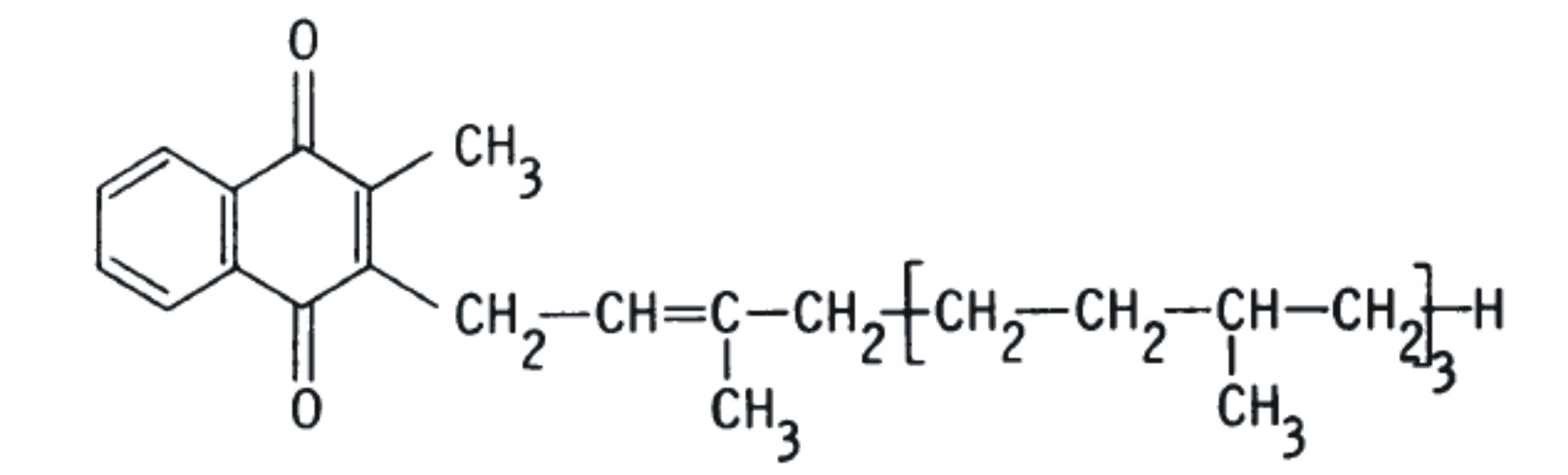Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Microsoft word - sandoz_final_word_text[1]
A MINI REVIEW
Viridis BioPharma Pvt. Ltd.
Viridis BioPharma Pvt. Ltd.
"VITAMIN K2-7"
A MINI REVIEW
Had this review been written just 25 years ago, the metabolism of vitamin K would have been
considered just in the context of its role in haemostasis. During the last 25 years there has been
intensive research with the vitamin K and its analogues which has surfaced the role of vitamin
K2-7 (natural) and K2-4 (synthetic) in the human physiology in multiple functions. The newly
discovered functions extend throughout the body in the Ca metabolism giving rise to the label
"Calcium Paradox" because Ca gets deposited in the vasculature instead of the required target
bones.
Vitamin K is an essential fat-soluble micronutrient which is needed for a unique posttranslational
chemical modification in a small group of proteins with calcium-binding properties, collectively
known as vitamin K – dependent proteins (VKD proteins) or Gla-proteins.
This review is general as well as specific to K2-7 to bring out its importance
amongst other vitamin K analogues.
INTRODUCTION
Historical:
In 1929, the Danish Nutritional scientist Dr. Henrik Dam discovered that
feeding chicks a totally fat–free diet caused uncontrolled bleeding under their skin [1]. Dr. Dam quickly discovered the reason for this disturbing effect: the diet was missing a previously
"koagulationsvitamin" – literally, the "clotting vitamin". The English name for the new vitamin was taken from Dr. Dam's Danish: "K", for "Koagulation".
Science was established that the gamma-glutamyl carboxylase is an
integral cellular glycoprotein that uses vitamin K, a cofactor, to modify clusters of glutamyl residues (glu's) to γ-carboxylated glutamyl residues (gla's) post-translationally in vitamin K–dependent (VKD) proteins as they pass through the endoplasmic reticulum. Carboxylation is required for VKD protein functions in hemostasis. The VKD proteins, in the coagulation cascade, needing vitamin K to transact from the Glu status to Gla, to be biologically active, were proteins II, VII, IX, X, C, S and Z. It is interesting to note the surprising fact that the carboxylase itself has been shown to be a VKD protein [2].
