Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Psichiatriaedipendenze.it
HEROIN ADDICTION &
Pacini Editore & AU CNS
Heroin Addict Relat Clin Probl 2011; 13(2): 5-40
Basics on Addiction: a training package for medical practitioners or
psychiatrists who treat opioid dependence
Icro Maremmani 1, Matteo Pacini 2, Pier Paolo Pani 3, on behalf of the 'Basics on Addiction Group'
1 Vincent P. Dole Dual Diagnosis Unit, Santa Chiara University Hospital, Department of Psychiatry, NPB, University
of Pisa, Italy
2 G. de Lisio Institute of Behavioural Sciences, Pisa, Italy
3 Social-Health Division, Health District 8 (ASL 8) Cagliari, Italy
Opioid dependence is a chronic, relapsing brain disease that causes major medical, social and economic problems to
both the individual and society. This seminar is intended to be a useful training resource to aid healthcare professionals
– in particular, physicians who prescribe opioid pharmacotherapies – in assessing and treating opioid-dependent indi-
viduals. Herein we describe the neurobiological basis of the condition; recommended approaches to patient assessment
and monitoring; and the main principles and strategies underlying medically assisted approaches to treatment, including
the pharmacology and clinical application of methadone, buprenorphine and buprenorphine–naloxone.
Key Words: Tolerance; physical dependence; addiction; clinical assessment; maintenance pharmacotherapies;
methadone; buprenorphine; suboxone.
broader impact on other budgets (e.g., social wel-
fare and criminal-justice services). In addition,
Opioid dependence is a chronic, relapsing opioid dependence affects productivity, due to
brain disease that causes major medical, social unemployment, absenteeism and premature mor-
and economic problems to both the individual tality [111]. In West and Central Europe, there are
and to society. Opioid-dependent individuals are estimated to be between 1 and 1.4 million opiate
subject to substantial health risks including over- users, corresponding to a prevalence of between
dose, transmission of infectious diseases, poor 0.4% and 0.5% of the population.
physical and mental health and frequent hospi-
Given the magnitude of these problems, it
talization [44]. For society as a whole, opioid de- has become crucial to ensure medical practition-
pendence incurs a significant economic burden, ers responsible for treating opioid dependence
both in terms of direct healthcare costs (i.e., treat- have access to evidence-based training pack-
ment and prevention services), and in terms of the ages. This supplement is intended to be a useful
Correspondence: Icro Maremmani, MD; Vincent P. Dole Dual Diagnosis Unit, Santa Chiara University Hospital,
Department of Psychiatry, University of Pisa, Via Roma, 67 56100 PISA, Italy, EU.
Phone +39 0584 790073 Fax +39 0584 72081 E-Mail:
[email protected]
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
how each of these treatment options can be used
Table 1. Actions of morphine [78]
to treat opioid dependence and the main efficacy
Central nervous system depression
and safety considerations that are relevant to the
Respiratory depression (death)
choice of treatment strategy.
2. Neurobiology of opioid dependence
Cough suppression
Pupillary constriction
Nausea and vomiting
2.1. Opioids and their mechanism of action
Increased respiratory tract secretions
2.1.1. What is an opioid?
training resource to aid healthcare professionals
Opium has been used for social and medici-
– in particular, physicians who prescribe opioid nal purposes for thousands of years to produce
pharmacotherapies – in assessing and treating euphoria, analgesia and sleep and to prevent di-
opioid-dependent individuals. It is based upon arrhoea [85]. Several pharmacologically active
the ‘Basics on Addiction' training package de- compounds are derived from the opium poppy
veloped as a collaborative initiative by leading
Papaver somniferum, including morphine, co-
treatment experts in Italy and led by Professor deine, papaverine, thebaine and noscapine [24].
Icro Maremmani (President of EUROPAD) and Opioids is the term given to natural or synthetic
Professor Pier Paolo Pani (President of the Italian drugs that have certain pharmacological actions
Society of Addiction Medicine) on behalf of the similar to those of morphine [84] by the interac-
Basics on Addiction (BoA) Group.
tion with some or all opioid receptors.
In order to optimally treat opioid-dependent
individuals it is first necessary to understand the
2.1.2. Acute opioid effects
neurobiological basis of the condition as a chron-
ic, relapsing disorder. The first article in this sup-
Morphine, the archetypal opioid, is a power-
plement, ‘Neurobiology of opioid dependence', ful analgesic and narcotic, and remains one of the
gives an overview of the effects of opioids on the most valuable analgesics for relief of severe pain
body at the cellular level and the physiological ef- [24]. It also induces a powerful sense of content-
fects of opioids and neurobiological adaptations ment and well-being, which is an important part
to opioids (including tolerance, physical depend- of its analgesic activity, as it reduces the anxiety
ence, withdrawal, craving and relapse). Effective and agitation associated with a painful illness or
treatment of opioid dependence requires thor- injury. Other opioid effects on the central nervous
ough, ongoing assessment of patients to ensure system include respiratory depression, depres-
therapeutic strategies are suited to their individu- sion of the cough reflex, nausea and vomiting and
al needs and circumstances. The second article in pupillary constriction [85]. Morphine also acts
this supplement describes approaches to clinical on the gut wall, reducing intestinal secretion and
assessment and monitoring that should be con- motility and lengthening gut transit time [21].
ducted in drug-dependent individuals to inform The actions of morphine are shown in Table 1.
choices regarding appropriate treatment. The fi-
Following elucidation of the chemical struc-
nal article discusses the main principles, goals ture of morphine at the beginning of the 20th
and strategies underlying medically assisted ap- century [95], many semi-synthetic and totally
proaches to opioid-dependence treatment, the synthetic opioids have been produced (including
unique pharmacological profiles of methadone, methadone, buprenorphine and pethidine) with
buprenorphine and buprenorphine-naloxone, the aim of harnessing the clinically useful proper-
I. Maremmani et al.: Basics on Addiction: a training package for medical practitioners or psychiatrists who treat opioid dependence
Table 2: Effects associated with the main types of opioid receptors [85]
mu (μ, MOP or OP3)
delta (δ DOP or OP2)
kappa (κ, KOP or OP1)
Respiratory depression
Pupil constriction
Reduced GI motility
Physical dependence
+: denotes activity; −: denotes weak or no activity
ties of the opioids without the less desirable side the brainstem, the medial thalamus, the spinal
effects (i.e. habit-forming propensity or nausea cord, the hypothalamus and the limbic system.
and vomiting) [24].
