role of cannabinoids in
palliative cancer care, but to delineate their role as potential anti-cancer agents with activityat a number of sites by way of multiple mechanisms of action.
Although long-recognized for its medicinal values and widely used by millions throughout the world,cannabis receives little attention in the standard literature because of its status as a controlledsubstance and classification in the United States as a Schedule I agent with a high potential for abuseand no known medical use. Data on the potential effectiveness of medicinal cannabis is difficult tofind due to the limited numbers of clinical trials that have been conducted to date. As a botanical,cannabis shares those difficulties encountered in the study of plants that are grown in many climatesand environments from diverse genetic strains and harvested under variable conditions. However, the
potential benefits of medicinal cannabis have not been lost on a large number of people living withcancer, some of whom have been quite vocal in attributing their ability to complete their prescribedcourse of chemotherapy to the anti-emetic effects of inhaled cannabis. In the practice of integrativeoncology, the provider is frequently faced with situations in which being able to recommendcannabinoids in cancer.
Cannabis as Medicine: A Brief History
subcontinent, cannabis was introduced into Western medicine in the 1840's by W. B. O'Shaughnessy,a surgeon who learned of its medicinal benefits first hand while working in the British East IndiesCompany. Promoted for reported analgesic, sedative, anti-inflammatory, antispasmodic andanticonvulsant properties, cannabis was said to be the treatment of choice for Queen Victoria'sdysmennorhea. In the early 1900s, medicines that were indicated for each of cannabis's purportedactivities were introduced into the Western armamentarium making its use less widespread.
Physicians in the United States were the main opponents to the introduction of the Marihuana Tax
Act by the Treasury Department in 1937. The legislation was masterminded by Harry Anslinger,director of the Federal Bureau of Narcotics from its inception in 1931 until 1962, who testified inCongress that "Marijuana is the most violence-causing drug in the history of mankind." The Actimposed a levy of one dollar an ounce for medicinal use and one hundred dollars an ounce forrecreational use, which in 1937 dollars was a prohibitive cost. By using the Mexican name for theplant and associating it with nefarious South-of-the-Border activities, the proponents fooled manyphysicians. The Act was singly opposed by the American Medical Association who felt that objectiveevidence that cannabis was harmful was lacking and that its passage would impede further researchinto its medical utility. In 1942, cannabis was removed from the U.S. Pharmacopoeia.
Mayor Fiorello LaGuardia of New York commissioned an investigation into the reality of the
potential risks and benefits of cannabis that reported in 1944 that the substance was not associatedwith any increased risk of criminal activity, addiction or insanity as had been claimed. TheLaGuardia Commission Report, as well as subsequent similar investigations that have beencommissioned nearly every decade since, went largely ignored.
In 1970 with the initiation of the Controlled Substances Act, marijuana was classified as a
Schedule I dug. Where both Schedule I and Schedule II substances have a high potential for abuse,Schedule I drugs are distinguished by having no accepted medical use. Other Schedule I substancesinclude heroin, LSD, mescaline, methylqualone and, most recently, gammahydroxybutyrate (GHB). In1973, President Nixon's investigation into the risks and benefits of marijuana, the ShaferCommission, concluded that it was a safe substance with no addictive potential that had medicinalbenefits. Despite the fact that it was deemed to have no medical use, marijuana was distributed topatients by the United States government on a case by case basis by way of a Compassionate UseInvestigational New Drug (IND) program established in 1978.
In the late 1980s and early 1990s, many people living with human immunodeficiency virus-1 (HIV)
10% body weight and frequently fever and diarrhea created hordes of cachectic individuals in searchprogram, the Bush administration shut it down in 1992, the same year that dronabinol (delta-9-tetrahydrocannabinol [THC], Marinol®) was approved for treatment of anorexia associated with theAIDS wasting syndrome.
Delta-9-THC is one of the approximately 100 cannabinoids found in the cannabis plant and is felt
to be the main psychoactive component. Overall, the plant contains about 400 compounds derivedfrom its secondary metabolism, many of which may contribute to its medicinal effect. Synthetic delta-9-THC in sesame oil was first licensed and approved in 1986 for the treatment of chemotherapy-associated nausea and vomiting. Clinical trials done at the time determined that dronabinol was aseffective, if not more so, than the available antiemetic agents (Sallen & Zinberg, 1975). The potentclass of serotonin 5-HT3 receptor antagonists which have subsequently revolutionized the ability toadminister emetogenic chemotherapy had not yet come to market.
Dronabinol was investigated for its ability to stimulate weight gain in patients with the AIDS
wasting syndrome in the late 1980's. Results from a number of trials suggested that although patientsdronabinol alone and together, the cannabinoid seemed to negate some of the weight increase seen inthose only receiving the hormone (.
Cannabinoid Chemistry and Biologic Effects
Cannabinoids are a group of 21 carbon terpenophenolic compounds produced uniquely by Cannabiscompounds, the plant compounds may also be referred to as phytocannabinoids. Although delta-9-THC is the primary active ingredient in cannabis, there are a number of non-THC cannabinoids andnoncannabinoid compounds that also have biologic activity. Cannabidiol (CBD), cannabinol,cannabichromene, cannabigerol, tetrahydrocannabivirin and delta-8-THC are just some of theadditional cannabinoids that have been identified. It is postulated that the secondary compounds mayenhance the beneficial effects of delta-9-THC, for example by modulating the THC-induced anxiety,anticholinergic or immunosuppressive effects, and may reduce the unwanted effects of delta-9-THC,for example by attenuating seizures, psychoses or motor discoordination. In addition, cannabisassociated terpenoids and flavonoids may increase cerebral blood flow, enhance cortical activity,kill respiratory pathogens and provide anti-inflammatory activity (.
The neurobiology of the cannabinoids has only been identified within the past 25 years during
research investigations. In 1986 it was discovered that cannabinoids inhibited the accumulation ofcyclic adenosine monophosphate (cAMP), suggesting the presence of a receptor-mediatedmechanism. By attaching a radiolabel to the synthetic cannabinoid, the first cannabinoid receptor,
CB1, was pharmacologically identified in the brain in 1988. The CB1 receptor is coupled to Giproteins. Its engagement inhibits adenylyl cyclase and voltage-gated calcium channels, and stimulatesrectifying potassium conductances and mitogen-activated protein kinase activity. By 1990,investigators had cloned the CB1 receptor, identified its DNA sequence and mapped its location inthe brain, with the largest concentration being in the basal ganglia, cerebellum, hippocampus andcerebral cortex. Nowadays CB1 is known to be an ubiquitous protein that is present in basically allbody tissues. In 1993 a second cannabinoid receptor, CB2, was identified outside the brain.
Originally detected in macrophages and the marginal zone of the spleen, the highest concentration ofCB2 receptors is located on the B lymphocytes and natural killer cells, suggesting a possible role inimmunity.
The existence of cannabinoid receptors has subsequently been demonstrated in most animal
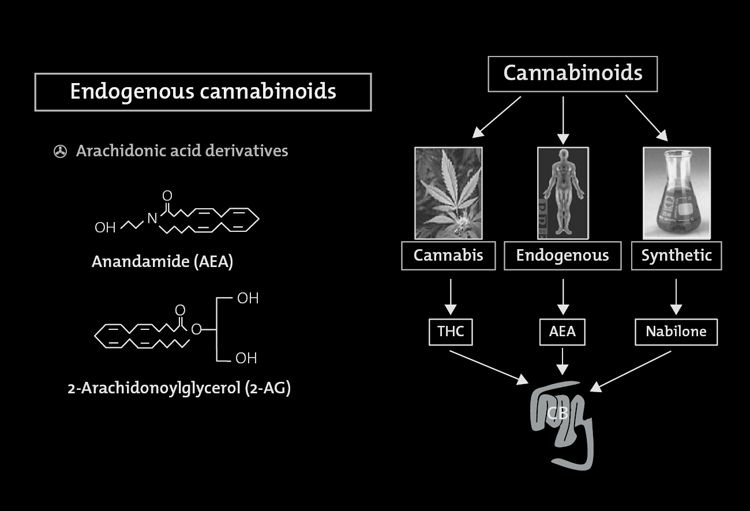
species, all the way down to invertebrates. Are these receptors present in the body solely to complexwith ingested phytocannabinoids? The answer came in 1992 with the identification of a brainconstituent that binds to the cannabinoid receptor. Named anandamide from the Sanskrit word forbliss, the first endocannabinoid had been discovered. Subsequently 2- arachidonoylglycerol (2-AG)has also been confirmed as part of the body's endogenous cannabinoid system. Theseendocanabinoids function as neuromodulators. As the ligands for the 7-transmembrane domaincannabinoid receptors located in presynaptic nerve terminals, binding of the endocannabinoid leadsto G-protein activation and the cascade of events transpires resulting in the opening of potassiumchannels which decreases cell firing and the closure of calcium channels which decreasesneurotransmitter release .
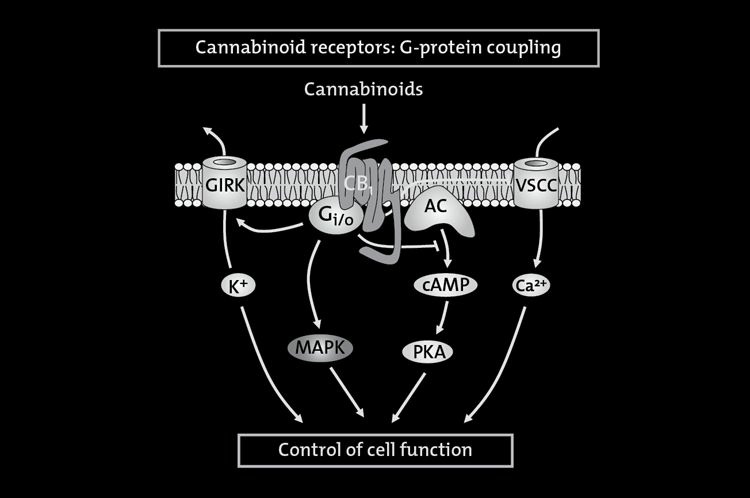
FIGURE 8.1. Cannabinoids and their receptors.
Cannabinoids are a group of 21 carbon terpenophenolic compounds produced by Cannabis species. The phytocannabinoids complexwith two receptors, CB1 and CB2, to produce their physiologic effects.
The functions of the endogenous cannabinoid system in the body are becoming more appreciated
through advances in cannabinoid pharmacology ( . Theidentification of the cannabinoid receptors has led to a host of agonists and antagonists beingsynthesized. Utilizing these tools, investigators are discovering that the system is likely to beimportant in the modulation of pain and appetite, suckling in the newborn and the complexities ofmemory (Michael Pollen in The Botany of Desire gives a particularly entertaining description of thenatural function of endocannabinoids in memory (2001)). In addition to being utilized to learn moreabout the natural function of the endocannabinoid system, a number of these cannabinoid receptoragonists and antagonists are being developed as potential pharmaceutical therapies. In the meantime,dronabinol, nabilone (Cesamet®, a synthetic cannabinoid) and cannabis are the currently availablecannabinoid therapies in the United States. Levonantradol (Nantrodolum®) is a synthetic cannabinoidadministered intramuscularly, not used as much clinically since the oral agents became available.