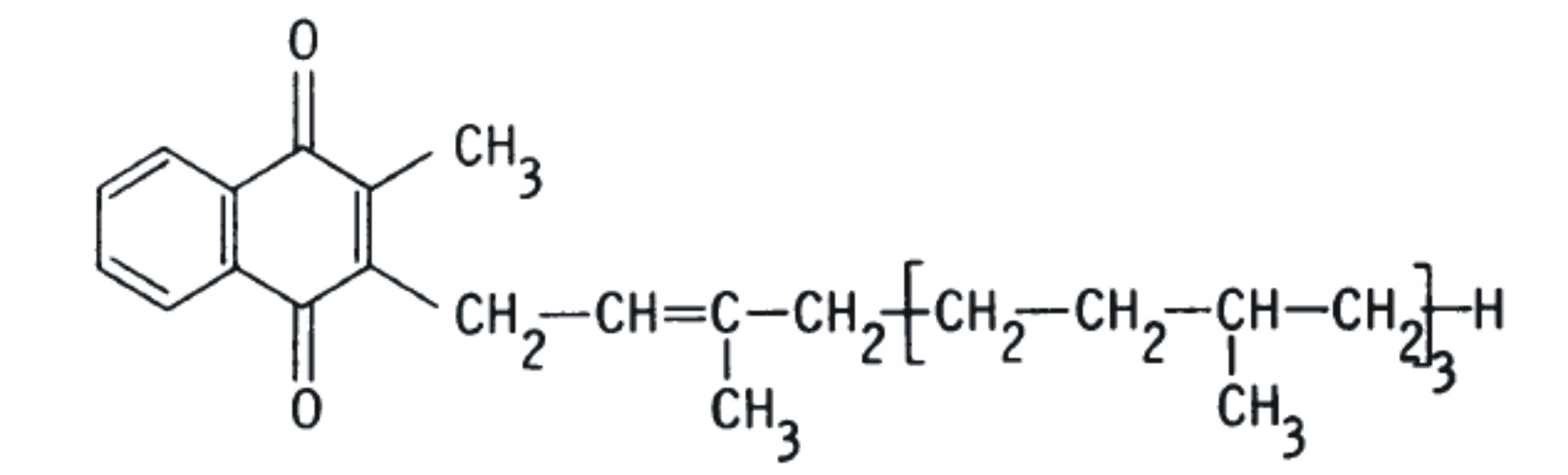
New Research –
For over fifty years, nearly everyone thought that the vitamin K
story began and ended with blood clotting. If doctors paid attention to it at all, it was only to make sure that it didn't interfere with the blood-thinning, anticoagulant drug warfarin (Coumadin®). But even as nutrition textbooks and mainstream medicine continued to think of vitamin K as a one-act show, a paradigm shift had been forced onto researchers in the late 1970's when new vitamin K-dependent protein associated with bone - building osteoblast cells was discovered [3, 4]. Lian et al [4] named it osteocalcin. Now, importance of osteocalcin is well established in osteopenia and osteoporosis. Similarly the role of Matrix Gla Protein (MGP), yet another VKD protein, is well recognized in the vascular calcification. What is important is high expression of MGP and its carboxylation by vitamin K for it to actively prevent deposition of calcium in vasa media and vasa intima [5-7].
Vitamin K Analogues -
Vitamin K is, in fact, a family of structurally similar, fat-soluble,
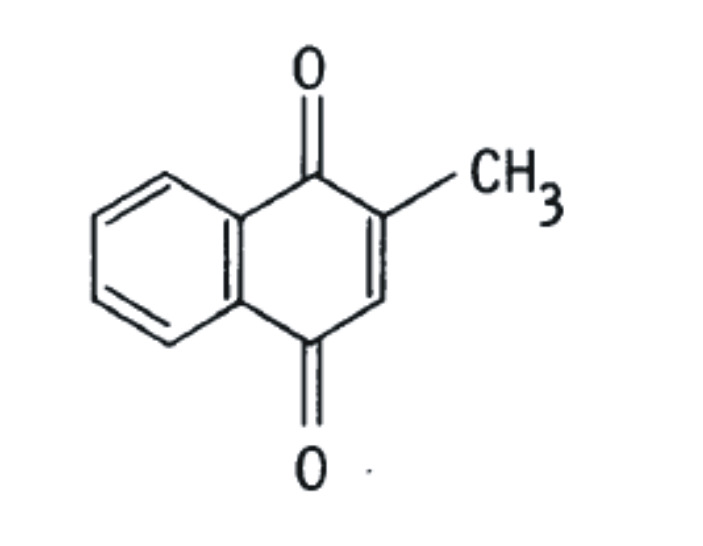
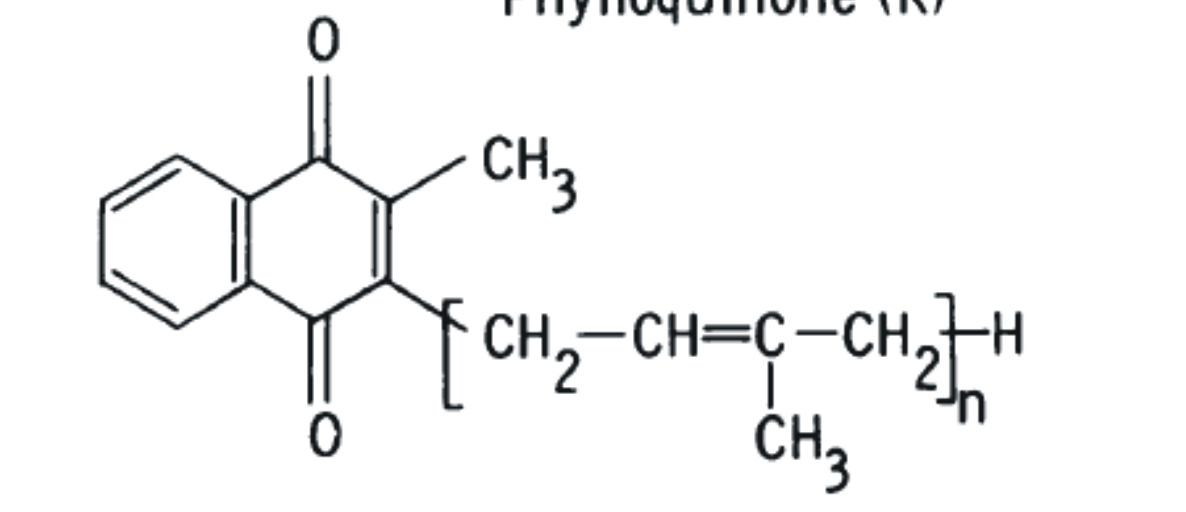
2-methyl-1,4-naphthoquinones, including phylloquinone (K1), menaquinones (K2), and menadione (K3). The structural difference is in the substituent side chain at the gamma position (Schematic A).
PHYLLOQUINONE (VITAMIN K1 = PK)
MENADIONE (VITAMIN K3)
MENAQUINONE (VITAMIN K2-N = MK-N)
Schematic A
The best-known member of the vitamin K family is phylloquinone (K1), also known as phytonadione because of its relationship with photosynthesis. Phylloquinone is found in higher plants and algae, with the highest concentrations found in green leafy vegetables [8]. Vitamin K1.derived from the food intake is selectively distributed in hepatic and non-hepatic tissues.