They have also been identified on peripheral
sensory nerve fibres and their terminals and on
2.1.3. Opioid receptors
immune cells [36]. Each receptor type is associ-
ated with specific functional effects, as shown
Pharmacologic studies performed in the 1970s in Table 2. The best-studied receptor type is the
had suggested the existence of three types of clas- mu receptor (also known as the μ, MOP or OP3
sic opioid receptor, termed mu, delta and kappa receptor), which is found in both spinal and su-
[68], and this was subsequently confirmed by praspinal structures as well as in the periphery.
receptor-cloning studies. Opioid receptors be- It plays an important role in nociception, as well
long to the large family of receptors possessing as respiration, cardiovascular function, intestinal
seven transmembrane domains of amino acids transit, feeding, learning and memory, locomotor
and are coupled to guanine nucleotide-binding activity, thermoregulation, hormone secretion,
proteins known as G-proteins [17]. They reduce and immune functions [25]. Kappa receptors
the intracellular cyclic adenosine monophosphate (also known as κ, KOP or OP2 receptors) have
(cAMP) content by inhibiting adenylate cyclase been implicated in the regulation of nociception,
and also exert effects on ion channels through diuresis, feeding and neuroendocrine secretion.
a direct G-protein coupling to the channel [85]. In addition, as kappa receptor agonists can pro-
The main effects of opioids at the membrane duce dysphoria in humans [25], they appear to
level are thus the promotion of the opening of play a role in regulation of mood. The olfactory
potassium channels and inhibition of the opening bulb, neocortex, caudate putamen and nucleus
of voltage-gated calcium channels [85]. These accumbens contain the highest densities of delta
membrane effects reduce neuronal excitability (δ, DOP or OP1) receptors, with lower densities
as the increased potassium conductance causes in the thalamus, hypothalamus and brainstem
hyperpolarisation of the membrane and reduces [25]. A fourth opioid receptor has been discov-
transmitter release due to inhibition of calcium ered more recently, the NOP receptor (formerly
entry [85]. The overall effect is inhibitory at the referred to as opiate receptor-like 1 [ORL1],
cellular level [85]. However, opioids do increase LC132 or OP4). Pharmacologically this is not a
activity in some neuronal pathways by suppress- classical opioid receptor, as non-selective opioid
ing the firing of inhibitory interneurones [85].
antagonists (e.g., naloxone) display negligible af-
High densities of opioid receptors are present finity; the International Union of Basic and Clini-
in five areas of the central nervous system (CNS): cal Pharmacology (IUPHAR) database of recep-
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
Table 3: Selectivity of opioid drugs and peptides for the three main opioid receptors [85]
mu (μ, MOP or OP3)
delta (δ, DOP or OP2)
kappa (κ, KOP or OP1)
Endogenous peptides
Morphine, codeine,
Etorphine, bremazocine
Fentanyl, sufentanil
Partial/mixed agonists
+: agonist activity; (): partial agonist activity; x: antagonist activity; −: weak or no activity
tors proposes that the NOP receptor is considered way [13]. The classification of opioid drugs and