Nabiximols (Sativex®), a whole plant extract delivered as an oro-mucosal spray with varyingcombinations of THC and cannabidiol, is available in Canada and some European countries andundergoing late phase testing in the US and other countries.
FIGURE 8.2. Signaling pathways coupled to the CB1 cannabinoid receptor.
Cannabinoids exert their effects by binding to specific Gi protein-coupled receptors. The CB1 cannabinoid receptor signals to a numberof different cellular pathways. These include, for example, (i) inhibition of the adenylyl cyclase (AC)–cyclic AMP–protein kinase A
(PKA) pathway; (ii) modulation of ion conductances, by inhibition of voltage-sensitive Ca2+ channels (VSCC) and activation of Gi
protein-coupled inwardly rectifying K+ channels (GIRK); and (iii) activation of mitogen-activated protein kinase (MAPK) cascades.
Other less established cannabinoid receptor effectors and the crosstalk among the different pathways have been omitted forsimplification.
Through the receptors described above, cannabis delivered by way of inhalation, orally or oro-
mucosally can produce a host of biologic effects (). The1999 Institute of Medicine report—Marijuana and Medicine: Assessing the Science Base—makesthe following general conclusions about the biology of cannabis and cannabinoids (
• Cannabinoids likely have a natural role in pain modulation, control of movement, and memory.
• The natural role of cannabinoids in immune systems is likely multifaceted and remains unclear.
• The brain develops tolerance to cannabinoids.
• Animal research has demonstrated the potential for dependence, but this potential is observed under a narrower
range of conditions than with benzodiazepines, opiates, cocaine, or nicotine.
• Withdrawal symptoms can be observed in animals but appear mild compared with those of withdrawal from
opiates or benzodiazepines.
Pharmacology of Cannabis
and remain elevated with a terminal half-life of 20–30 hours. When consumed orally, delta-9-THC isinitially metabolized in the liver to 11-OH-THC, also a potent psychoactive metabolite. On the otherhand, when inhaled, the cannabinoids are rapidly absorbed into the bloodstream with a peakconcentration in 2–10 minutes, which rapidly declines over the next 30 minutes. Thus, smokingachieves a higher peak concentration with a shorter duration of effect. Less of the psychoactive 11-OH-THC metabolite is formed. When nabiximols is taken oro-mucosally, no pharmacokineticinteractions seem to occur between its two major cannabinoid constituents, namely THC and CBD,and the pharmacokinetic properties of the THC present in nabiximols are similar to those of oral THC(.
may induce P450 isoforms. The effects are predominantly related to the CYP1A2, CYP2C andCYP3A isoforms. The potential for a cannabinoid interaction with cytochrome P450 and, hence,possibly metabolism of chemotherapeutic agents has led to a small amount of data on the possibilityof botanical:drug interactions. In one study, 24 cancer patients were treated with intravenousirinotecan (600 mg, n = 12) or docetaxel (180 mg, n = 12), followed 3 weeks later by the same drugsconcomitant with medicinal cannabis taken as an herbal tea for 15 consecutive days, starting 12 daysbefore the second treatment . The carefully conducted pharmacokinetic analysesshowed that cannabis administration did not significantly influence exposure to and clearance ofirinotecan or docetaxel.
Cannabinoids and Cancer Symptom Management
The nausea and vomiting related to cancer chemotherapy continues to be a significant clinicalproblem even in light of the newer agents that have been added to our armamentarium since the 1970sand 1980s when clinical trials of cannabinoids were first conducted ( Inthose days, phenothiazines and metoclopropramide were the main antiemetic agents used. Dronabinol,synthetic THC, and nabilone, a synthetic analog of THC, were both tested as novel oral agents in anumber of controlled clinical trials. Nabilone was approved in Canada in 1982, but only recentlybecame available in the United States. Dronabinol was approved as an antiemetic to be used incancer chemotherapy in the United States in 1986.
Numerous meta-analyses confirm the utility of these THC-related agents in the treatment of
chemotherapy-induced nausea and vomiting. conducted a systematic review of30 randomized comparisons of cannabis with placebo or antiemetics from which dichotomous data onefficacy and harm were available. Oral nabilone, oral dronabinol, and intramuscular levonantradolwere tested. No trials of smoked cannabis were included. There were 1,366 patients involved in thesystematic review. Cannabinoids were found to be significantly more effective antiemetics thanprochlorperazine, metoclopramide, chlorpromazine, thiethylperazine, haloperidol, domperidone, oralizapride. In this analysis, the number needed to treat (NNT) for complete control of nausea was 6;the NNT for complete control of vomiting was 8. Cannabinoids were not more effective in patientsreceiving very low or very high emetogenic chemotherapy. In crossover trials, patients preferredcannabinoids for future chemotherapy cycles. Tramer identified some "potentially beneficial sideeffects" that occurred more often with cannabinoids including the "high," sedation or drowsiness, andeuphoria. Less desirable side effects that occurred more frequently with cannabinoids includeddizziness, dysphoria or depression, hallucinations, paranoia, and hypotension.
A later analysis by Ben Amar reported that 15 controlled studies compared nabilone to placebo or
available antiemetic drugs (. In 600 patients with a variety of malignant diagnoses,nabilone was found to be superior to prochlorperazine, domperidone and alizapride, with patientsclearly favoring the nabilone for continuous use. Nabilone has also been shown to be moderatelyeffective in managing the nausea and vomiting associated with radiation therapy and anesthesia afterabdominal surgery ; ). In thesame meta-analysis, Ben Amar reports that in 14 studies of dronabinol involving 681 patients, thecannabinoid antiemetic effect was equivalent or significantly greater than chlorpromazine andequivalent to metochlopramide, thiethylperazine and haloperidol. It is noted that the efficacy of thecannabinoids in these studies was sometimes outweighed by the adverse reactions and that none of thecomparator antiemetics were of the serotonin receptor antagonist class that is the mainstay oftreatment today.
A small pilot, randomized, double-blind, placebo-controlled phase II trial was conducted to
investigate the whole-plant cannabis-based medicine, nabiximols, added to standard antiemetics inthe treatment of chemotherapy-induced nausea and vomiting (). Seven patients wererandomized to receive the mixture of delta-9-THC and CBD and 9 added placebo to their standard ofcare antiemetic regimen. Of the nabiximols recipients, 5 of the 7 compared to 2 of the 9 on placeboexperienced a complete response with a mean daily dose of 4.8 sprays in both groups without seriousadverse effects. Further larger studies of the potential of nabiximols as an antiemetic are warranted.
There have been only three controlled trials evaluating the efficacy of smoked cannabis in
chemotherapy-induced nausea and vomiting (. In two of the studies, the smoked
cannabis was only made available after patients failed dronabinol. The third trial was a randomized,double-blind, placebo-controlled, cross-over trial involving 20 adults where both smoked cannabisand oral THC were evaluated. One-quarter of the patients reported a positive antiemetic response tothe cannabinoid therapies. On direct questioning of the participants, 35% preferred the oraldronabinol, 20% preferred the smoked marijuana and 45% did not express a preference. Fourparticipants receiving dronabinol alone experienced distorted time perception or hallucinations,which were also reported by two with smoked marijuana and one with both substances. Bothdronabinol and nabilone are FDA-approved for the treatment of nausea and vomiting associated withcancer chemotherapy in patients who have failed to respond adequately to conventional antiemetictherapy. Nabilone's extended duration of action allows for twice-a- day dosing of one or twomilligrams commencing 1 to 3 hours prior to receiving chemotherapy. A dose of 1 or 2 milligrams thenight before administration of chemotherapy might also be useful. It is recommended to commencedronabinol at an initial dose of 5 mg/m2, also 1 to 3 hours prior to the administration ofchemotherapy, then every 2 to 4 hours after chemotherapy, for a total of 4 to 6 doses/day. Should the 5mg/m2 dose prove to be ineffective, and in the absence of significant side effects, the dose may beescalated by 2.5 mg/m2 increments to a maximum of 15 mg/m2 per dose. Nabilone, with fewermetabolites and a lower dose range, may be associated with fewer side effects. The need to dose oneto three hours prior to chemotherapy is one factor that drives patients to prefer inhaled cannabiswhere the delivery and effect peak within minutes. Patients also prefer the ability to more tightlytitrate the dose of cannabinoids they receive when inhaling compared to oral ingestion.
The National Comprehensive Cancer Network antiemesis guidelines recommend cannabinoids
among other therapies to consider as a breakthrough treatment for chemotherapy-induced nausea andvomiting ().
Anorexia, early satiety, weight loss, and cachexia are some of the most daunting symptommanagement challenges faced by the practicing oncologist. There are very few tools in the tool-boxfor addressing these concerns. Patients are not only disturbed by the disfigurement associated withwasting, but also by their inability to engage in the social interaction associated with breaking breadand partaking of a meal. For many, the hormonal manipulation with megestrol acetate (syntheticallyderived progesterone) may be contraindicated or the side effects undesirable. Two small controlledtrials demonstrated that oral THC stimulates appetite and may slow weight loss in patients withadvanced malignancies () In a larger randomized, double-blind, parallel group studyof 469 adults with advanced cancer and weight loss, patients received either 2.5 mg of oral THCtwice daily, 800 mg of oral megestrol daily, or both. In the megestrol monotherapy group, appetiteincreased in 75% and weight in 11% compared to 49% and 3%, respectively, in the oral THC group.
These differences were statistically significant. The combined therapy did not confer additionalbenefits. A smaller randomized placebo-controlled trial of dronabinol in cancer patientsdemonstrated enhanced chemosensory perception in the treatment group . In thepatients receiving cannabinoids, food was reported to taste better, appetite improved and theproportion of protein calories was increased compared to the placebo group.
Many animal studies have previously demonstrated that THC and other cannabinoids have a
stimulatory effect on appetite and increase food intake. It is felt that the endogenous cannabinoidsystem may serve as a regulator of feeding behavior. For example, anandamide in mice leads to a
potent enhancement of appetite (). It is felt thatthe CB1 receptors, present in the hypothalamus where food intake is controlled and in the mesolimbicreward system, may be involved in the motivational or reward aspects of eating. This led to thedevelopment of the pharmaceutical CB1 antagonist rimonabant (Acomplia ®), which was approvedin Europe for the treatment of obesity on the basis of Phase III clinical trials where it was shown toUnited States and was ultimately withdrawn from the European market because it was found to induceanxiety and depressive disorders that were deemed high risk, often leading to patient suicide.
Anecdotal as well as clinical trial evidence also supports the appetite- stimulating effect of
inhaling cannabis. In classic trials conducted in the 1970s in healthy controls, it was found that,especially when smoked in a social/communal setting, cannabis inhalation led to an increase incaloric intake, predominantly in the form of between-meal snacks, mainly in the form of fatty andsweet foods. In cancer patients with anorexia as well as chemotherapy-induced nausea, it is worthnoting that cannabis is the only antiemetic that also has orexigenic action. Although cannabis thusprovides two potential benefits to the patient with cancer, the appetite-stimulation does not alwaysreverse the cancer cachexia that is a function of energy wasting in addition to decreased food intake.