Menaquinones (K2) also occur naturally, but are produced by an array of bacteria, generally not by higher plants, except for K2-4. Recent studies have determined menaquinones are produced in limited quantities by animals, and by humans, from the conversion of other forms of vitamin K [9, 10] The most common form of vitamin K2 in animals is menaquinone 4 (menatetrenone; MK-4), produced by the processing of exogenous and bacterial naphthoquinones [11]. Vitamins K1 and K2 differ only in the side chain in gamma position. Vitamin K1 possesses a phytyl group (partially saturated polyisoprenoid group) at position 3, while vitamin K2 possesses a repeating, unsaturated trans-poly-isoprenyl group.
The IUPAC-IUB Commission on Biochemical Nomenclature abbreviates
phylloquinone (K1) as "K" while menaquinone (K2) is abbreviated as "MK-n." The "n" signifies the number of unsaturated isoprene units that compose the side chain at the 3-position which may vary between 1 and 14. Here on we shall denote phylloquinone as PK and menaquinones as MK-n.
Biochemistry -
Only known function of vitamin K is that it serves as a cofactor for γ-
glutamylcarboxylase, an endoplasmic enzyme involved in the posttranslational carboxylation of glutamate residues into γ-carboxyglutamate (Gla). Hence, the vitamin K-dependent step is a carboxylation reaction taking place during the later stages of protein biosynthesis. Vitamin K hydroquinone (KH2) is the active coenzyme, the oxidation of which to vitamin K 2, 3 epoxide (KO) provides the energy to drive the carboxylation reaction. The resulting Gla-residues are found in a limited number of proteins, and in these proteins only at certain well-defined positions [12].Specific staining techniques or HPLC detection (after hydrolysis) identify the Gla-proteins as unique products of vitamin K action. In vitamin K-deficiency the carboxylation reaction cannot proceed, hence the Gla-proteins are released in an undercarboxylated form. Gla-residues form calcium-binding groups in proteins. So the main physico-chemical difference between normal and decarboxy proteins is their large difference in both binding of calcium from solution and the adsorption of these proteins to insoluble calcium salts.
Naturally occurring vitamin K1 ( phylloquinone), a plant product is distinguished by its phytyl chain at position 3 in menadione ( see schematic A) whereas a series of bacterial menaquinones (MKs i.e. vitamin K2) have isoprenoid residues at the position 3 in menadione.
Absorption
Bioavailibilty -
Booth et al [13] quotes Gijsbers et al [14] reported that the bioavailability
(the area under an absorption curve) in human subjects of 1 mg phylloquinone in spinach
was only 4% that of pure phylloquinone. Adding butter to the spinach increased this to
13%. Schurgers et al [15], through their experimental studies of PK absorption from
spinach and MK-7 absorption from Natto, one week apart to exclude mutual interference
of absorption, and also broccoli as source for PK and curd cheese and egg yolk as sources
for higher menaquinones (MK-8 and MK-9) and MK-4 respectively, conclude that "In
all cases it was found that PK absorption from vegetables was very poor (5–10%
without concomitant fat intake and 10–15% if taken together with 30 g fat), whereas
menaquinone absorption from dairy produce and natto was much better, probably
almost complete." This fact makes a large section of the population susceptible to
vitamin K deficiency, when these MK vitamins are lacking in the diet and due to poor
absorption of PK.
Dietary Sources -
In absolute amounts PK forms well over 80% of the total amount of
vitamin K in the human diet In absolute amounts PK forms well over 80% of the total amount of vitamin K in the human diet. However, it must be noted that due to different bioavailability, MK's absolute absorption in the system will be higher. Considering 10% bioavailability for PK and 80% bioavailability for MK, ratio of PK/MK absorbed by the body is 8/16 i.e. 1 as to 2. This means that for the practical purposes there is a greater amount of MK coming into the body compared to PK .
Non-dietary sources -
There has been a great debate over the role of the intestinal flora
produced K vitamins as a source sufficient to meet the human requirements. Many bacteria that populate the microbial ecosystem of the human intestine synthesize Menaquinones. Bacterial numbers and composition vary considerably along the human gastrointestinal tract. In the small intestine , growth is limited by the rapid transit times. In humans, by far the largest reservoir of bacteria occupies the large intestine. Quantitatively, the most important genera of intestinal bacteria are the bacteroides and bifidobacteria. They differ in that only bacteroides synthesize menaquinones. Most synthesize MK-10 and MK-11 and minor amounts of MK-7, MK-8, Mk-9 and Mk-12. Suttie et al [16] and Shearer et al [17] have examined the evidence of the contribution of gut menaquinones and concluded that while they do contribute to the human nutrition but not significantly.
Phylloquinone Transport - Dietary vitamin K , after digestion, and triglycerides (TG) are
emulsified by bile salts to form mixed micelles which are taken up by the enterocytes of the intestinal epithelium and processed into chylomicrons (CM) which are secreted into lymph lacteals and enter the blood via thoracic duct [18]. As CM looses their TG they(CM) becomes CM remnants (CR).Together with very low-density lipoproteins





(VLDL) and with the triglyceride-rich lipoprotein(TRL) that are the major carriers of phylloquinone in the circulation. There ia lesser but significant amount carried also by low- and high- density lipoprotein (LDL and HDL). Available data suggests that some 50-70% of phylloquinone is associated with TRL with the remainder being approximately equally distributed between LDL and HDL [19-21].