as a non-opioid branch of the opioid receptor endogenous peptides in terms of their agonist,
family [27].
partial agonist or antagonist activity and their
selectivity for the three main opioid receptors is
2.1.4. Agonists and antagonists
shown in Table 3.
The overall effect of an opioid depends on
2.1.5. Endogenous opioids
its activity at each of the opioid receptors; some
opioids act as agonists on one type of receptor
The search for endogenous compounds that
and antagonists or partial agonists at another. Ag- mimicked the actions of morphine in the 1970s
onist potency depends on two parameters: i) the led to the discovery of the endogenous opioids
affinity of the agonist for the receptor, that is, its [43]. Four classes of endogenous opioids have
tendency to bind to the receptor; and ii) the effi- now been identified: endorphins, enkephalins,
cacy (commonly indicated as intrinsic activity) of dynorphins and endomorphins [56]. Endogenous
the agonist, that is, its ability to initiate changes opioids function as neuromodulators to influ-
which lead to effects once bound. Full agonists ence the actions of other neurotransmitters such
(which can produce maximal effects) have high as dopamine or glutamate [94]. The endogenous
efficacy whereas partial agonists (which can pro- opioid system has been found to be important in
duce only submaximal effects) have intermedi- the modulation of pain, mood, blood-pressure
ate efficacy [87]. The relationship of a drug with regulation and other cardiovascular functions,
its receptor is often likened to that of the fit of a control of respiration, appetite, thirst and sexual
key into its lock – the drug represents the key and activity [94]. There are high concentrations of re-
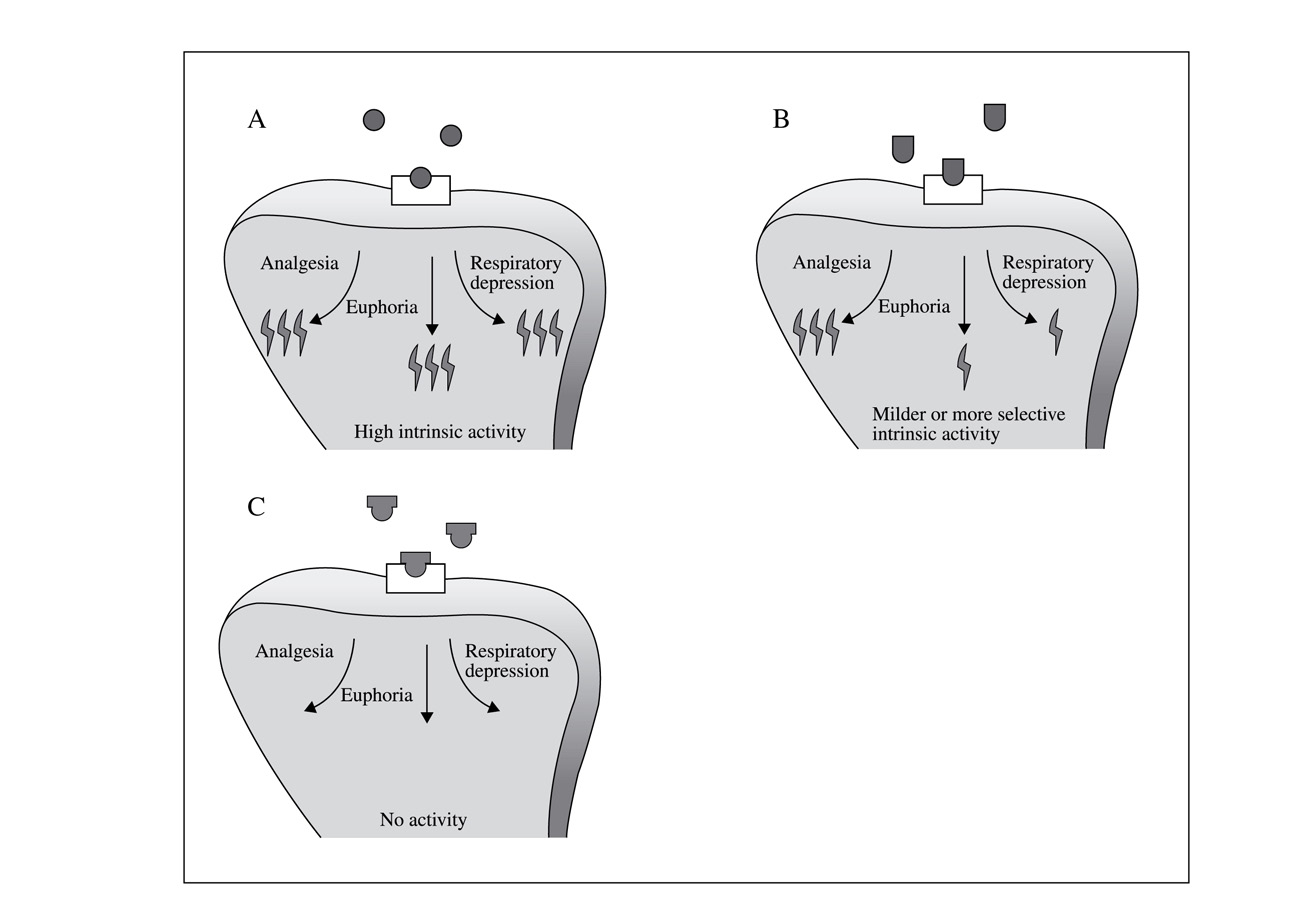
the receptor represents the lock (Figure 1). Hor- ceptors for endorphins and enkephalins in many
mones, neurotransmitters, drugs or intracellular areas of the CNS, particularly in the periaqueduc-
messengers may all interact with receptors in this tal grey matter of the midbrain, in the limbic sys-
I. Maremmani et al.: Basics on Addiction: a training package for medical practitioners or psychiatrists who treat opioid dependence
Table 4: Clinical features of opioid intoxication and
Drowsiness, stupor or coma
Symmetric, pinpoint, reactive pupils
Decreased peristalsis
Skin cool and moist
Hypoventilation (respiratory slowing, irregular
breathing, apnea)
Reversal with naloxone
Figure 1: Action of opioid agonists, antagonists
Anxiety, restlessness
and partial agonists. A: Opioid agonist; B: Opioid
partial agonists; C: Opioid antagonists
Chills, hot flushes
tem and at interneurones in the dorsal horn areas.
Myalgias, arthralgias
These areas are involved in pain transmission
or perception and the endogenous opioids are
Abdominal cramping
Vomiting, diarrhoea
thought to be the body's natural pain-relieving
chemicals, which act by enhancing inhibitory ef-
fects at opioid receptors. Opioid drugs elicit their
Tachycardia, hypertension (mild)
effects by mimicking the actions of the endog-
Hyperthermia (mild), diaphoresis, lacrimation,
enous opioids on opioid receptors [13].
Spontaneous ejaculation
2.2. Chronic opioid use: tolerance, physical de-
pendence and addiction
for effects such as constipation and miosis [85].