Our understanding of the possible mechanisms of cannabinoid-induced analgesia has been greatlyincreased through study of the cannabinoid receptors, endocannabinoids and synthetic agonists andantagonists. The CB1 receptor is found in the central nervous system as well as in peripheral nerveterminals. Elevated levels of the CB1 receptor—like opioid receptors—are found in areas of thebrain that modulate nociceptive processing ( . Incontrast, CB2 receptors are located in peripheral tissue and are present at very low expression levelsin the CNS. Of the endogenous cannabinoids identified, anandamide has high affinity for CB1receptors, whereas 2-AG has affinity for both CB1 and CB2 receptors. With the development ofreceptor-selective antagonists (SR141716 for CB1 and SR144528 for CB2), additional informationhas been obtained regarding the roles of the receptors and endogenous cannabinoids in modulation of
Cannabinoids may also contribute to pain modulation through an anti-inflammatory mechanism—a
CB2 effect with cannabinoids acting on mast cell receptors to attenuate the release of inflammatoryagents such as histamine and serotonin and on keratinocytes to enhance the release of analgesicopioids (Facci & Meng, 1998; ).
Cannabinoids are effective in animal models of both acute and persistent pain. The central analgesicmechanism differs from the opioids in that it cannot be blocked by opioid antagonists. The potentialfor additive analgesic effects with opioids as well as the potential for cannabinoids to reduce nauseaand increase appetite make a strong case for the evaluation of marijuana as adjunctive therapy forpatients on morphine (.
Medical literature cites evidence of cannabinoids' ability to reduce naturally occurring pain, but
few human studies have been performed. Early studies of cannabinoids on experimental pain in
human volunteers produced inconsistent results. In some cases, the administration of cannabinoidsfailed to produce observable analgesic effects; in others, cannabinoids resulted in an increase of painsensitivity (hyperalgesia). Institute of Medicine reviewers noted that these studies suffered from poordesign and methodological problems and dubbed their findings inconclusive (.
Encouraging clinical data on the effects of cannabinoids on chronic pain come from three studies of
cancer pain. Cancer pain results from inflammation, mechanical invasion of bone or other pain-sensitive structure, or nerve injury. It is severe, persistent, and often resistant to treatment withopioids. Noyes and colleagues conducted two studies on the effects of oral THC on cancer pain. Bothstudies used standard single-dose analgesic study methodology and met the criteria for well-controlled clinical trials of analgesic efficacy.
The first experiment measured both pain intensity and pain relief in a double-blind, placebo
controlled study of 10 subjects (. Observers compared theeffects of placebo and 5, 10, 15, and 20 mg doses of delta-9-THC over a 6-hour period. Researchersreported that 15 and 20 mg doses produced significant analgesia, as well as anti-emesis and appetitestimulation. Authors cautioned that some subjects reported unwanted side effects such as sedation anddepersonalization at the 20 mg dose level. In a follow-up single-dose study of 36 subjects, Noyes etal. reported that 10 mg of THC produced analgesic effects over a seven-hour observation periodcomparable to 60 mg of codeine, and that 20 mg of THC induced effects equivalent to 120 mg ofcodeine Authors noted that respondents found higher doses ofTHC to be more sedative than codeine. However, in a separate publication, Noyes et al reported thatpain yielded similar results (. Authors found the THC analogueequivalent to 50 mg of codeine and superior to both placebo and 50 mg of secobarbital in subjectswith mild, moderate and severe pain.
A more recent study investigated the effects of whole-plant extract preparations in patients with
intractable cancer pain (). One hundred seventy-seven patients experiencinginadequate analgesia despite chronic opioid use were randomized to receive the THC:CBD extract(N=60), the THC extract (N = 58) or placebo (N = 59) in a two-week, multicenter, double-blind trial.
Pain relief was superior in the THC:CBD group with twice as many patients in the combination armachieving a greater than 30% reduction in pain when compared to placebo. The THC alone groupfared more or less the same as the placebo recipients. No change from baseline in median dose ofopioids or need for breakthrough medications was seen.
Cannabinoids have also been shown to be of potential benefit in an animal model of neuropathic pain(. Neuropathic pain is a troubling symptom in cancerpatients, especially those treated with platinum-based chemotherapy or taxanes. A painful sensoryperipheral neuropathy is also commonly encountered in patients with HIV infection either as aconsequence of HIV itself or antiretroviral drugs used in treatment of the infection. We completed arandomized, controlled trial of smoked cannabis compared to placebo in 50 subjects with HIV-related peripheral neuropathy (). Smoked cannabis reduced daily pain by 34%compared to 17% with placebo (p = 0.03). Greater than 30% reduction in pain was reported by 52%in the cannabis group and by 24% in the placebo group (p = 0.04). The first cannabis cigarette
reduced chronic pain by a median of 72% compared to 15% with placebo (p < 0.001). Cannabis alsoreduced experimentally induced hyperalgesia to both brush and von Frey hair stimuli (p ≤ 0.05) in aheat-capsaicin experimental pain model used to anchor the more subjective response of the chronicneuropathic pain. No serious adverse events were reported. The number needed to treat in this studywas 3.6, which was virtually identical to the number needed to treat in other studies of inhaled
Two recent placebo-controlled studies of cannabinioids for central neuropathic pain associated
with multiple sclerosis produced results similar to the present study. In a crossover trial of syntheticdelta-9-THC up to 10 mg/day, an NNT of 3.5 was reported () A trialof the sublingual spray containing delta-9-THC alone or combined with CBD showed a 41% painsleep quality was also reported with the sublingual spray. Nabiximols is currently approved inCanada for treatment of neuropathic pain related to multiple sclerosis as well as cancer-related pain.
A small clinical trial has been conducted investigating nabiximols in 16 patients with chemotherapy-induced neuropathic pain with results suggesting that larger follow-on clinical trials in this patientpopulation are warranted (.
In an animal model of paclitaxel induced neuropathic pain, chronic administration of the
nonpsychoactive cannabinoid CBD prevents the onset of chemotherapy induced neurotoxicity in mice(. The investigators suggest that adjunct treatmentwith CBD during taxane chemotherapy may be safe and effective in the prevention or attenuation ofchemotherapy-induced neuropathic pain, although human studies are certainly required.
Synergism between opioids and cannabinoids has been postulated and subsequently demonstrated in aantinociceptive effects of morphine are predominantly mediated by mu receptors but may be enhancedby delta-9-THC activation of kappa and delta opioid receptors (. It has beenfurther postulated that the cannabinoid:opioid interaction may occur at the level of their signaltransduction mechanisms (; ).
Receptors for both classes of drugs are coupled to similar intracellular signaling mechanisms thatlead to a decrease in cAMP production by way of Gi protein activation ). There has also been some evidence thatcannabinoids might increase the synthesis or release of endogenous opioids, or both. With thisbackground, we conducted a pharmacokinetic interaction study to investigate the effect of concomitantsustained-release oxycodone had their opioid concentration over time curves evaluated before andafter 4 days exposure to vaporized cannabis. No adverse side effects of combining cannabinoids andopioids were observed over the course of the in-patient evaluation. There were no significantalterations in the area under the curves for the opioids after the addition of vaporized cannabis.
Although the study was not powered for pain as an endpoint, evidence of potential synergistic relief
of pain was appreciated. If cannabinoids and opioids are shown to be synergistic in a larger follow-on controlled clinical trial, it is possible that lower doses of opioids may be effective for longerperiods of time with fewer side effects, clearly a benefit to the cancer patient with pain.
ANXIETY, DEPRESSION, AND SLEEP
In clinical trials of cannabis, euphoria is often scored as an adverse effect. Although not all patientsexperience mood elevation after exposure to cannabis, it is a frequent outcome. Much depends on the"set and setting" and the individual's prior experience with cannabis. Some people developdysphoria with or without paranoia upon exposure to cannabis; for them cannabis or its constituentsmay not be clinically useful. Sleepiness is another common side effect that can easily be recast asimproved sleep quality as has been reported in trials of nabiximols as well as inhaled cannabis(; ). For the cancer patient suffering from anorexia,nausea, pain, depression, anxiety, and insomnia, a single agent that can address all these symptomswould be a valuable addition to the armamentarium. Cannabis may be particularly useful insupportive- or palliative-care situations (.
Safety and Side Effects
Cannabinoids have an extremely favorable drug safety profile (; . Unlike opioid receptors,cannabinoid receptors are not located in brainstem areas controlling respiration, so lethal overdosesdue to respiratory suppression do not occur. The LD50 has been estimated to be 1,500 poundssmoked in 15 minutes as extrapolated from animal studies where the median lethal dose wasestimated to be several grams per kilogram of body weight (
The administration of cannabinoids to laboratory animals and humans does result in psychoactive effects. In humans,
the central nervous system effects are both stimulating and depressing and are divided into four groups: affective
(euphoria and easy laughter); sensory (temporal and spatial perception alterations and disorientation); somatic
(drowsiness, dizziness and motor incoordination); and cognitive (confusion, memory lapses and difficulty
concentrating).
As cannabinoid receptors are not just located in the central nervous system but are present in
tissues throughout the body, additional side effects of note include tachycardia and hypotension,conjunctival injection, bronchodilation, muscle relaxation, and decreased gastrointestinal motility.
Tolerance to the unwanted side effects of cannabis appears to develop rapidly in laboratory animalsand humans. This is felt to occur due to a decrease in the number of total and functionally coupledcannabinoid receptors on the cell surface with a possible minor contribution from increasedcannabinoid biotransformation and excretion with repeated exposure.
Although cannabinoids are considered by some to be addictive drugs, their addictive potential is
considerably lower than other prescribed agents or substances of abuse. The brain develops toleranceto cannabinoids. Animal research demonstrates a potential for dependence, but this potential isobserved under a narrower range of conditions than with benzodiazepines, opiates, cocaine, ornicotine. Withdrawal symptoms—irritability, insomnia with sleep EEG disturbance, restlessness, hotflashes, and rarely nausea and cramping—have been observed, but appear mild compared with the
withdrawal from opiates or benzodiazepines and usually dissipate after a few days. Unlike othercommonly used drugs, cannabinoids are stored in adipose tissue and excreted at a low rate (half-life1-3 days), so even abrupt cessation of THC intake is not associated with rapid declines in plasmaconcentration that would precipitate withdrawal symptoms or drug craving.
The 1999 Institute of Medicine report addressed the frequent concern that marijuana is a "gateway
first most people encounter. Not surprisingly, most users of other illicit drugs have used marijuanafirst. However, most drug users begin with alcohol and nicotine before marijuana; hence marijuana isnot the most common and is rarely the first "gateway" drug. The report summarizes that there is noconclusive evidence that the drug effects of marijuana are causally linked to the subsequent abuse ofother illicit drugs and cautions that data on drug use progression cannot be assumed to apply to theuse of drugs for medical purposes, which is certainly pertinent to the discussion of cannabis in cancerpatients.
Cannabis and Cancer Risk
A study conducted by the National Toxicology Program of the U.S. Department of Health and HumanServices on mice and rats suggested that cannabinoids may have a protective effect against tumordevelopment (U.S. Dept. of Health and Human Services, 1996). In this 2-year evaluation, rats andmice were given increasing doses of THC by gavage. A dose-related decrease in the incidence ofboth benign and malignant tumors was observed. Animals receiving THC dosing also survived longerthan those receiving vehicle alone.