Phylloquinone is found mainly in TRL and most is cleared from the circulation within 8 hrs. All the studies of phylloquinone disappearance from the circulation suggests a dual phase transport with a half-life of 0.3 hrs in the rapid phase and a half-life of 2.5 hrs in the slower phase [22]
Menaquinone Transport - When three different forms of vitamin K (K1, MK-4, MK-7) were
ingested simultaneously they were found to behave differently [21]. Mk-4 concentrations were lower at all times than those of phylloquinone and were distributed equally between TRL,LDL and HDL up to 4 hrs. The reason for lower serum concentrations, authors speculate, is faster uptake of phylloquinone by tissues compared to at least for MK-4. Figure 1 and Figure 2 is a comparison of phylloquinone vs. MK-7 serum concentration-time profile and the steady state concentrations of these two analogues [23] whereas Figutre 3 is the ratio of cOC to ucOC wrt days progression demonstrating superiority and continuing advantage of VVitamin K2-7.
Circulating vitamin K concentrations following a single oral dose of
1 mg each of vitamin K1 and MK-7. Points are means from 15 subjects ;
error bars represent SD. Baseline levels (1 nM[0.45 µg/L] for K1 and < 0.07
nM [ <0.05 µg/L] for MK-7) were subtracted from all values. Indicates K1
Accumulation and efficacy of K vitamins during long-term daily administration.
Participants received in a crossover design either K1 (O) or MK-7 ( ) or placebo;
in the latter case only K1 ( ) could be detected. Points are means of 18 values;
error bars represent SD. Circulating levels of vitamin K; baseline levels for K1
were subtracted; no MK-7 could be detected at baseline. Ratio between
circulating carboxylated and undercarboxylated osteocalcin (cOC/ucOC);
at baseline the ratio was 1.07 for MK-7, 1.8 for K1, and 1.7 for the placebo group.
Tissue stores of Vitamin K -
Cellular uptake -Several studies have shown that the transport of vitamin K into different
cell types is not uniform. Liver and bones, for example, differ in their requirements of
vitamin K implying different modes and differences in uptake efficiency [24-26].
Tissue Stores - Liver, being the site of synthesis of VKD coagulation proteins, was
originally thought of to be the major site of vitamin K storage. It turns out that livers
major VK content is MKs rather then PK., the ratio being 9 as to 1 [27, 28]. Vitamin K is
present in every cell of the body. Its presence in heart, pancreas and at a lower level in
brain and kidney.
Metabolism
Bioactivity - Ushiroyama et al [29] state that compared to other vitamin K analogues, MK's
have the most potent gamma-carboxylation activity.
Metabolic transformation of PK and MK – Its worth mentioning that what complicates the
study of vitamin K types and stores is continuous transformation of PK to Mk via K3 mechanism.
Vitamin K Catabolism - Most of our knowledge in this area comes from PK studies. Vitamin K
builds relatively low stores and circulation levels compared to vitamins A, D and E. Early isotopic work shows that regardless of dose, 45 mcg/day or 1mg/day, 60 to 70% of a single dose of vitamin K is ultimately excreted as catabolic products in the bile and urine [30].
Excretion –
In tracer studies mentioned earlier [30], it was found that 20% of an injected dose of PK was in the urine whereas about 40-50% was excreted in the faeces via the bile irrespective of the dose size. The metabolites are biologically inactive.
K2-7 and OSTEOPOROSIS
Relationship between Vitamin K2-7 status to ucOC to BMD
The original stimulus for studying the relationship between dietary intakes of vitamin K and bone health had been some small patient-based studies suggesting a possible link between low serum K1 and osteoporosis [31, 32] and even more convincing epidemiological evidence obtained by Szulc and co-workers showing a positive association between ucOC and fracture risk [33, 34] and an inverse association between ucOC and bone mass [35] in French elderly women. Another prospective study performed in elderly home-dwelling French women (EPIDOS) showed an increased hip fracture risk in the highest quartile of serum ucOC [36]. Similar associations linking an impaired carboxylation of OC with bone mass and fracture risk were subsequently reported from The Netherlands [37] and Finland [38]. Since, as already discussed, the carboxylation of OC is sensitive to vitamin K intakes in the usual dietary range, these associations with ucOC give credence to the hypothesis that adequate intakes of vitamin K are necessary to maintain healthy bones. Lately, this hypothesis has been reinforced by studies of patients with chronic gastrointestinal disorders in whom malabsorption of fat-soluble vitamins is
common. Thus patients with primary biliary cirrhosis and Crohn
s disease are known to be at
high risk of osteoporosis and often have both low serum K1 concentrations and a high circulating ucOC [39-42]. Importantly, the study by Schoon et al. [39] provided evidence of an inverse
relationship between ucOC and BMD in Crohn
s disease. There are several studies, human and
animal that relate higher levels of ucOC (under carboxylated OsteoCalcin ) to higher fracture rates. Vitamin K2-7 has been shown to decrease ucOC dose dependently, and consequently fracture rates.
K2-7 in vitro STUDIES
K2-7 & Osteoblastic Activity: Experiments with osteoblasts in a serum free culture containing
K2-7(10-7 to 10-5 M) have confirmed significant activity increase in protein content, alkaline
phosphatase activity, and DNA content [43]. Katsuyama , in his experiments, used K2-7 directly
overcoming confounding effects of calcium and isoflavones and recorded stimulation of
osteoblastic differentiation, not proliferation[44]
K2-7 & Osteoclastic Bone Resorption: PTH and PGE2 are bone-resorbing factors and cause
significant decrease in calcium content in the femoral-metaphyseal tissues from rats in vitro [45,
46]. This decrease was completely prevented with K2-7 (10-7 to 10-5 M) culture, indicating K2-
7s inhibitory effect on bone resorption. Additionally K2-7 prevented the PTH- and PGE2-
induced increase in medium glucose consumption and lactic acid production by bone tissue in
vitro [46].