When the drug is stopped or when its effect
2.2.1. Effects of chronic opioid exposure
is counteracted by a specific antagonist [80], un-
pleasant physical effects occur, which indicates
Although possessing valuable properties (e.g., the occurrence of the withdrawal (abstinence)
analgesia), repeated and chronic exposure to syndrome. Withdrawal symptoms generally rep-
opioids can lead to development of tolerance and resent physiologic actions opposite to the acute
physical dependence. The rate of development of actions of opioid drugs. For example, pupillary
tolerance varies from one opioid to another.
constriction and constipation occur with opiate
Tolerance describes the need to progressive- use, whereas pupillary dilatation and diarrhoea
ly increase the drug dose to produce the effect occur in the withdrawal state [54]. The most
originally achieved with smaller doses, following common symptoms of opioid intoxication and
repeated exposure to opioid agonists. It may de- withdrawal are shown in Table 4. Individuals
velop at different rates for the different effects of who abruptly stop taking morphine are extremely
opioids and can occur over days, weeks or years restless and distressed and have a strong crav-
[90]. Tolerance develops to the analgesic and ing for the drug. Although not life-threatening,
euphoric effects of opioids, and to some of the opioid withdrawal is associated with severe psy-
adverse effects such as respiratory depression, chological and moderate physical distress [54].
nausea and sedation, but does not fully develop The onset of withdrawal symptoms typically
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
Figure 2: Experience of the opioid-dependent individual depending on opioid
concentrations in the body. Reproduced with permission from Newman et al.,
1995 [78]
occurs 8–16 hours after cessation of the use of diction, and it is also referred to as ‘psychological
heroin or morphine, with autonomic symptoms dependence' [86].
appearing first. By 36 hours, severe restlessness,
piloerection, lacrimation, abdominal cramps and 2.2.2. Criteria for opioid dependence/addiction
diarrhoea become apparent. Symptoms reach
their peak intensity at 48–72 hours and resolve
The key criteria indicating that an individual
over 7–10 days [54]. However, negative mood is addicted is when they no longer have control
states and craving may persist for up to 2 years over their drug use and demonstrate a persistent
after abstinence [37, 69]. Symptoms experienced change in reward-seeking behaviour, with an irre-
by the opioid-dependent patient depend on the sistible desire to repeat the drug experience or to
concentration of opioids in their body and their avoid the discontent of not having it. Such an in-
own individual levels of tolerance: the patient stinctive drive is contrary to the person's declared
will experience euphoria when the concentration intentions and underlies relapsing behaviour (re-
of opioids in the body exceeds the tolerance level cidivism). It is the key aspect of addiction, and
and will experience withdrawal symptoms when is also referred to as ‘psychological dependence'
the concentration of opioids in the body is below [86]. A joint statement by the World Health Or-
the dependence level. When the opioid concen- ganization (WHO), the United Nations Office on
tration is in between these two levels the opioid- Drugs and Crime (UNDOC) and the Joint United
dependent patient will look and feel normal (Fig- Nations Programme on HIV/AIDS (UNAIDS)
ure 2) [78]. Evidence of tolerance/withdrawal is defines the key elements of opioid dependence as
termed ‘physical dependence', although it is not a follows: a strong desire or sense of compulsion
constant or exclusive feature of addiction. Addic- to take opioids; difficulties in controlling opioid-
tion manifests with a persistent change in reward- taking behaviour; a withdrawal state when opioid
seeking behaviour, with an irresistible desire to use has ceased or been reduced; evidence of tol-
repeat the drug experience or to avoid the discon- erance, such that increased doses are required to
tent of not having it. Such an instinctual drive is achieve effects originally produced by lower dos-
contrary to the person's declared intentions and es; progressive neglect of alternative pleasures or
underlies recidivism. It is the key aspect of ad- interests; and persistence with opioid use despite
I. Maremmani et al.: Basics on Addiction: a training package for medical practitioners or psychiatrists who treat opioid dependence
clear evidence of overtly harmful consequences bile organisms [35]. Drugs of abuse mediate their
acute reinforcing effects by enhancing dopamine
activity in this neural network, which consists
2.2.3. Neurobiology of opioid- and drug-addiction
of dopamine projections from cell bodies in the
ventral tegmental area to limbic structures and
Advances in knowledge of the neurobiological cortical areas of the brain [35]. It has been pro-
processes that occur following acute and chronic posed that a network of four circuits within the
opioid administration have helped to improve sci- mesolimbic system are involved in drug abuse
entific understanding of how drug addiction de- and addiction: the nucleus accumbens and the
velops, including the role of the specific neuronal ventral pallidum, which are associated with re-
circuits in mediating the reinforcing effects of ward; the orbitofrontal cortex and the subcallosal
opioids and the development of uncontrolled use cortex, which are associated with motivation/