Mice and rats are not people, and gavage is not equivalent to smoking a combusted botanical
product. Many would find the combustion and inhalation of a therapeutic agent to be an undesirableand perhaps counterintuitive way to deliver a drug. Most of the evidence available on the risk ofcancer from marijuana smoking comes from epidemiologic studies, naturally, because prospective,randomized control trials are not possible. Over the years, reports of increased risks of lung cancer,oropharyngeal cancers, and prostate and cervical cancer have been most consistently reported. Foreach trial suggesting a possible increase in cancer incidence in chronic marijuana users, others havebeen published that appear to refute the association. A retrospective cohort study of 64,855 KaiserPermanente health care members seen between 1979 and1985 and followed through 1993 yielded aninteresting finding (. Men aged 15–49 weredivided into four cohorts based on their use of tobacco and marijuana: never smoked either, smokedonly cannabis, smoked only tobacco, smoked tobacco and cannabis. There were 5,600–8,200 men ineach cell followed for an average of nearly nine years. In the men who never smoked, there were 2cases of lung cancer diagnosed over the follow-up period. In the men who smoked tobacco, eitheralone or in addition to marijuana, the risk of lung cancer was increased 10-fold. In the over 50,000person-years of follow-up of men who only smoked marijuana, there were no documented cases oflung cancer; less than in the never smokers!
A systematic review evaluating 19 studies that involved persons 18 years or older who smoked
marijuana and examined premalignant or cancerous lung lesions concluded that observational studiesfailed to demonstrate significant associations between marijuana smoking and lung cancer afteradjusting for tobacco use (. The authors site theselection bias, small sample size, limited generalizability and overall young participant age in stating
that because of the biological plausibility of an association of marijuana smoking and lung cancer,physicians should still caution patients regarding potential risks until further rigorous studies permitdefinitive conclusions.
A population-based case-control study of the association between marijuana use and the risk of
lung and upper aerodigestive tract cancers was performed in Los Angeles ().
There were 1,112 incident cancer cases (611 lung, 303 oral, 108 esophagus, 100 pharynx, 90 larynx)matched to 1,040 cancer-free controls on age, gender, and neighborhood. A standardizedquestionnaire used during face-to-face interview collected information on marijuana use expressed injoint-years, where 1 joint-year is the equivalent of smoking one marijuana cigarette per day for oneyear. The interviews also requested information on the use of other drugs including hashish, tobacco(all forms) and alcohol, sociodemographic factors, diet, occupational history, environmental factorsincluding exposure to smoke, medical history and family history of cancer. Data were presented ascrude odds ratios and adjusted odds ratios using three models of covariate adjustment (with onlyModel 3 including tobacco use and pack/years). The results showed that although using marijuana for≥ 30 joint-years was positively associated in the crude analysis with each cancer except pharyngeal,no positive associations were found when adjusting for several confounders including cigarettesmoking. In fact, in the Model 3 analysis for lung cancer, the cohort who reported > 0 to < 1 joint-years of marijuana use had a 37% reduction in the risk of developing lung cancer compared to thosewho never smoked marijuana. Although this was the only cohort in which the reduction in lung cancerrisk reached statistical significance, in the model, all levels of marijuana use (including ≥ 60 joint-years) had adjusted odds ratios less than 1.0. The authors report adjusted ORs <1 for all cancersexcept oral cancer and found no consistent association of marijuana use with any malignant outcome.
In what appears to be an overly aggressive attempt to delineate the possible limitations of their workthat could have led to such a consistent yet startling result, the authors mention that "it is possible thatmarijuana use does not increase cancer risk … Although the adjusted ORs < 1 may be chancefindings, they were observed for all non-reference exposure categories with all outcomes except oralcancer. Although purely speculative, it is possible that such inverse associations may reflect aprotective effect of marijuana."
Postulating that chronic use of cannabis impacts negatively on endocrine and reproductive systems,
population-based case control studies report an association between marijuana use and elevated riskof especially nonseminomatous germ cell tumors. Although lacking good dose information andadequate sample sizes, the trends warrant further follow-up. Of note, a comprehensive review fromHealth Canada concluded that although concerns exist, the epidemiologic evidence of a link betweenuse of cannabis and cancer remains inconclusive (.
Cannabinoids as Anticancer Agents
There has been an increasing body of evidence over the past decade that cannabinoids may have aanimal models have suggested that THC and other cannabinoids may inhibit the growth of sometumors by the modulation of signaling pathways that lead to growth arrest and cell death as well as by
inhibition of angiogenesis and metastasis. The antiproliferative effects were originally reported in1975 by Munson and colleagues, who demonstrated that delta-9-THC, delta-8-THC and cannabinolinhibited Lewis lung adenocarcinoma cell growth in vitro as well as in mice. Curiously, there was noreal follow-up of these findings for 20 years, when the line of investigation was picked up byscientists in Spain and Italy; those countries have remained at the forefront of this emerging fieldthe late 1990s, several plant-derived (THC and CBD), synthetic (WIN-55,212-2 and HU-210), andendogenous cannabinoids (anandamide and 2-arachidonoylglycerol) have been shown to exertantiproliferative effects of a wide variety of tumor cells in culture systems. In addition to the originallung adenocarcinoma study, other tumor cells that have been shown to be sensitive to cannabinoid-induced growth inhibition include glioma, thyroid epithelioma, leukemia/lymphoma, neuroblastoma ; Vaccani, Massi, Colombo, Rubino, & Parolaro, 2005;. Perhaps even more compelling,cannabinoid administration to nude mice slows the growth of various tumor xenografts or geneticallyinitiated tumors including lung, breast, colorectal, and skin carcinomas, thyroid epitheliomas,melanomas, pancreatic carcinomas, lymphomas, and gliomas. The requirement of CB1 and/or CB2receptors for the antitumor effect has been shown by various biochemical and pharmacologicalapproaches already mentioned, and the cumulative effects of cannabinoid receptor signaling in thecontrol of cell fate are expected to have important implications in the potential of cannabinoids forregulating tumor cell growth.
Cannabinoids may exert their antitumor effects by a number of different mechanisms including
direct induction of transformed cell death (, direct inhibition of transformed-cell growth,and inhibition of tumor angiogenesis and metastasis ). Adesirable property of antitumor compounds is their preferential targeting of malignant cells.
Cannabinoids appear to kill tumor cells but do not affect their nontransformed counterparts and mayeven protect them from cell death. This is best exemplified by glial cells. Cannabinoids have beenshown to induce apoptosis of glioma cells in culture and induce regression of glioma cells in miceand rats. In contrast, cannabinoids protect normal glial cells of astroglial and oligodendrogliallineages from apoptosis mediated by the CB1 receptor ().
Immunohistochemical and functional analyses in mouse models of gliomas, skin carcinomas, and
other tumors have demonstrated that cannabinoid administration alters the vascular hyperplasiacharacteristic of actively growing tumors into a pattern characterized by small, differentiated,impermeable capillaries, thus thwarting angiogenesis. This is accompanied by a reduced expressionof vascular endothelial growth factor (VEGF) and other pro-angiogenic cytokines, as well as ofVEGF receptors. Activation of cannabinoid receptors in vascular endothelial cells inhibits cellmigration and survival, also contributing to impaired tumor vascularization. Cannabinoidadministration to tumor-bearing mice decreases the activity and expression of matrixmetalloproteinase 2, a proteolyic enzyme that allows tissue breakdown and remodeling duringangiogenesis and metastasis. This supports the inhibitory effect of cannabinoids in inhibiting tumorinvasion in animal models (.
Further support comes from studies in human non–small cell lung cancer cell lines that overexpress
epidermal growth factor receptor, in which THC inhibits epidermal growth factor-induced growth,chemotaxis, and chemoinvasion (. In an in vivo model using severecombined immunodeficient mice, subcutaneous tumors were generated by inoculating the animalswith the same cell lines. Tumor growth in THC-treated animals was inhibited by 60% compared withvehicle-treated controls. The inhibition was significant both regarding the subcutaneous xenograft aswell as the number and weight of lung metastases. Tumor specimens revealed antiproliferative andantiangiogenic effects of THC.
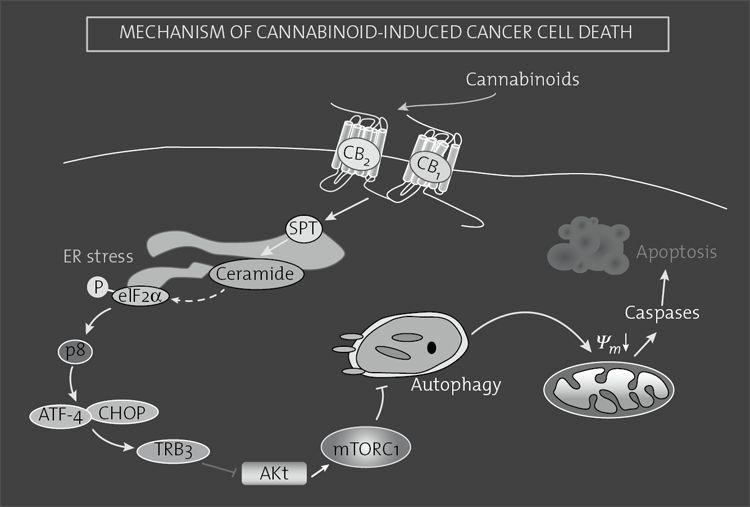
FIGURE 8.3. Mechanism of cannabinoid-induced cancer cell death.
Cannabinoid agonists bind to CB1 and/or CB2 receptors to stimulate de novo synthesis of ceramide via induction of the enzyme serinepalmitoyltransferase (SPT). This triggers the induction of an eIF2α-mediated endoplasmic reticulum (ER) stress response that promotesthe up-regulation of the transcription factor p8 and several of its downstream targets, including the transcription factors ATF-4 andCHOP and the pseudokinase TRB3. This favors the interaction of TRB3 with the prosurvival protein AKT, thus leading to the inhibitionof the AKT–mTORC1 axis and the subsequent induction of autophagy. Autophagy is upstream of intrinsic mitochondrial apoptosis in theprocess of cannabinoid-induced cell death.
Another potential anticancer and particularly antimetastasis mechanism for cannabinoids has been
significant reductions in breast cancer cell proliferation and invasiveness in in vitro models andmetastases in mice. Reducing Id-1 expression with antisense technology is not a possible interventionin humans with breast cancer at this time, however. CBD has been demonstrated to down-regulate Id-1 expression in aggressive human breast cancer cells (McAllister, 2011). The investigators,
therefore, suggest that CBD represents the first nontoxic exogenous agent that can significantlydecrease Id-1 expression in metastatic breast cancer cells leading to the down-regulation of tumoraggressiveness in vitro.