K2-7 ANIMAL STUDIES
Early animal studies with K2-7 have shown to prevent bone loss in ovariectomized rats, an animal model for osteoporosis [47, 48]. K2-7 has been found to have a stimulatory
effect on calcification in the femoral tissues obtained from normal young rats in vitro [49]. Culture with MK-7 (10-6 or 10-5 M) caused a significant increase in biochemical components ( calcium content, alkaline phosphatase activity, and DNA content) in the femoral-diaphyseal (cortical bone) and meta-physeal (trabecular bone) tissues obtained from aged rats in vitro [50].
K2-7 in Bone Loss:
As reviewed K2-7 has stimulatory effect on osteoblastic bone formation
and inhibitory effect on bone resorption, thereby increasing bone mass, as depicted in Figure 4. The preventive effect of dietary K2-7 on bone loss was investigated using OVX (Ovariectomy) rats [47, 48]. K2-7 (18.1 mcg/100 gm diet) was administered for 24 days. This feeding caused K2-7 increases in serum and femur of OVX rats, and femoral dry weight and femoral calcium content were prevented from decrease. In yet another experiment this effect of bone parameters was confirmed by feeding diets with and without K2-7. Further experiments involved 150 days trials confirming earlier observations.
K2-7 HUMAN STUDIES
Circulating K2-7 and cOC (� � ������
Tsukamoto et al [51, 52]
examined effect of K2-7 in fermented Soybeans on normal individuals. Serum K2-7 was not found in normal individualds who had not eaten natto. After the administration of K2-7 natto serum concentration and cOC were significantly raised after 7 to 14 days. The changes in these levels were dose dependent. This illustrates that intake of K2-7 can stimulate � carboxylation, which plays an important role in bone formation in normal individuals.
For Japanese fermented natto(soybeans) is a traditional food. It contains 100 times as much K2-7 as various cheeses. K2-7 is considered to promote γ carboxylation. Therefore natto may play preventive role in osteoporosis. Katsuyama et al [53] in a study of 117 premenopausal volunteers conclude that natto (K2-7) may improve the bone health of even people who have low affinity receptor for vitamin D. Katsuyama [54] in a 6 months and one year study concludes that natto (K2-7) intake contributes to promotion of bone formation.
Epidemiologic data suggest that intake of natto may play a role in prevention of osteoporosis. Japanese natto contains many positive factors for the bone health, however, there was a significant positive correlation between the level of vitamin K2-7 in serum and the habit of eating Natto in postmenopausal women in the Tokyo area. Natto may contribute to the prevention of osteoporosis. This cautious statement was in the initial stage of findings [55]. Japanese study comparing circulating K2-7 levels between various geographies attribute low fracture risk in postmenopausal women in Eastern Japan, compared to British women, to their higher levels of circulating K2-7 [56]. JPOS study (Japanese Population-based Osteoporosis Study) measured BMD at the spine, hip, and forearm in 944 women (20-79 y old) at baseline and follow-up conducted 3 years later. A significant correlation was determined between K2-7 and the rates of change in BMD at the femoral neck and at the distal third of the radius in the postmenopausal women [57].
Combined Treatment with K2 and D3:
It is known that both vitamin K2 and vitamin D affect bone metabolism. In particular, vitamin D increases intestinal calcium absorption via the action of 1, 25 (OH)2 vitamin D3 [58-60] while vitamin K2 increases renal calcium absorption [61, 62]. Furthermore, a synergistic additive effect of vitamin K2 and vitamin D supplementation is demonstrated in adult ovariectomized rats, young rats and postmenopausal women with osteoporosis [63-65].
Combined Treatment with K2 and Bisphosphonates:
Work of Ito [66] has demonstrated that risedronate prevented the deterioration in the connectivity of the trabeculae in the proximal tibial metapysis in ovariectomiszed rats, whereas vitamin K2 increased trabecular thickness. Thus, the combined treatment of resendronate [67, 68]and vitamin K2 had an additive effect in preventing the deterioration of the trabecular bone architecture in ovariectomized rats.
Osteoporosis Study 1:
A clinical study conducted by Cees Vermeer, Ph.D., at the University of Maastricht, the Netherlands, found vitamin fermentation K2-7 superior to ordinary vitamin K1 supplements in the levels of K absorbed into the bloodstream, as well as reducing several risk factors for osteoporosis.
According to lead researcher Vermeer, "This study is the first of its kind comparing a vitamin K2-7 extract in supplement form to ordinary vitamin K supplements for bone health. These findings build on the existing body of evidence supporting vitamin K2-7 as an important form of vitamin K with benefits that extend beyond K1 alone." [Business Wire, March 22, 2004].
Participants received vitamin K1 or K2-7 supplements in equivalent doses over a six-week period. The following conclusions were drawn:
Much higher circulating vitamin K levels were reached with K2-7 than with K1. Circulating under-carboxylated osteocalcin (a marker for hip fracture risk) was decreased much more by K2-7 than by K1. - The ratio between carboxylated and under-carboxylated osteocalcin (this ratio being a marker for bone vitamin K status) was improved similarly by both forms of vitamin K, although in the case of K1 a clear plateau was reached, whereas in the case of K2-7 the curve was still rising after six weeks of administration and would have continued further. The data suggest that K2-7 and not K1 contributes to decreasing bone turnover. Since high bone turnover is a strong risk factor for accelerated bone loss, it seems that K2-7 has a second protective effect on bone.
Yamaguchi [69] in his various studies concludes "Menaquinone-7, an analogue of vitamin K(2) which is abundant in fermented soybeans, has been demonstrated to stimulate osteoblastic bone formation and to inhibit osteoclastic bone resorption. "
Osteoporosis Study 2:
Study 1 establishes superiority of vitamin K2-7 over vitamin K1 in terms of bioavailability, bioactivity and extrahepatic tissue specificity. It is relevant to note the Nurses' Health Study [70] that recruited 72,327 women aged between 38 and 63 years, i.e. relatively young women who have a markedly lower incidence of hip fracture than elderly ones. The study period was ten-
years and concluded a significant protective effect of vitamin K on subjects who had never used HRT.