drive; the amygdala and the hippocampus, which
are associated with memory and learning; and the
2.2.3.1. The reward pathway
prefrontal cortex and the anterior cingulate gyrus,
which are associated with control [101]. These
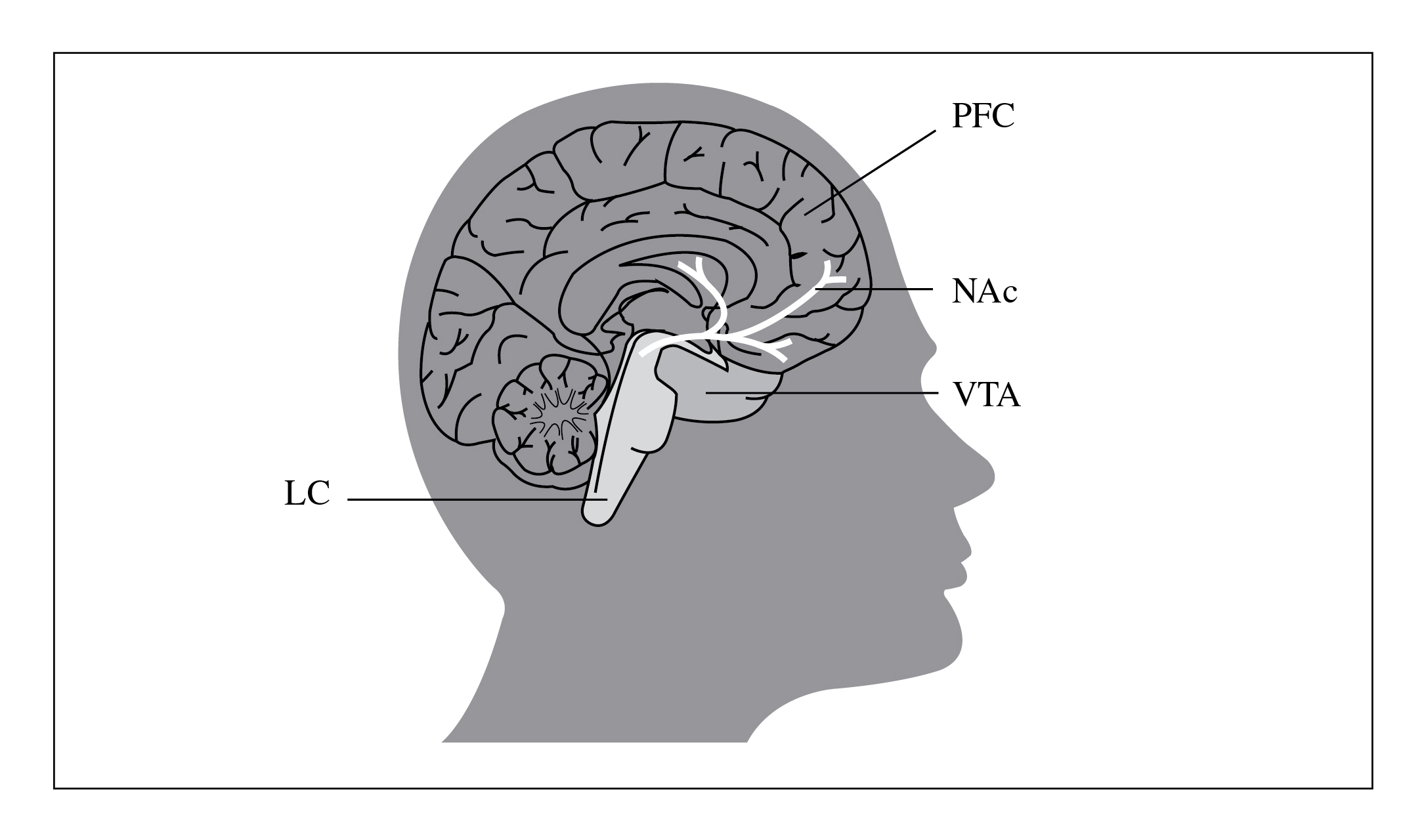
Increased dopamine activity in the mesocorti- four circuits receive direct innervations from
colimbic system (Figure 3) is intimately involved dopamine neurones but are also connected with
in eliciting and reinforcing responses to natural one another through direct or indirect projections
stimuli (e.g., food, drink and sex), which is impor- (mostly glutamatergic), confirming observations
tant to drive behaviour necessary for survival and from preclinical studies indicating that modifica-
reproduction [55]. From an evolutionary point of tions in glutamatergic projections mediate many
view, the capacity to seek rewards as goals is es- of the adaptations observed with addiction [101].
sential for the survival and reproduction of mo- As may be expected from such a complex system,
Figure 3: The mesolimbic reward system. Reproduced with permission from Kosten and George, 2002 [57]
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
other brain regions are thought to be involved in [100]. For excellent reviews of the neurobiology
these circuits (e.g., the thalamus and insula), one underpinning addiction, see Felkenstein, 2008
region may participate in more than one circuit and Volkow, 2003 [35, 101].
(e.g., the cingulate gyrus plays a role in both con-
trol and motivation/drive circuits) and other brain 2.2.4. Relapse
regions (e.g., the cerebellum) and circuits (e.g.,
attention and emotion circuits) are likely to be
A defining feature of drug dependence is the
affected in drug addiction [101]. In the case of incidence of relapse to drug-seeking and drug-
addiction to opioids it is predominantly the inter- taking behaviours following months or years of
action of opioids with mu receptors in the meso- abstinence [116]. It has been estimated that be-
corticolimbic system that appears to mediate the tween 40 and 60% of drug-addicted patients will
behavioural and reinforcing properties [35].
relapse within a year [72] even though they may
have achieved abstinence temporarily alone or
2.2.3.2. Uncontrolled use and craving
through detoxification or environmental interven-
tions. Such a pattern is common to most chronic
Tolerance may develop with repeated opioid relapsing disorders, such as diabetes or hyperten-
use to the extent that the user no longer experi- sion. The relapsing course illustrates the chronic
ences the euphoric effects once achieved with the nature of opioid addiction and the need for long-
drug, despite ingesting higher and higher doses of term approaches to treatment. An important focus
opioids [31]. Chronic opioid users will typically of addiction research has been to identify the be-
continue to exhibit a strong drive to engage in havioural, environmental and neural mechanisms
further drug-seeking and -using behaviours de- underlying drug relapse. Three types of trigger
spite developing tolerance to the euphoric effects have been identified to cause craving and relapse
of opioids. It has been postulated that repeated following extended periods of abstinence: a small
exposure to drugs of abuse disrupts the function ‘priming' dose of the drug; cues previously asso-
of the striato-thalamo-orbitofrontal circuit. This ciated with drug use (e.g., people, places, things,
dysfunction leads to a conditioned response when moods); and stress (e.g., stressful life events as
the addicted subject is exposed to the drug and/or well as anger, anxiety and depression) [114]. As
drug-related stimuli that activates the circuit and opioid-using individuals invariably relapse fol-
results in the intense drive to get the drug (con- lowing opioid withdrawal, detoxification alone
sciously perceived as craving) and uncontrolled does not constitute an adequate intervention for
self-administration of the drug (consciously per- substance dependence; maintenance treatment is
ceived as loss of control). This model of addic- a more effective option for opioid-addicted in-
tion postulates that the drug-induced perception dividuals to resume a normal life and achieve a
of pleasure is particularly important for the initial favourable outcome [78]. Detoxification is, how-
stage of drug self-administration but that with ever, a first step for many forms of shorter- or
chronic administration, pleasure alone cannot longer-term abstinence-based approaches, i.e.,
account for the compulsive drug intake. Rather, those in which no opioid agonist pharmacother-
dysfunction of the striato-thalamo-orbitofrontal apy is used. Both detoxification with subse-
circuit, which is known to be involved in pers- quent abstinence-oriented treatment and agonist
erverative behaviours, accounts for the compul- maintenance treatment are considered essential
sive intake [100]. During withdrawal and without components of an effective treatment system for
drug stimulation, the striato-thalamo-orbitofron- people with opioid dependence [113]. Overcom-
tal circuit becomes hypofunctional, resulting in ing opioid dependence is not easy: at the cellular
a decreased drive for goal-motivated behaviours level, the pathological changes that occur as a re-
I. Maremmani et al.: Basics on Addiction: a training package for medical practitioners or psychiatrists who treat opioid dependence
sult of drug use can persist even after drug use lapse and results from enduring cellular changes.