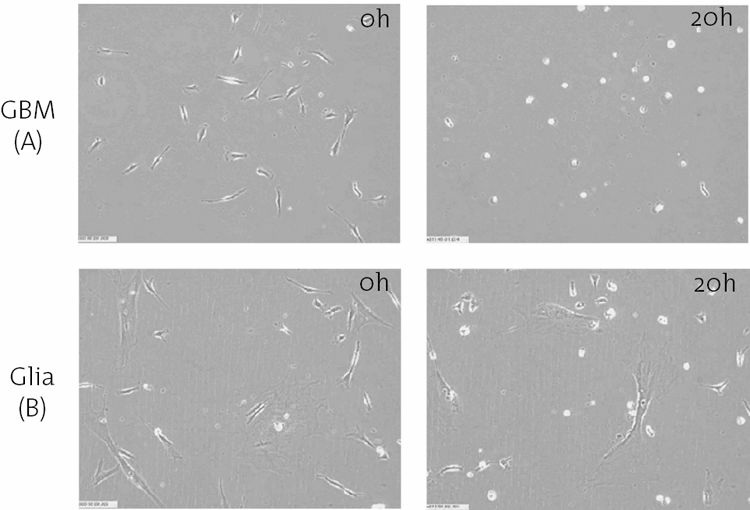
FIGURE 8.4. Delta-9-THC kills brain tumor cells at a concentration that is nontoxic to normal brain cells. Images obtained through a
time-lapse microscope illustrate the selective induction of cell death in cultures of human glioblastoma multiforme (GBM) cells (A)
compared to normal human glial cells (B). After 20 hours of treatment, death of nearly all of the GBM cells is evidenced by cells
shrinking to inanimate white spheres. The normal cells exposed to the same concentration of delta-9-THC continue to migrate and divide.
(Photo courtesy of McAllister and Yount).
Two additional potential mechanisms of anticancer activity warrant brief mention. Cannabinoids,
both plant-derived and endogenous, are believed to have anti-inflammatory effects. Inflammation isbeing increasingly linked to the development of various malignancies. Perhaps one of the mostobvious associations is the development of colorectal carcinoma in patients with inflammatory boweldisease. A mouse study has demonstrated that signaling of the endogenous cannabinoid system islikely to provide intrinsic protection against colonic inflammation (). This has ledto the development of a hypothesis that phytocannabinoids and endocannabinoids may be useful in theformation. Treatment with CBD 1 mg/kg decreased these azoxymethane effects.
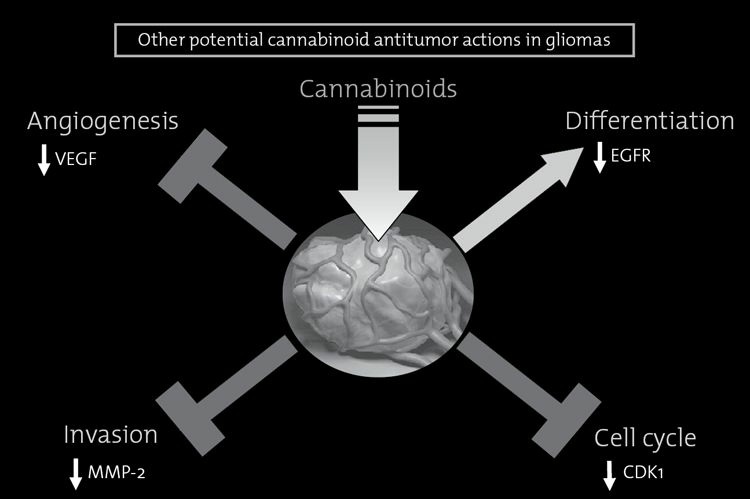
FIGURE 8.5. Other antitumor effects of cannabinoids.
Besides inducing apoptosis of tumor cells, cannabinoid administration can decrease the growth of gliomas by other mechanisms, includingat least: (i) reduction of tumor angiogenesis, by inhibition of the vascular endothelial growth factor (VEGF) pathway; (ii) inhibition oftumor cell invasion, by down-regulation of matrix metalloproteinase-2 (MMP-2) expression; (iii) induction of tumor cell differentiation, bydown-regulation of epidermal growth factor (EGF) receptor expression; and perhaps (iv) arrest of the cell cycle, by down-regulation ofcyclin-dependent kinase-1 (CDK1) expression. The relative contribution of these processes to the inhibition of tumor growth depends onvarious factors such as the type of tumor under study, the experimental model used and the intensity of cannabinoid signaling.
Kaposi's sarcoma-associated herpesvirus/Human herpesvirus-8 (KSHV/HHV-8) and Epstein-Barr
virus (EBV) are related and implicated in the cause of a number of malignant diseases includingKaposi's sarcoma and primary effusion lymphoma (KSHV) and Burkitt's lymphoma, primary centralnervous system lymphoma, Hodgkin's disease, and nasopharyngeal carcinoma (EBV). A group ofinvestigators has demonstrated that THC is a potent and selective antiviral agent against KSHV(. It is felt that THC may inhibit KSHVreplication through the activation of cannabinoid receptors. The authors conclude that further studieson cannabinoids and herpesviruses are important as they may lead to development of drugs thatinhibit reactivation of these oncogenic viruses. Counter to these findings, however, is the suggestionthat delta-9-THC may actually enhance KSHV infection and replication and foster KSHV-mediatedendothelial transformation (). These investigators cautionthat use of cannabinoids may thus place individuals at greater risk for the development andprogression of Kaposi's sarcoma, although epidemiologic data have not supported these in vitrofindings ().
So with the body of evidence increasing, where are the clinical trials in humans with malignant
disease. True, cannabinoids have psychoactive side effects, but these could be considered to be
within the boundaries of tolerance for the toxicity profiles of cytotoxic chemotherapeutic and targetedsmall molecule therapies widely used in oncology. Ten years ago, the Spanish Ministry of Healthapproved a pilot clinical trial carried out in collaboration between the Tenerife University Hospitaland the Guzman laboratory in Madrid to investigate the effect of local administration of THCintracranially through an infusion catheter on the growth of recurrent glioblastoma multiforme(). In this ground-breaking pilot study, THC administration was shown to be safeand associated with decreased tumor cell progression –as assessed by magnetic resonance imagingand biomarker expression criteria- in at least two of nine patients studied.
Cannabinoid-Based Combination Anticancer Therapies
The use of combination anticancer therapies has a number of theoretical advantages over single agent-based strategies as they allow the simultaneous targeting of tumor growth, progression, and/orspreading at different levels. In line with this idea, recent observations suggest that the combinedadministration of cannabinoids with other anticancer drugs acts synergistically to reduce tumorgrowth in mice. For example, the administration of THC and temozolomide (the benchmark agent forthe management of glioblastoma) exerts a strong antitumor action in glioma xenografts, an effect thatis also evident in temozolomide-resistant tumors (. Of interest, no toxicity wasobserved in mice treated with combinations of THC and temozolomide. As most patients withglioblastoma receive temozolomide treatment, these findings indicate that the combinedadministration of temozolomide and cannabinoids could be therapeutically exploited in themanagement of glioblastoma.
Likewise, another study has recently shown that the combined administration of gemcitabine and
different cannabinoid agonists synergistically reduces the viability of pancreatic cancer cells(). Other reports indicate that anandamide and HU-210 may also enhance the
An additional approach has been to combine THC with CBD, a cannabinoid that reduces the
growth of several types of tumor xenografts in mice through a still poorly defined mechanism.
Combined administration of THC and CBD enhances the anticancer activity of THC and reduces theadministration, such as seizures, incoordination, and psychotic events, and, therefore, improves thetolerability of cannabis-based medicines (). As Cannabis sativa contains an estimated100 different cannabinoids, some of the other cannabinoids present in addition to CBD might alsoattenuate the psychoactive side-effects of THC or even produce other therapeutic benefits. Thus, webelieve that clinical studies aimed at analyzing the efficacy of cannabinoids as antitumor agentsshould be based not only on the use both of pure substances, such as THC and CBD, but also ofcannabis products containing controlled amounts of THC, CBD, and other cannabinoids.
Despite these impressive in vitro and animal-model findings regarding the potential antitumor
effects of cannabinoids, there is still no solid basis for ongoing claims by proponents of highlyconcentrated cannabis extracts or oils that these preparations can "cure cancer." Increasing numbersof patients in North America are seeking oils high in THC and/or CBD due to testimonials that
patients have used these preparations either topically to eradicate skin cancers or systemically toeliminate nonskin cancers. This has led to a number of patients seeking to forego or postponepotentially curative conventional cancer therapies in favor of self-medicating with high-potencycannabis oils. Many patients claiming to be cured of their cancers have used the products in additionto conventional cancer therapies obfuscating the issue further. Although the in vitro and animalevidence is intriguing, there have not yet been any robust human studies investigating cannabis as ananticancer agent that would warrant advising patients to forego conventional therapy in favor of usinga high-potency cannabis extract. Patients who chose to delay conventional therapies in hopes ofbenefitting from a trial of cannabis oil against their cancer risk the possibility of having a potentiallytreatable cancer become incurable. As the preclinical evidence suggests that cannabinoids mightenhance the antitumor activity of conventional chemotherapeutic agents as well as ameliorateassociated side effects, the addition of cannabinoid-based preparations to standard cancer therapyshould not be discouraged by the treating oncologist.
Alternative Delivery Systems
What if clinical trials were to demonstrate that inhaled cannabis may be of benefit to patients with acondition like, for example, recurrent glioblastoma multiforme? It is not likely that even a meta-analysis of a number of similar studies in any condition would convince the necessary regulatorybodies that cannabis should be re-instated to the U.S. Pharmacoepia and made widely available topatients who may benefit from its use. The Institute of Medicine Report in 1999 clearly stated that theaccumulated data indicate a potential therapeutic value for cannabinoid drugs particularly in the areasof pain relief, control of nausea and vomiting and appetite stimulation. They went on to suggest thatthe "goal of clinical trials of smoked marijuana would not be to develop it as a licensed drug, but as a
To this end, we conducted a trial in healthy marijuana smoker volunteers comparing the blood
levels of cannabinoids achieved upon inhaling marijuana that has been vaporized in a device thatheated the plant product to below the temperature of combustion and collected the volatilized gaseswith those obtained upon smoking a comparable dosed cigarette (). Eighteenhealthy subjects were evaluated. One dose (1.7, 3.4 or 6.8% tetrahydrocannabinol) and deliverysystem (smoked cannabis cigarette or vaporization system) was randomly assigned for each of the sixinpatient study days. The peak plasma concentrations and six-hour area under the plasmaconcentration-time curve of THC after inhalation of vaporized cannabis were similar to those ofsmoked cannabis.
Carbon monoxide levels were substantially reduced with vaporization suggesting less exposure to
noxious substances. Neuropsychologic effects were equivalent and participants expressed a clearpreference for vaporization as a delivery method. No adverse events were observed. Vaporization ofcannabis is a safe and effective mode of delivery of THC. Numerous vaporization devices are nowavailable to patients accessing medicinal cannabis.
Another nonsynthetic alternative to smoked or inhaled cannabis is the oromucosal preparation of
whole-plant extract ( ; . Nabiximols was firstapproved as a prescription medication in Canada in 2005 for symptomatic relief of neuropathic painin multiple sclerosis and subsequently as adjunctive therapy for patients with cancer pain on otheranalgesic medications. The cannabis-based medication is now available in Canada, the United
Kingdom, Spain, and other European countries, and being evaluated in large-scale Phase III clinicaltrials in patients with cancer-related pain in the United States.
Guidelines for Providers
The Institute of Medicine is aware that the development and acceptance of smokeless marijuanadelivery systems "may take years; in the meantime there are patients with debilitating symptoms forwhom smoked marijuana may provide relief." So what is a provider to do? Patients with cancer havea number of symptoms that may be responsive to cannabinoid therapies. As enumerated, these includenausea, vomiting, anorexia, pain, insomnia, anxiety and depression. Many providers would frownupon the use of a relatively benign inhaled psychotropic agent while freely writing prescriptions forpharmaceutical agents with significantly greater cost, potential for addiction or abuse, and morenegative societal impact overall.