Osteoporosis Study 3:
The Framingham study investigated vitamin K intake in 1479 women (average age 75 years) and 1112 men [71]. The conclusion was "Low dietary vitamin K intake was associated with low BMD in women, consistent with previous reports that low dietary vitamin K intake is associated with an increased risk of hip fracture. However in men, a positive correlation was found etween BMD and blood parameters of vitamin K status (serum K1 and ucOC) [72].
Dam H: The antihemorrhagic vitamin of the chick: occurrence and chemical nature. Nature 1935, 135:652-653.
Berkner KL, Pudota BN: Vitamin K-dependent carboxylation of the carboxylase. Proc Natl Acad Sci U S A 1998, 95(2):466-471.
Stenflo J: A new vitamin K-dependent protein. Purification from bovine plasma and preliminary characterization. J Biol Chem 1976, 251(2):355-363.
Lian JB, Hauschka PV, Gallop PM: Properties and biosynthesis of a vitamin K-dependent calcium binding protein in bone. Fed Proc 1978, 37(12):2615-2620.
Schurgers LJ, Cranenburg EC, Vermeer C: Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thromb Haemost 2008, 100(4):593-603.
Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G: Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997, 386(6620):78-81.
Schurgers LJ, Dissel PE, Spronk HM, Soute BA, Dhore CR, Cleutjens JP, Vermeer C: Role of vitamin K and vitamin K-dependent proteins in vascular calcification. Z Kardiol 2001, 90 Suppl 3:57-63.
Thomson RH: Naturally Occurring Quinones. New York, N.Y.: Academic Press; 1971.
Davidson RT, Foley AL, Engelke JA, Suttie JW: Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteria. J Nutr 1998, 128(2):220-223.
Thijssen HH, Drittij-Reijnders MJ: Vitamin K status in human tissues: tissue-specific accumulation of phylloquinone and menaquinone-4. Br J Nutr 1996, 75(1):121-127.
Seegers WH, Bang NU: Blood Clotting Enzymology. New York, NY: Academic Press; 1967.
Furie B, Furie BC: Molecular and cellular biology of blood coagulation. N Engl J Med 1992, 326(12):800-806.
Booth SL, Suttie JW: Dietary intake and adequacy of vitamin K. J Nutr 1998, 128(5):785-788.
Gijsbers BL, Jie KS, Vermeer C: Effect of food composition on vitamin K absorption in human volunteers. Br J Nutr 1996, 76(2):223-229.
Schurgers LJ, Vermeer C: Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis 2000, 30(6):298-307.
Suttie JW: The importance of menaquinones in human nutrition. Annu Rev Nutr 1995, 15:399-417.
Shearer MJ: Vitamin K metabolism and nutriture. Blood reviews 1992, 6(2):92-104.
Blomstrand R, Forsgren L: Vitamin K1-3H in man. Its intestinal absorption and transport in the thoracic duct lymph. Int Z Vitaminforsch 1968, 38(1):45-64.
Kohlmeier M, Salomon A, Saupe J, Shearer MJ: Transport of vitamin K to bone in humans. J Nutr 1996, 126(4 Suppl):1192S-1196S.
Lamon-Fava S, Sadowski JA, Davidson KW, O'Brien ME, McNamara JR, Schaefer EJ: Plasma lipoproteins as carriers of phylloquinone (vitamin K1) in humans. Am J Clin Nutr 1998, 67(6):1226-1231.
Schurgers LJ, Vermeer C: Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim Biophys Acta 2002, 1570(1):27-32.
Jones KS, Bluck LJ, Wang LY, Coward WA: A stable isotope method for the simultaneous measurement of vitamin K1 (phylloquinone) kinetics and absorption. Eur J Clin Nutr 2008, 62(11):1273-1281.
Schurgers LJ, Teunissen KJ, Hamulyak K, Knapen MH, Vik H, Vermeer C: Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood 2006.
Sato T, Ohtani Y, Yamada Y, Saitoh S, Harada H: Difference in the metabolism of vitamin K between liver and bone in vitamin K-deficient rats. Br J Nutr 2002, 87(4):307-314.
Vermeer C, Shearer MJ, Zittermann A, Bolton-Smith C, Szulc P, Hodges S, Walter P, Rambeck W, Stocklin E, Weber P: Beyond deficiency: potential benefits of increased intakes of vitamin K for bone and vascular health. Eur J Nutr 2004, 43(6):325-335.
Price PA, Kaneda Y: Vitamin K counteracts the effect of warfarin in liver but not in bone. Thromb Res 1987, 46(1):121-131.
Shearer MJ, McCarthy PT, Crampton OE, Mattock MB: The assessment of human vitamin K status from tissue measurement. Current advances in vitamin K research (ed JW Suttie) 1988, Elsevier:437-452.
Usui y Th, N Nishimura, N Kobayashi, T Okanoue and K Ozawa Vitamin K concentrations in the plasma and liver of surgical patients. 1990, 51:846-852.
Ushiroyama T, Ikeda A, Ueki M: Effect of continuous combined therapy with vitamin K(2) and vitamin D(3) on bone mineral density and coagulofibrinolysis function in postmenopausal women. Maturitas 2002, 41(3):211-221.
Shearer MJ, McBurney A, Barkhan P: Studies on the absorption and metabolism of phylloquinone (vitamin K1) in man. Vitam Horm 1974, 32:513-542.
Hart JP, Shearer MJ, Klenerman L, Catterall A, Reeve J, Sambrook PN, Dodds RA, Bitensky L, Chayen J: Electrochemical detection of depressed circulating levels of vitamin K1 in osteoporosis. J Clin Endocrinol Metab 1985, 60(6):1268-1269.
Hodges SJ, Akesson K, Vergnaud P, Obrant K, Delmas PD: Circulating levels of vitamins K1 and K2 decreased in elderly women with hip fracture. J Bone Miner Res 1993, 8(10):1241-1245.