has ceased [45, 51] and the likelihood of relapse Changes in protein content and/or function often
actually increases during a period of abstinence become greater with increasing periods of with-
(a process called ‘incubation') as a result of the drawal, which is consistent with the possibility
neuroadaptations that occur in drug dependence that the more temporary changes in protein ex-
[40, 93]. Pharmacotherapies should ideally be ac- pression that mediate the transition to addiction
companied with motivation, social support, and may induce changes in protein expression that
positive coping strategies to fully achieve reha- convert vulnerability to relapse from a temporary
bilitative goals [61].
and reversible phase into permanent features of
addiction [52].
2.2.5. Stages of addiction
2.2.6. Risk factors for opioid dependence
The development of addiction may be consid-
ered to consist of three stages: (1) acute (immedi-
Dependence is not an inevitable consequence
ate) drug effects; (2) transition from recreational of opioid use, as demonstrated by their wide-
use to patterns of use consistent with addiction; spread use as a treatment for chronic pain [79]. It
and (3) end-stage addiction, which is character- has been proposed that addictive disease does not
ised by an overwhelming desire to obtain the drug, begin with the onset of substance use, but that an
a diminished ability to control drug seeking and individual's complex history of risk and protec-
reduced pleasure from biological rewards [52]. tive factors increase or decrease the likelihood of
These stages are associated with neurobiological their developing an addictive disorder when they
adaptations, including a switch from dopamine- use a substance for the first time [12]. A large
to glutamate-based behaviour as different parts number of risk and protective factors have been
of the neural circuitry play the key role [52]. identified, the most important of which is genet-
The first stage of addiction, acute drug effects, is ics, with some research suggesting that between
caused by supraphysiological levels of dopamine 40% and 60% of the vulnerability to addictive
being released throughout the motive circuit disease is accounted for by genetic factors [60].
which induces changes in cell signalling. These However, exposure to certain substances can be
changes lead to short-term neuroplastic changes, sufficient to induce dependence in the absence
persisting for a few hours or days after drug in- of risk factors. Associations have been found
take, which initiate cellular events involved in the between substance abuse and polymorphisms in
process of addiction. The second stage of addic- genes encoding opioid (OPRM1 and OPRK1),
tion, the transition from recreational drug use to serotonin (5-hydroxytryptamine-1B [HTR1B]
addiction, is associated with changes in neuronal and melanocortin (MC2R) receptors, endogenous
function that accumulate with repeated drug use opioids (prodynorphin [PDYN]) and neurotrans-
and diminish with drug discontinuation over days mitter enzymes (catechol-O-methyltransferase
or weeks. There are also alterations in the con- [COMT] and tryptophan hydroxylase [TPH])
tent and function of various proteins that are in- [115]. Other factors known to play a role in the
volved in dopamine transmission (e.g., tyrosine development of addictive disorders include an
hydroxylase, dopamine transporters, RGS9-2 and individual's temperament, psychopathology, at-
D2 autoreceptors) that persist for a few days af- titudes and perceptions. Society, including fam-
ter drug discontinuation. However, these changes ily, peer group, school and community, also have
appear to be compensatory and may not directly important implications for the development of
mediate the transition to addiction. End-stage addictive disease [12]. Prevention strategies have
addiction is characterised by vulnerability to re- been demonstrated to play an important part in
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
reducing the risk of opioid dependence among dependence in the same way, treatment success
vulnerable groups [76].
may be defined as a decrease in drug use with
only occasional relapses or abstinence from drug
2.2.7. Opioid dependence as a chronic, relapsing brain use with only occasional relapses rather than total
abstinence. Total abstinence develops gradually,
is rarely achieved soon after initiating treatment,
Individuals who are drug dependent have and depends on ongoing treatment rather than
historically been considered to be ‘bad', ‘weak' being self-maintaining in the absence of chronic
people who are unable to control their behav- treatment.
iour and do not deserve treatment. Among the
Optimal management of opioid dependence
scientific community, however, advances in our requires a multi-faceted approach in order to ad-
understanding of the neurobiology of addiction, dress the neurobiological, social, behavioural and
the pharmacology of opioids and their receptors, psychological aspects of the condition [62]. The
and the discovery that some individuals may be pharmacotherapy of opioid dependence will be
particularly susceptible to drug dependence have discussed in more detail in Part 3 of this supple-
led to greater appreciation of the condition being ment.