A Medical Board of California Action Report from 2004 provides a model for how states with
medical marijuana legislation should advise physicians (Medical Board of California, 2004) "Theintent of the board at this time is to reassure physicians that if they use the same proper care inrecommending medical marijuana to their patients as they would any other medication or treatment,their activity will be viewed by the Medical Board just as any other appropriate medicalintervention…. If physicians use the same care in recommending medical marijuana to patients as theywould recommending or approving any other medication or prescription drug treatment, they havenothing to fear from the Medical Board."
The Board recommends following the accepted standards that would be used in recommending any
medication. A history and physical examination should be documented. The provider should ascertainthat medical marijuana use is not masking an acute or treatable progressive condition. A treatmentplan should be formulated. A patient need not have failed all standard interventions before marijuanacan be recommended. The physician may have little guidelines in actually recommending a concretedose for the patient to use (. Because there are somany variables associated with effect, the physician and patient should develop an individual self-titration dosing paradigm that allows the patient to achieve the maximum benefit with tolerable sideeffects. Discussion of potential side effects and obtaining verbal informed consent are desirable.
Periodic review of the treatment efficacy should be documented. Consultation should be obtainedwhen necessary. Proper record keeping that supports the decision to recommend the use of medicalmarijuana is advised. Despite all these guidelines, the California Medical Board still remindsphysicians that making a written recommendation "could trigger a federal action."
On a more positive note, in a unanimous vote, the Assembly of the American Psychiatric
Association approved a strongly worded statement supporting legal protection for patients usingmedical
(). The APA action paper reiterates that "thethreat of arrest by federal agents, however, still exists. Seriously ill patients living in these states withmedical marijuana recommendations from their doctors should not be subjected to the threat ofpunitive federal prosecution for merely attempting to alleviate the chronic pain, side effects, orsymptoms associated with their conditions or resulting from their overall treatment regimens….[We]support protection for patients and physicians participating in state approved medical marijuanaprograms."
It behooves the integrative oncologist to follow closely future studies of cannabinoids and cancer.
It is likely that these agents will not only prove to be useful in symptom management and palliativecare, but as anti-tumor agents as well.
Abel, E. L. (1980). Marijuana: The first twelve thousand years. New York: Plenum Press.
Abrams, D. I. (2000). Potential interventions for HIV/AIDS wasting: An overview. JAIDS, 25, S74–S80.
Abrams, D. I., Child, C. C., & Mitchell, T. (1995). Marijuana, the AIDS wasting syndrome and the US government. New England
Journal of Medicine, 333, 670–671.
Abrams, D. I., Couey, P., Shade, S. B., Kelly, M. E., & Benowitz, N. L. (2011). Cannabinoid: Opioid interaction in chronic pain. Clinical
Pharmacology and Therapeutics 90, 844–851.
Abrams, D. I., Jay, C., Shade, S., Vizoso, H., Reda, H., Press, S., … Petersen K. (2007). Cannabis in painful HIV-associated sensory
neuropathy: A randomized, placebo-controlled trial. Neurology 68, 515–521.
Abrams, D. I., Vizoso, H. P., Shade, S. B., Jay, C., Kelly, M. E., Benowitz, N. (2007). Vaporization as a smokeless cannabis delivery
system: A pilot study. Clinical Therapeutics and Pharmacology 82, 572–578.
Adams, I. B., & Martin, B. R. (1996). Cannabis: Pharmacology and toxicology in animals and humans. Addiction, 91, 1585–1614.
Adler, J. N., & Colbert, J. A. (2013). Medicinal use of marijuana—polling results. New England Journal of Medicine, 369, e30.
Agurell, S., Halldin, M., Lindgren, J., Ohlsson, A., Widman, M., Gillespie, H., & Hollister, L. (1986). Pharmacokinetics and metabolism of
delta1- tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacology Review, 38(1), 21–43.
Aviello, G., Romano, B., Borrelli, F., Capasso, R., Gallo, L., Piscitelli, F, … Izzo, A. A. (2012). Chemopreventive effect of the non-
psychotropic phytocannabinoid cannabidiol on experimental colon cancer. Journal of Molecular Medicine, 90, 925–934.
Bar-Sela, G., Vorobeichik, M., Drawsheh, S., Omer, A., Goldberg, V., & Muller, E. (2013). The medical necessity for medicinal
cannabis: Prospective, observational study evaluating treatment in cancer patients on supportive or palliative care. Evidence-BasedComplementary and Alternative Medicine, 510392. doi: 10.1155/2013/510392
Beal, J. E., Olson, R., Laubenstein, L., Morales, J. O., Bellman, P., Yangco, B., … Shepard, K. V. (1995). Dronabinol as a treatment for
anorexia associated with weight loss in patients with AIDS. Journal of Pain and Symptom Management, 10, 89–97.
Ben Amar, M. (2006). Cannabinoids in medicine: a review of their therapeutic potential. Journal of Ethnopharmacology, 105, 1–25.
Bifulco, M., & DiMarzo, V. (2002). Targeting the endocannabinoid system in cancer therapy: A call for further research. Nature
Medicine, 8, 547–550.
Bifulco, M., Laezza, C., Pisanti, S., & Gazzerro, P. (2006). Cannabinoids and cancer: Pros and cons of an antitumour strategy. British
Journal of Pharmacology, 148, 123–135.
Blazquez, C., Gonzalez-Feria, L., Alvarez, L., Haro, A., Llanos Casanova, M., & Guzman, M. (2004). Cannabinoids inhibit the vascular
endothelial growth factor pathway in gliomas. Cancer Research, 64, 5617–5623.
Blazquez, C., Casanova, M. L., Planas, A., Gomez del Pulgar, T., Villanueva, C., Fernandez-Acenero, M. J., … Guzman, M. (2003).
Inhibition of tumor angiogenesis by cannabinoids. FASEB Journal, 17, 529–531.
Booth, M. (2003). Cannabis: A history. New York: St. Martin's.
Borgelt, L. M., Franson, K. L., Nussbaum, A. M., & Wang, G. S. (2013). The pharmacologoic and clinical effects of medical cannabis.
Bostwick, J. M., Reisfield, G. M., & DuPont, R. L. (2013). Medicinal use of marijuana. New England Journal of Medicine, 368, 866–
Bowles, D. W., O'Bryant, C. L., Camidge, R., & Jimeno, A. (2012). The intersection between cannabis and cancer in the United States.
Critical Reviews in Oncology/Hematology, 83, 1–10.
Brisbois, T. D., de Kock, I. H., Watanabe, S. M., Mirhosseini. M., Lamoureux, D. C., Chasen, M., … Wismer, W. V. (2011). Delta-9-
tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: results of a randomized, double-blind, placebo-controlled pilot trial. Annals of Oncology, 22(9), 2086–2093.
Caffarel, M. M., Andradas, C., Pérez-Gómez, E., Guzmán, M., & Sánchez, C. (2012). Cannabinoids: A new hope for breast cancer
therapy? Cancer Treatment Review, 38, 911–918.
California Physicians and Medical Marijuana. Action Report. Medical Board of California 2004; 90:1–4 available at
Calignano, A., LaRana, G., Giuffrida, A., & Piomelli, D. (1998). Control of pain initiation by endogenous cannabinoids. Nature, 394, 277–
Carter, G. T., Weydt, P., Kyashna-Tocha, M., & Abrams, D. I. (2004). Medicinal cannabis: rational guidelines for dosing. IDrugs, 7,
Chan, I. S. F., Naton, J. D., Saravolatz, L. D., Crane, L. R., & Osterberger, J. (1995). Frequencies of opportunistic diseases prior to
death among HIV-infected persons. AIDS, 9, 1145–1151.
Chao, C., Jacobson, L. P., Jenkins, F. J., Tashkin, D., Martinez-Maza, O., Roth, M. D., … Detels, R. (2009). Recreational drug use and
risk of Kaposi's sarcoma in HIV- and HHV-8-coinfected homosexual men. AIDS Research and Human Retroviruses 25(2), 149–156.
Christensen, R., Kristensen, P. K., Bartals, E. M., Bliddal, H., & Astrup, A. (2007). Efficacy and safety of the weight-loss drug
rimonabant: A meta-analysis of randomised trials. Lancet, 370, 1706–1713.
Cichewicz, D. L. (2004). Synergistic interactions between cannabinoid and opioid analgesics. Life Science, 74(11), 1317–1324.
Cichewicz, D. L., & McCarthy, E. A. (2003). Antinociceptive synergy between delta(9)-tetrahydrocannabinol and opioids after oral
administration. Journal of Pharmacology and Experimental Therapy, 304(3), 1010–1015.
Cichewicz, D. L., Martin, Z. L., Smith, F. L., & Welch, S. P. (1999). Enhancement mu opioid antinociception by oral delta9-
tetrahydrocannabinol: Dose-response analysis and receptor identification. Journal of Pharmacology and Experimental Therapy,289(2), 859–867.
Daling, J. R., Doody, D. R., Sun, X., Trabert, B. L., Weiss, N. S., Chen, C., … Schwartz, S. M. (2009). Association of marijuana use and
the incidence of testicular germ cell tumors. Cancer, 115(6), 1215–1223.
De Petrocellis, L., Melck, D., Palmisano, A., Bisogno, T., Laezza, C., Bifulco, M., & Di Marzo, V. (1998). The endogenous cannabinoid
anandamide inhibits human breast cancer cell proliferation. Proceedings of the National Academy of Science, 95, 8375–8380.
Devane, W. A., Dysarc, F. A., Johnson, M. R., Melvin, L. S., & Howlett, A. C. (1988). Determination and characterization of a
cannabinoid receptor in rat brain. Molecular Pharmacology, 34, 605–613.
Devane, W. A., Hanus, L., Breuer, A., Pertwee, R. G., Stevenson, L. A., Griffin, G., … Mechoulam, R. (2002). Isolation and structure
of a brain constituent that binds to the cannabinoid receptor. Science, 258, 1946–1949.
Donadelli, M., Dando, I., Zaniboni, T., Costanzo, C., Dalla Pozza, E., Scupoli, M. T., … Palmiera, M. (2011). Gemcitabine/cannabinoid
combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Diseases, 2, e152.
Duran, M., Perez, E., Abanades, S., Vidal, X., Saura, C., Majem, M., … Capella, D. (2010). Preliminary efficacy and safety of an
oromucosal standardized cannabis extract in chemotherapy-induced nausea and vomiting. British Journal of Clinical Pharmacology,70(5), 656–663.
Ellis, R. J., Toperoff, W., Vaida, F., van den Brande, G. Gonzales, J., Gouaux, B., … Atkinson, J. H. (2009). Smoked medicinal cannabis
for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychoparmacology, 34(3), 672–680.
Elikottil, J., Gupta, P., & Gupta, K. (2009). The analgesic potential of cannabinoids. Journal of Opioid Management, 5(6):341–357.