Szulc P, Chapuy MC, Meunier PJ, Delmas PD: Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J Clin Invest 1993, 91(4):1769-1774.
Szulc P, Chapuy MC, Meunier PJ, Delmas PD: Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture: a three year follow-up study [see comments]. Bone 1996, 18(5):487-488.
Szulc P, Arlot M, Chapuy MC, Duboeuf F, Meunier PJ, Delmas PD: Serum undercarboxylated osteocalcin correlates with hip bone mineral density in elderly women. J Bone Miner Res 1994, 9(10):1591-1595.
Vergnaud P, Garnero P, Meunier PJ, Breart G, Kamihagi K, Delmas PD: Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: the EPIDOS Study. J Clin Endocrinol Metab 1997, 82(3):719-724.
Knapen MH, Nieuwenhuijzen Kruseman AC, Wouters RS, Vermeer C: Correlation of serum osteocalcin fractions with bone mineral density in women during the first 10 years after menopause. Calcif Tissue Int 1998, 63(5):375-379.
Luukinen H, Kakonen SM, Pettersson K, Koski K, Laippala P, Lovgren T, Kivela SL, Vaananen HK: Strong prediction of fractures among older adults by the ratio of carboxylated to total serum osteocalcin. J Bone Miner Res 2000, 15(12):2473-2478.
Schoon EJ, Muller MC, Vermeer C, Schurgers LJ, Brummer RJ, Stockbrugger RW: Low serum and bone vitamin K status in patients with longstanding Crohn's disease: another pathogenetic factor of osteoporosis in Crohn's disease? Gut 2001, 48(4):473-477.
Szulc P, Meunier PJ: Is vitamin K deficiency a risk factor for osteoporosis in Crohn's disease? Lancet 2001, 357(9273):1995-1996.
Bernstein CN, Blanchard JF, Leslie W, Wajda A, Yu BN: The incidence of fracture among patients with inflammatory bowel disease. A population-based cohort study. Ann Intern Med 2000, 133(10):795-799.
Jahnsen J, Falch JA, Aadland E, Mowinckel P: Bone mineral density is reduced in patients with Crohn's disease but not in patients with ulcerative colitis: a population based study. Gut 1997, 40(3):313-319.
Yamaguchi M, Sugimoto E, Hachiya S: Stimulatory effect of menaquinone-7 (vitamin K2) on osteoblastic bone formation in vitro. Mol Cell Biochem 2001, 223(1-2):131-137.
Katsuyama H, Otsuki T, Tomita M, Fukunaga M, Fukunaga T, Suzuki N, Saijoh K, Fushimi S, Sunami S: Menaquinone-7 regulates the expressions of osteocalcin, OPG, RANKL and RANK in osteoblastic MC3T3E1 cells. International journal of molecular medicine 2005, 15(2):231-236.
Yamaguchi M, Ma ZJ: Inhibitory effect of menaquinone-7 (vitamin K2) on osteoclast-like cell formation and osteoclastic bone resorption in rat bone tissues in vitro. Mol Cell Biochem 2001, 228(1-2):39-47.
Yamaguchi M, Uchiyama S, Tsukamoto Y: Inhibitory effect of menaquinone-7 (vitamin K2) on the bone-resorbing factors-induced bone resorption in elderly female rat femoral tissues in vitro. Mol Cell Biochem 2003, 245(1-2):115-120.
Yamaguchi M, Kakuda H, Gao YH, Tsukamoto Y: Prolonged intake of fermented soybean (natto) diets containing vitamin K2 (menaquinone-7) prevents bone loss in ovariectomized rats. J Bone Miner Metab 2000, 18(2):71-76.
Yamaguchi M, Taguchi H, Gao YH, Igarashi A, Tsukamoto Y: Effect of vitamin K2 (menaquinone-7) in fermented soybean (natto) on bone loss in ovariectomized rats. J Bone Miner Metab 1999, 17(1):23-29.
Ehara Y, Takahashi H, Hanahisa Y, Yamaguchi M: Effect of vitamin K2 (menaquinone-7) on bone metabolism in the femoral-metaphyseal tissues of normal and skeletal-unloaded rats: enhancement with zinc. Research in experimental medicine 1996, 196(3):171-178.
Yamaguchi M, Uchiyama S, Tsukamoto Y: Stimulatory effect of menaquinone-7 on bone formation in elderly female rat femoral tissues in vitro: prevention of bone deterioration with aging. International journal of molecular medicine 2002, 10(6):729-733.
Tsukamoto Y, Ichise H, Kakuda H, Yamaguchi M: Intake of fermented soybean (natto) increases circulating vitamin K2 (menaquinone-7) and gamma-carboxylated osteocalcin concentration in normal individuals. J Bone Miner Metab 2000, 18(4):216-222.
Tsukamoto Y, Ichise H, Yamaguchi M: Prolonged Intake of Dietary Fermented Soybeans (Natto) with the Reinforced Vitamin K2 (Menaquinone-7) Enhances Circulating γ - Carboxylated Osteocalcin Concentration in Normal Individuals. Journal of Health Science 2000, 46(4):317-321.
Katsuyama H, Ideguchi S, Fukunaga M, Saijoh K, Sunami S: Usual dietary intake of fermented soybeans (Natto) is associated with bone mineral density in premenopausal women. J Nutr Sci Vitaminol (Tokyo) 2002, 48(3):207-215.
Katsuyama H, Ideguchi S, Fukunaga M, Fukunaga T, Saijoh K, Sunami S: Promotion of bone formation by fermented soybean (Natto) intake in premenopausal women. J Nutr Sci Vitaminol (Tokyo) 2004, 50(2):114-120.
Hosoi T: [Recent progress in treatment of osteoporosis]. Nippon Ronen Igakkai Zasshi 1996, 33(4):240-244.