a chronic, relapsing brain illness. In addition, the
substantial changes in brain structure and func- 2.3. Conclusion
tion observed in drug dependence that persist
after individuals have stopped drug use provide
An understanding of the mechanisms respon-
further evidence that the condition should be con- sible for opioid addiction is critical for optimal
sidered a medical condition rather than a moral treatment of this chronic brain condition. Im-
weakness. Viewing drug dependence as a chronic provements in our understanding of the cellular
illness akin to diabetes or chronic hypertension processes responsible for opioid dependence,
changes the way in which treatment success is addiction and relapse have helped to inform the
recognised. In the case of diabetes, for example, now widespread view that opioid dependence
complete cure is not currently a feasible outcome is a chronic disease requiring medical treatment
and a decrease in blood glucose would therefore rather than a purely moral or social problem that
be indicative of treatment success. Considering can be ‘cured' by criminal-justice solutions. Ulti-
Key learning points
Opioids are drugs that share some of the pharmacological effects of opium
Opioid receptors are widely distributed in the nervous system
Mu-receptor activation produces direct opioid effects, including euphoria
Opioids promote the release of dopamine in the reward pathway (ventral tegmental area, nu-
cleus accumbens, prefrontal cortex)
Opioids are classified as agonists (complete, partial) or antagonists according to their intrinsic
activity at different receptors
Neuroadaptations that occur in response to chronic opioid lead to:
Tolerance: reduced effect of drug for a given dose
Withdrawal: emergence of withdrawal syndrome upon abstinence or reduced drug levels
Cravings and vulnerability to relapse
Opioid dependence is a chronic, relapsing brain disorder
Relapse is a symptom of the disorder and not a sign of abstinence failure
I. Maremmani et al.: Basics on Addiction: a training package for medical practitioners or psychiatrists who treat opioid dependence
mately, increased understanding of the neurobiol- heterogeneity of the opioid-dependent popula-
ogy of addiction should help to optimise the way tion makes treatment standardisation implausi-
we manage drug-dependent individuals with the ble [108]. A comprehensive, long-term treatment
treatment options we currently have at our dis- plan should be developed based on a multi-facto-
posal and also inform the development of new rial assessment and the best available clinical evi-
dence. All decisions should be made in concert
with principles of medical ethics and considera-
3. Clinical assessment of opioid
tion of patient preferences [111].
3.1. Key components of patient assessment in
Effective management of opioid dependence
includes a comprehensive patient assessment.
The goals of the assessment are to confirm a
A detailed patient assessment should consider
diagnosis of opioid dependence, determine the specific physical, psychological and social fac-
appropriate course of therapy and identify any tors, in addition to past and current drug use, in
co-existing physical or psychosocial conditions order to assess the patient's condition and treat-
that may affect treatment outcomes [108, 111]. ment options (Table 5). Psychological assess-
As the number of options to treat opioid addic- ment of patients is critical as psychosocial fac-
tion increases across a range of clinical settings, tors, including co-existing psychiatric disorders
it becomes possible and desirable to tailor ther- and cognitive impairment, patient readiness and
apy to individual needs [111]. Furthermore, the motivation for treatment, contribute to non-com-
Table 5: Key features of patient assessment [108, 111]
Demographics and family history
Psychiatric history
Past and current drug use
Past treatment experience
Clinical examination
Assessment of intoxication/withdrawal
Presence of opportunistic infection(s)
Presence of co-morbidities
Lab investigation(s)
Urine and plasma drug screen, LFTs, HIV, hepatitis B
Co-existing conditions
Infectious diseases
HIV, hepatitis C and B, sexually-transmitted diseases,
Other substance abuse
Alcohol, benzodiazepines, stimulants, barbiturates,
cocaine, marijuana, hallucinogens
Psychiatric disturbance
Depression, anxiety, personality disorders, cognitive
Living conditions
Extent of integration into drug community,
Legal/criminal issues
Past/present involvement with legal system and
Occupational situation
Current and past employment
Social/cultural factors
Language barriers, education level, religion
Support for treatment and avoidance
Patient motivation
Short-term and long-term goals, and reason for
seeking treatment
LFT: liver function tests; TB: tuberculosis; CBC: complete blood count; HIV: human immunodeficiency virus
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
pliance and treatment failure [108].
it does not distinguish addictive use from thera-
peutic dependence on prescribed drugs. We use
3.2. Diagnosis of opioid dependence
the term dependence here to mean addiction (i.e.,
a persistent change in reward-seeking behaviour,
As of 1964, the World Health Organization with an irresistible desire to repeat the drug ex-
has recommended the term ‘substance addiction' perience or avoid the discontent of not having it,
be replaced by the term ‘substance dependence' which is contrary to the person's declared inten-
[111] (www.who.int). The term ‘substance de- tions). Differentiating between opioid use, abuse
pendence' is somewhat ambiguous, however, as and dependence is critical to establishing the
Table 6: Definitions of substance abuse and dependence [2,109]
Substance abuse (DSM-IV-TR)(2)/Harmful use (ICD-10) (109)
A maladaptive pattern of substance use leading to clinically A pattern of psychoactive substance use that is causing
significant impairment or distress, as manifested by one (or damage to physical or mental health; adverse social
more) of the following, occurring at any time in the same
consequences are also common, but not sufficient to
12-month period:
establish a diagnosis of harmful use
Recurrent substance use resulting in a failure to fulfil major
role obligations at work, school, or home
Recurrent substance use in situations in which it is
physically hazardous
Recurrent substance-related legal problems
Continued substance use despite persistent or recurrent
social or interpersonal problems caused or exacerbated by
the effects of the substance
In addition, the individual must never have met the criteria
for substance dependence for the substance in question
A maladaptive pattern of substance use leading to clinically A cluster of physiological, behavioural, and cognitive
significant impairment or distress. Three (or more) of the
phenomena in which the use of a substance or a class of
following, occurring at any time in the same 12-month
substances takes on a much higher priority for a given
individual than other behaviours that once had greater
value. Three or more of the following have been present
together at some time during the previous year:
Taking the substance in larger amounts or over a longer
Strong desire or compulsion to take the substance
period than was intended
Difficulty controlling substance use (onset, termination,
Persistent desire or unsuccessful efforts to cut down or
or levels of use)
control substance use
A physiological withdrawal state when substance use is
Spending a great deal of time in activities necessary to
stopped or reduced
obtain, use, or recover from the substance
Evidence of tolerance (increased doses are required in
Giving up or reducing important social, occupational, or
order to achieve the effects originally produced by lower
recreational activities because of substance use
Continued use despite knowledge of having a persistent or Progressive neglect of alternative pleasures or interests
recurrent physical or psychological problem likely to have because of time spent to obtain, use, or recover from the
been caused or exacerbated by the substance
Persisting with substance use despite clear evidence of
overtly harmful consequences
DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision;
ICD, International Classification of Diseases
I. Maremmani et al.: Basics on Addiction: a training package for medical practitioners or psychiatrists who treat opioid dependence
most effective course of treatment, if any. A diag- often a temporary stage of opioid usage; depend-
nosis of any opioid disorder is made using criteria ence develops rapidly as a result of the powerful
similar to other substance abuse disorders [108]. reinforcing qualities of the opioid and the emer-
The Diagnostic and Statistical Manual of Mental gence of tolerance [28, 108].