Engels, F. K., de Jong, F. A., Sparreboom, A., Mathot, R. A., Loos, W. J., Kitzen, J. J., … Mathijssen, R. H. (2007). Medicinal cannabis
does not influence the clinical pharmacokinetics of irinotecan and docetaxel. Oncologist, 12(3), 291–300.
Facci, L., Dal Toso, R., Romanello, S., Buriani, A., Skaper, S. D., & Leon, A. (1995). Mast cells express a peripheral cannabinoid
receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proceedings of the National Academy of ScienceUSA, 92, 3376–3380.
Felder, C. C., & Glass, M. (1998). Cannabinoid receptors and their endogenous agonists. Annual Review of Pharmacology and
Toxicology, 38, 179–200.
Fields, H. L., & Meng, I. D. (1998). Watching the pot boil. Nature Medicine, 4, 1008–1009.
Fine, P. G., & Rosenfeld, M. J. (2013). The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Medical Journal,
4(4), eooXX. doi:10.5041/RMMJ.100XX
Gorter, R., Seefried, M., & Volberding P. (1992). Dronabinol effects on weight in patients with HIV infection. AIDS, 6, 127.
Grothenhermen, F., & Russo, E. (Eds.). (2002). Cannabis and cannabinoids: Pharmacology, toxicology, and therapeutic potential.
Binghamton, NY: Haworth Press.
Guindon, J., & Hohmann, A. G. (2011). The endocannabinoid system and cancer: Therapeutic implication. British Journal of
Pharmacology, 163, 1447–1463.
Gustafsson, S. B., Lindgren, T., Jonsson, M., & Jacobsson, S. (2009). Cannabinoid receptor-independent cytotoxic effects of
cannabinoids in human colorectal carcinoma cells: synergism with 5-fluorouracil. Cancer Chemotherapy and Pharmacology, 63,691–701.
Guzman, M. (2003). Cannabinoids: Potential anticancer agents. Nature Reviews/Cancer,3, 745–755.
Guzman, M., Duarte, M. J., Blazquez, C., Ravina, J., Rosa, M. C., Galve-Roperh, I., … Gonzalez-Feria, L. (2006). A pilot study of delta-
9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. British Journal of Cancer, 95, 1–7.
Hashibe, M., Morgenstern, H., Cui, Y., Tashkin, D. P., Zhang, Z-F., Cozen, W., … Greeland, S. (2006). Marijuana use and the risk of
lung and upper aerodigestive tract cancers: Results of a population-based case-control study. Epidemiol Biomarkers and Prevention ,15, 1829–1834.
Health Canada. (2010). Marihuana (Marijuana, Cannabis): Dried plant for administration by ingestion or other means. Ottawa, Canada:
Herzberg, U., Eliav, E., Bennett, G. J., & Kopin, I. J. (1997). The analgesic effect of R(+)-WIN 55,212–2 mesylate, a high affinity
cannabinoid agonist, in a rat model of neuropathic pain. Neuroscience Letters, 221, 157–160.
Ibrahim, M. M., Porreca, F., Lai, J., Albrecht, P. J., Rice, F. L., Khodorova, A., … Malan, T. P. Jr. (2005). CB2 cannabinoid receptor
activation produces antinociception by stimulating peripheral release of endogenous opioids. Proceedings of the National Academyof Science, 102, 3093–3098.
Johnson, J. R., Burnell-Nugent, M., Lossignol, D., Ganae-Motan, E. D., Potts, R., & Fallon, M. T. (2010). Multicenter, double-blind,
randomized, placebo-controlled, parallel-group study of the efficacy, safety and tolerability of THC:CBD extract and THC extract inpatients with intractable cancer-related pain. Journal of Pain and Symptom Management 39(2), 167–178.
Joy, J. E., Watson, S. J., & Benson, J. A. (Eds.). (1999). Marijuana and medicine: Assessing the science base. Washington, DC:
National Academy Press.
Karschner, E. L., Darwin, W. D., Goodwin, R. S., Wright, S., & Huestis, M. A. (2011). Plasma cannabinoid pharmacokinetics following
controlled oral ∆-9tetrahydrocannabinol and oromucosal cannabis extract administration. Clinical Chemistry, 57, 66–75.
Katona, I., & Freund, T. F. (2012). Multiple functions of endocannabinoid signaling in the brain. Annu Review of Neurosciene, 35, 529–
Lacson, J. C., Carroll, J. D., Tuazon, E., Castelao, E. J., Bernstein, L., & Cortessis, V. K.,…(2012). Population-based case-control study
of recreational drug use and testis cancer risk confirms an association between marijuana use and nonseminoma risk. Cancer,118(21), 5374–5383.
Ligresti, A., Moriello, A. S., Starowicz, K., Matias, I., Pisanti, S., De Petrocellis, L., … Di Marzo, V. (2006). Anti-tumor activity of plant
cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. Journal of Pharmacology and ExperimentalTherapy, 318, 1375–1387.
Llanos Casanova, M., Blazquez, C., Martinez-Palacio, J., Villanueva, C., Fernandez-Acenero, M. J., … Guzman, M. (2003). Inhibition of
skin tumor growth and angiogenesis in vivo by activation of cannabinoid recptors. Journal of Clinical Investigation, 111, 43–50.
Lynch, M. E., Cesar-Rittenberg, P., & Hohmann, A. G. (2014). A double-blind, pacebo-controlled, cross-over pilot with extension using
an oral mucosal cannabinoid-extract for treatment of chemotherapy-induced neuropathic pain. Journal of Pain SymptomManagement, 47, 166–173.
Mack, A., & Joy, J. E. (2001). Marijuana as medicine?—The science beyond the controversy. Washington, DC: National Academy
Manzanares, J., Corchero, J., Romero, J., Fernandez-Ruiz, J. J., Ramos, J. A., & Fuentes, J. A. (1999). Pharmacological and biochemical
interactions between opioids and cannabinoids. Trends in Pharmacology Science, 20(7), 287–294.
Marcu, J. P., Christian, R. T., Lau, D., Zielinski, A. T., Horowitz, M. P., Lee J., … McAllister, S. D. (2010). Cannabidiol enhances the
inhibitory effects of delta9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Molecular Cancer Therapy, 9,180–189.
Massa, F., Marsicano, G., Hermann, H., Cannich, A., Monory, K., Cravatt, B. F., … Lutz, B. (2004). The endogenous cannabinoid
system protects against colonic inflammation. Journal of Clinical Investigation, 113, 1202–1209.
Massi, P., Solinas, M., Cinquina, V., & Parolaro, D. (2012). Cannabidiol as potential anticancer drug. British Journal Clinical
Pharmacology, 75(2), 303–312.
McAllister, S. D., Chan, C., Taft, R. J., Luu, T., Abood, M. E., & Moore, D. (2005). Cannabinoids selectively inhibit proliferation and
induce death of cultured glioblastoma multiforme cells. Journal of Neuro-Oncology, 74, 31–40.
McAllister, S. D., Christian, R. T., Horowitz, M. P., Garcia, A., & Desprez, P-Y. (2007). Cannabidiol as a novel inhibitor of Id-1 gene
expression in aggressive breast cancer cells. Molecular Cancer Therapy, 6, 2921–2927.
McAllister, S. D., Murase, R., Christian, R. T., Lau, D., Zielinski, A. J., Allison, J., … Desprez, P. Y. (2011). Pathways mediating the
effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion and metastasis. Breast Cancer Research &Treatment, 129(1), 37–47.
McKallip, R. J., Lombard, C., Fisher, M., Martin, B. R., Ryu, S., Grant, S., … Nagarkatti, M. (2002). Targeting CB2 cannabinoid
receptors as a novel therapy to treat malignant lymphoblastic disease. Blood, 100, 627–634.
Mechoulam, R., Berry, E. M., Avraham, Y., DiMarzo, V., & Fride, E. (2006). Endocannabinoids, feeding and suckling—from our
perspective. International Journal of Obesity (London), 30(Suppl 1), S24–S28.
Medveczky, M. M., Sherwood, T. A., Klein, T. W., Friedman, H., & Medveczky, P. G. (2004). Delta-9-tetrahydrocannabinol (THC)
inhibits lytic replication of gamma oncogenic herpesviruses in vitry. BMC Medicine, 2, 34.
Mehra, R., Moore, B. A., Crothers, K., Tetrault, J., & Fiellin, D. A. (2006). The association between marijuana smoking and lung cancer:
a systematic review. Archives of Internal Medicine, 166, 1359–1367.
Meng, I. D., Manning, B. H., Martin, W. J., & Fields, H. J. (1998). An analgesic circuit activated by cannabinoids. Nature, 395, 381–
Miller, L. K., & Devi, L. A. (2011). The highs and lows of cannabinoid receptor expression in disease: mechanisms and their therapeutic
implications. Pharmacology Review, 63, 461–470.
Miyato, H., Kitayama, J., Yamashita, H., Souma, D., Asakage, M., Yamada, J., & Nagawa, H. (2009). Pharmacological synergism
between cannabinoids and paclitaxel in gastric cancer cell lines. Journal of Surgical Research, 155, 40–47.
Noyes, R., & Baram. D. (1974). Cannabis analgesia. Comprehensive Psychiatry, 15, 531.
Noyes, R., Brunk, S., Avery, D, & Canter, A. (1975). The analgesic properties of delta-9-tertrahydrocannabinol and codeine. Clinical
Pharmacology and Therapeutics, 18, 84–89.
Noyes, R., Brunk, S., Baram, D., & Canter, A. (1975). Analgesic effect of delta-9-tetrahydrocannabinol. Journal of Clinical
Pharmacology, 15, 139–143.
Patsos, H. A., Hicks, D. J., Greenhough, A., Williams, A. C., & Paraskeva, C. (2005). Cannabinoids and cancer: potential for colorectal
cancer therapy. Biochemical Society Transactions, 33, 712–714.
Pertwee, R. G. (1997). Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacology Therapy, 74, 129–180.
Pertwee, R. G. (2009). Emerging strategies for exploiting cannabinoid receptor agonists as medicines. British Journal of
Pharmacology, 156, 397–411.
Pertwee, R. G., Howlett, A. C., Abood, M. E., Alexander, S. P., Di Marzo, V., Elphick, M. R., … Ross, R. A. (2010). Cannabinoid
receptors and their ligands: beyond CB1 and CB2. Pharmacology Review, 62, 588–631.
Pisanti, S., Picardi, P., D'Alessandro, A., Laezza, C., & Bifulco, M. (2013). The endocannabinoid signaling system in cancer. Trends in
Pharmacological Science, 34, 273–282.
Pollan, M. (2001). The Botany of desire: A plant's eye view of the world. New York: Random House.
Preet, A., Ganju, R. K., & Groopman, J. E. (2007). Delta-9-tetrahydrocannabinol inhibits epithelial growth-factor-induced lung cancer
cell migration in vitro as well as its growth and metastasis in vivo. Oncogene, 2007, 1–8.
Pugh, G., Welch, S. P., & Bass, P. P. (1994). Modulation of free intracellular calcium and cAMP by morphine and cannabinoids, alone
and in combination in mouse brain and spinal cord synaptpsomes. Pharmacology Biochemical Behavior. 49, 1093–1100.
Pugh, G., Jr., Smith, P. B., Dombrowski, D. S., & Welch, S. P. (1996). The role of endogenous opioids in enhancing the antinociception
produced by the combination of delta 9-tetrahydrocannabinol and morphine in the spinal cord. Journal of Pharmacology andExperimental Therapy, 279(2), 608–616.