Kaneki M, Hedges SJ, Hosoi T, Fujiwara S, Lyons A, Crean SJ, Ishida N, Nakagawa M, Takechi M, Sano Y et al: Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: possible implications for hip-fracture risk. Nutrition 2001, 17(4):315-321.
Ikeda Y, Iki M, Morita A, Kajita E, Kagamimori S, Kagawa Y, Yoneshima H: Intake of fermented soybeans, natto, is associated with reduced bone loss in postmenopausal women: Japanese Population-Based Osteoporosis (JPOS) Study. J Nutr 2006, 136(5):1323-1328.
Baeksgaard L, Andersen KP, Hyldstrup L: Calcium and vitamin D supplementation increases spinal BMD in healthy, postmenopausal women. Osteoporos Int 1998, 8(3):255-260.
Ooms ME, Roos JC, Bezemer PD, van der Vijgh WJ, Bouter LM, Lips P: Prevention of bone loss by vitamin D supplementation in elderly women: a randomized double-blind trial. J Clin Endocrinol Metab 1995, 80(4):1052-1058.
Shiraishi A, Higashi S, Ohkawa H, Kubodera N, Hirasawa T, Ezawa I, Ikeda K, Ogata E: The advantage of alfacalcidol over vitamin D in the treatment of osteoporosis. Calcif Tissue Int 1999, 65(4):311-316.
Kobayashi M, Hara K, Akiyama Y: [Effect of menatetrenone (V.K2) on bone mineral density and bone strength in Ca/Mg deficient rats]. Nippon yakurigaku zasshi 2002, 120(3):195-204.
Robert D, Jorgetti V, Lacour B, Leclerq M, Cournot-Witmer G, Ulmann A, Drueke T: Hypercalciuria during experimental vitamin K deficiency in the rat. Calcif Tissue Int 1985, 37(2):143-147.
Hirano J, Ishii Y: Effects of vitamin K2, vitamin D, and calcium on the bone metabolism of rats in the growth phase. J Orthop Sci 2002, 7(3):364-369.
Iwamoto J, Takeda T, Ichimura S: Effect of combined administration of vitamin D3 and vitamin K2 on bone mineral density of the lumbar spine in postmenopausal women with osteoporosis. J Orthop Sci 2000, 5(6):546-551.
Matsunaga S, Ito H, Sakou T: The effect of vitamin K and D supplementation on ovariectomy-induced bone loss. Calcif Tissue Int 1999, 65(4):285-289.
Ito M: Bone mass, microstructure, and quality with bone strength- The effects of antisorptive agents. J Jpn Soc Bone Morphom 2002, 12:51-54.
Iwamoto J, Takeda T, Sato Y, Yeh JK: Additive effect of vitamin K2 and risedronate on long bone mass in hypophysectomized young rats. Experimental animals / Japanese Association for Laboratory Animal Science 2007, 56(2):103-110.
Iwamoto J, Takeda T, Sato Y, Shen CL, Yeh JK: Beneficial effect of pretreatment and treatment continuation with risedronate and vitamin K2 on cancellous bone loss after ovariectomy in rats: a bone histomorphometry study. J Nutr Sci Vitaminol (Tokyo) 2006, 52(5):307-315.
Yamaguchi M: Regulatory mechanism of food factors in bone metabolism and prevention of osteoporosis. Yakugaku Zasshi 2006, 126(11):1117-1137.
Feskanich D, Weber P, Willett WC, Rockett H, Booth SL, Colditz GA: Vitamin K intake and hip fractures in women: a prospective study. Am J Clin Nutr 1999, 69(1):74-79.
Booth SL, Broe KE, Gagnon DR, Tucker KL, Hannan MT, McLean RR, Dawson-Hughes B, Wilson PW, Cupples LA, Kiel DP: Vitamin K intake and bone mineral density in women and men. Am J Clin Nutr 2003, 77(2):512-516.
Booth SL, Broe KE, McLean RR, Gagnon DR, Peterson JW, Hannan MT, Cupples LA, Cheng DM, Wilson PWF, Dawson-Hughes B et al: Low vitamin K status is associated with low bone mineral density and quantitative ultrasound in men. J Bone Miner Res 2002, 17 (Suppl 1).
Source: http://www.pharmacos.co.za/downloads/New%20Vitamin%20K2-7%20Mini%20Review.pdf
Corporate Information Biographical Details of Directors and Senior Management Chairman's Statement Corporate Governance Report Management Discussion and Analysis Directors' Report Independent Auditor's Report Consolidated Financial Statements Consolidated Income Statement Consolidated Statement of Comprehensive Income Consolidated Statement of Financial Position
En el nombre de Dios A la juventud de los países occidentales Los tristes acontecimientos que provocaron el terrorismo ciego en Francia, una vez más, han motivado este diálogo con ustedes. Para mí resulta muy lamentable que tales acontecimientos generen estas intervenciones, no obstante, es una realidad que si estos dolorosos temas no allanan el terreno para encontrar una solución, los daños serían mayores. El sufrimiento de todo ser humano, en cualquier parte del mundo, es triste para los demás. La imagen de la muerte de un niño ante los ojos de sus seres queridos, de una madre cuya alegría familiar se transforma en luto, de un esposo que traslada con prisa el cadáver de su esposa, o una audiencia que desconoce que dentro de poco verá cómo culmina la última escena de su vida, son imágenes que despiertan el sentimiento humano. Cualquiera que sepa de bondad, se siente consternado por tales escenas, sea en Francia, en Palestina, Irak, El Líbano o Siria. Seguramente, los mil millones y medio de musulmanes del mundo comparten este sentimiento y repudian a los autores de tales tragedias. Yo estoy convencido de que solo ustedes, los jóvenes, al ver tales calamidades del mundo de hoy, serán capaces de encontrar nuevas vías para construir el futuro y evitarán el camino incorrecto que ha conducido a Occidente hacia su situación actual.