Richardson, J., Kilo, S., & Hargreaves, K. (1998). Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral
CB1 receptors. Pain, 75, 111–119.
Robson, P. (2001). Therapeutic effects of cannabis and cannabinoids. British Journal of Psychiatry, 178, 107–115.
Rog, D. J., Nurmikko, T. J., Fride, T., & Young, C. A. (2005). Randomized, controlled trial of cannabis-based medicine in central pain in
multiple sclerosis. Neurology, 65, 812–819.
Russo, E. B. (2011). Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. British Journal of
Pharmacology, 163(7), 1344–1364.
Russo, E. B., Guy, G. W., & Robson, P. J. (2007). Cannabis, pain, and sleep: Lessons from therapeutic clinical trials of Sativex®, a
cannabis-based medicine. Chemistry & Biodiversity, 4, 1729–1743.
Sallen, S. E., Zinberg, N. E., & Frei, E. (1975). Antiemetic effect of delta-9-THC in patients receiving cancer chemotherapy. New
England Journal of Medicine, 293, 795–797.
Sanchez, C., de Ceballos, M. L., Gomez del Pulgar, T., Rueda, D., Corbacho, C., Velasco, G.…(2001). Inhibition of glioma growth in
vivo by selective activation of the CB2 cannabinoid receptor. Cancer Research, 61, 5784–5789.
Sarfaraz, S., Afaq, F., Adhami, V. M., & Mukhtar, H. (2005). Cannabinoid receptor as a novel target for the treatment of prostate
cancer. Cancer Research, 65, 1635–1641.
Sidney, S., Quesenberry, C. P., Friedman, G. D., & Tekewa, I. S. (1997). Marijuana use and cancer incidence (California, USA).
Cancer Causes and Control, 8, 722–728.
Smith, F. L., Cichewicz, D., Martin, Z. L., & Welch, S. P. (1998). The enhancement of morphine antinociception in mice by delta9-
tetrahydrocannabinol. Pharmacology Biochemistry and Behavior, 60(2), 559–566.
Staquet, M., Gantt, C., & Machin, D. (1978). Effect of a nitrogen analog of tetrahydrocannabinol on cancer pain. Clinical
Pharmacology and Therapeutics, 23, 397–401.
Sutton, I. R., & Daeninck, P. (2006). Cannabinoids in the management of intractable chemotherapy-induced nausea and vomiting and
cancer-related pain. Journal of Supportive Oncology, 4, 531–535.
Svendsen, K. B., Jensen, T. S., & Back, F. W. (2004). Does the cannabinoid dronabinol reduce central pain in multiple sclerosis?
Randomised double blind placebo controlled crossover trial. British Medical Journal, 329, 253–260.
Timpone, J. G., Wright, D. J., Li, N., Egorin, M. J., Enama, M. E., Mayers, J., & Galleto, G. (1997). The safety and pharmacokinetics of
single-agent and combination therapy with megestrol acetate and dronabinol for the treatment of the HIV wasting syndrome. AIDSResearch in Human Retroviruses, 13, 305–315.
Torres, S., Lorente, M., Rodriguez-Fornes, F., Hernandez-Tiedra, S., Salazar, M., Garcia-Toboada, C., … Velasco, G. (2011). A
combined preclinical therapy of cannabinoids and temozolomide against glioma. Molecular Cancer Therapy, 10, 90–103.
Trabert, B., Sigurdson, A. J., Sweeney, A. M., Strom, S. S., & McGlynn, K. A. (2011). Marijuana use and testicular germ cell tumors.
Cancer, 117(4), 848–853.
Tramer, M. R., Carroll, D., Campbell, F. A., Reynolds, D. J., Moore, R. A., & Mc Quay, H. J. (2001). Cannabinoids for control of
chemotherapy-induced nausea and vomiting: quantitative systematic review. British Medical Journal, 323, 16–21.
U.S. Department of Health and Human Services NTP Technical Report on the Toxicology and Carcinogenesis Studies of 1-Trans-
Vaccani, A., Massi, P., Colombo, A., Rubino, T., & Parolaro, D. (2005). Cannabidiol inhibits human glioma cell migration through a
cannabinoid receptor-independent mechanism. British Journal of Pharmacology, 144, 1032–1036.
Velasco, G., Galve-Roperh, I., Sanchez, C., Blazquez, C., & Guzman, M. (2004). Hypothesis: Cannabinoid therapy for the treatment of
gliomas? Neuropharmacology, 47, 315–323.
Velasco, G., Sánchez, C., Guzmán, M. (2012). Towards the use of cannabinoids as antitumour agents. National Review of Cancer, 12,
Walker, J. M., Hohmann, A. G., Martin, W. J., Strangman, N. M., Huang, S. M., & Tsou, K. (1999). The neurobiology of cannabinoid
analgesia. Life Science, 65, 665–673.
Walker, J. M., Huang, S. M., Strangman, N. M., Tsou, K., & Sanudo-Pena, M. C. (1999). Pain modulation by release of the endogenous
cannabinoid anandamide. Proceedings of the National Academy of Science, USA, 96, 12198–12203.
Walsh, D., Nelson, K. A., & Mahmoud, F. A. (2003). Established and potential therapeutic applications of cannabinoids in oncology.
Support Care Cancer, 11, 137–143.
Ward, S. J., McAllister, S. D., Neelakantan, H., & Walker, E. A. (2014). Cannabidiol inhibits paclitaxel-induced neuropathic pain through
5-HT1A receptors without diminishing nervous system function or chemotherapy efficacy. British Journal of Pharmacology, 171(3),636–645.
Ware, M. A., Wang, T., Shapiro, S., Robonson, A., Ducruet, T., Huynh, T., … Collet, J. P. (2010). Smoked cannabis for chronic
neuropathic pain: A randomized controlled trial. CMAJ, 182(14), E694–701.
Watanabe, K., Matsunaga, T., Yamamoto, I., Funae, Y., & Yoshimura, H. (1995). Involvement of CYP2C in the metabolism of
cannabinoids by human hepatic microsomes from an old woman. Biology of Pharmacology Bulletin, 18, 1138–1141.
Welch, S. P., & Eads, M. (1999). Synergistic interactions of endogenous opioids and cannabinoid systems. Brain Research , 848(1–2),
Welch, S. P., & Stevens, D. L. (1992). Antinociceptive activity of intrathecally administered cannabinoids alone, and in combination with
morphine, in mice. Journal of Pharmacology and Experimental Therapy, 262(1), 10–18.
Welch, S. P., Thomas, C., & Patrick, G. S. (1995). Modulation of cannabinoid-induced antinociception after intracerebroventricular
versus intrathecal administration to mice: possible mechanisms for interaction with morphine. Journal of Pharmacology andExperimental Therapy, 272, 310–321.
Werner, C. (2011). Marijuana gateway to health: How cannabis protects us from cancer and Alzheimer's disease . San Francisco:
Dachstar Press.
Wilsey, B., Marcotte T., Deutsch, R., Gouaux, B., Sakai, S., & Donaghe, H. (2013). Low-dose vaporized cannabis significantly improves
neuropathic pain. Journal of Pain, 14(2), 136–148.
Wilsey, B., Marcotte, T., Tsodikov, A., Millman, J., Bewntley, H., Gouaux, B., & Fishman, S. (2008). A randomized, placebo-controlled
crossover trial of cannabis cigarettes in neuropathic pain. Journal of Pain, 9(6), 506–521.
Wright, S. (2007). Cannabinoid-based medicines for neurological disorders—clinical evidence. Molecular Neurobiology, 36, 129–136.
Yamamoto, I., Watanabe, K., Narimatsu, S., & Yoshimura, H. (1995). Recent advances in the metabolism of cannabinoids.
International Journal of Biochemistry and Cell Biology, 27, 741–746.
Young, F. L. (1988). In the matter of marijuana medical rescheduling petition. Excerpt from US Federal Court Decision,
Zhang, X., Wang, J. F., Kunos, G., & Groopman, J. (2007). Cannabinoid modulation of Kaposi's sarcoma-associated herpesvirus
infection and transformation. Cancer Research, 67, 7230–7237.
Title Page
Copyright Page
1. Why Integrative Oncology?
2. An Integrative Approach to Reducing the Risk of Cancer
3. Molecular Targets of Botanicals Used for Chemoprevention
4. Research Methodology Challenges in Integrative Oncology
5. Diet and Cancer: Epidemiology and Risk Reduction
6. Nutritional Interventions in Cancer
7. Botanical and Mycological Medicine in Integrative Oncology
9. Botanical-Drug Interactions in Oncology—What Is Known?
10. The Antioxidant Debate
11. Physical Activity and Cancer
12. Massage Therapy
13. Mind–Body Medicine in Integrative Cancer Care
14. Music and Expressive Arts Therapies
15. Energy Medicine and Cancer
16. The Role of Spirituality
17. Naturopathic Oncology
18. Traditional and Modern Chinese Medicine
19. Ayurveda and Yoga for Cancer Supportive Care
20. Anthroposophic Medicine, Integrative Oncology, and Mistletoe Therapy of Cancer
21. Integrative Medicine and Breast Cancer
22. Prostate Cancer: An Integrative Approach
23. Integrative Medicine in Colorectal Cancer: Role of Energy Balance as Treatment
24. Integrative Medicine and Lung Cancer
25. Integrative Therapies in Cancer-Symptom Management
26. Alternative Therapies as Primary Treatments for Cancer
27. Communication Issues in Integrative Oncology
28. Tending the Spirit in Cancer
29. A Patient's Perspective
Source: http://www.greenbuddha.us/resources/2014%20Integrative%20Oncology%20Abrams%20Weil%20Cancer%20and%20Cannabinoids.pdf
Asthma Policy Purpose To outline requirements for Asthma Scope All operational levels of Life Saving Victoria Policy Introduction Bronchial asthma or wheezy bronchitis, is a common condition within the community and may affect all groups from infancy to advanced old age. In its most severe form it requires hospitalisation in an Intensive Care Ward but in its milder form does not prevent sporting competition at the highest level. There are several examples of world and Olympic champions who have suffered asthma for years and whose case histories have been described in Medical Journals. There is considerable evidence that exercise is very helpful in the overall management of people with asthma1. A history of asthma by itself should be no deterrent to participation in any aquatic activity including teaching, being taught, examining, competing, or practical lifeguarding providing the lifeguard or student concerned is following the advice of a medical practitioner who is fully conversant with the implications of these activities. Asthma is a very variable condition and LSV recognises that a lifeguard with asthma may be fully fit at some times but not fit for various lifeguarding activities at other times. The responsibility for the
Airflow Uniformity through Perforated Tiles in a Raised-Floor Data Center by James W. VanGilder, P.E., APC by Schneider Electric Roger R. Schmidt, Ph.D., P.E., IBM Corporation Executive summary Perforated tiles on a raised floor often deliver sub-stantially more or less airflow than expected, resulting in inefficiencies and even equipment failure due to inadequate cooling. In this paper, the impact of data center design parameters on perforated tile airflow is quantified and methods of improving airflow unifor-mity are discussed. This paper was written jointly by APC and IBM for the ASME InterPACK '05 conference.