Disorders, Fourth Edition, Text Revision (DSM-
Notably, the DSM-IV-TR requires criteria for
IV-TR) [2] describes two distinct categories for dependence to be fulfilled within a 12-month pe-
substance-use disorders: abuse and dependence riod, although possibly on different occasions.
In other words, a diagnosis can be based on a
The most important feature that differentiates relatively recent period of physical dependence
substance abuse from dependence is a loss of (e.g., in the past month) evidenced by signs of
control (e.g., persistent or strong desire to take withdrawal and tolerance, as long as features of
the substance, unsuccessful efforts to cut down previous escalating substance use or abuse have
or control substance use, continued usage despite occurred in the same 12-month period. In addi-
knowledge of harmful consequence and neglect tion, even if the pattern of use is not currently
of other activities). It should be noted that toler- problematic, the recurrence of problems within
ance and withdrawal are included in the potential the same 12-month period is (from a diagnostic
criteria for substance dependence in both DSM- perspective) considered equivalent to a constant
IV-TR and ICD definitions but neither tolerance problematic pattern of use. Conceptually, a diag-
nor withdrawal are required to establish the diag- nosis of substance dependence can also be made
nosis of abuse or dependence [2, 109]. Converse- for a past period, although the patient may be un-
ly, the sole presence of tolerance and withdrawal dergoing a remission phase. Therefore, a progno-
in the absence of other criteria, may indicate sis of long-lasting remission in the presence of a
what might be termed a ‘normal', medical sta- retrospective diagnosis of drug addiction is unre-
tus corresponding to habitual, controlled use of alistic.
a tolerance-inducing substance (e.g., nicotine or
alcohol) or therapeutic dependence on a toler- 3.3. Assessing opioid intoxication and withdrawal
ance-inducing prescribed drug (e.g., methadone
or buprenorphine). The DSM-IV-TR requires
The documentation of the signs of opioid in-
the clinician to specify whether the substance toxication or withdrawal is part of establishing a
dependence is with or without physiological de- diagnosis of opioid dependence (Table 7). The de-
pendence (manifested by evidence of tolerance or gree of opioid intoxication or withdrawal should
withdrawal) [2].
be evaluated with the reported time of last use.
A diagnosis of abuse is subordinate to that of
Clinical assessment is complicated by the
dependence: in other words, all dependent pa- fact that opioid users commonly abuse several
tients are also abusers, whereas abusers can be substances including alcohol, benzodiazepines,
assessed as such after ruling out a diagnosis of stimulants, marijuana, cocaine and nicotine,
dependence. Furthermore, patients who do not which may result in additional symptoms such
meet the criteria for abuse may fall into a cate- as tremors, delirium or seizures [1]. Care must
gory of non-pathologic use, comprising irregular be taken to make a differential diagnosis against
or habitual use, with possible features of toler- other conditions that may share similar symp-
ance and dependence. Typically, dependence is toms [108], such as panic attack, gastroenteritis,
the culmination of a pattern of abuse which starts peptic ulcer and pancreatitis.
with occasional, social or recreational drug use or
Injection sites are valuable indicators when
as part of a legitimate medical regimen, such as determining the chronology of drug use [111].
with the treatment of pain [1]. Abuse is, however, The most common sites for injection include
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
Table 7: Signs of opioid intoxication and withdrawal [108, 111]
Signs of opioid intoxication
Signs of opioid withdrawal
Drooping eyelids
Constricted pupils
Muscle aches and abdominal cramps
Reduced respiratory rate
Itching and scratching
Difficulty sleeping
Dry mouth and nose
Vomiting and diarrhoea
Agitation and restlessness
Elevated respiratory rate, blood pressure and pulse
the cubital fossa (area on the inside of the elbow some form of immunoassay, are generally recom-
joint) and the groin although superficial veins in mended before making treatment decisions. Gas–
the extremities and neck are also used [28, 111]. liquid chromatography (GLC) and gas chroma-
Recent injection marks are usually small and red tography–mass spectrometry (GC–MS) are very
and are sometimes inflamed or surrounded by sensitive and specific tests, but are labour inten-
slight bruising. Older injection sites are usually sive and expensive and are thus often reserved
not inflamed, but may show pigmentation chang- for confirmation of other forms of testing, such as
es (either lighter or darker) and the skin may have urinalysis [98, 108].
atrophied. A combination of recent and old injec-
Urinalysis is an inexpensive, although not
tion sites would normally be seen in an opioid- sensitive, form of screening for opioids and other
dependent patient with current neuroadaptation. substances of abuse. Interpretation of urinalysis
The visible injection sites should be consistent results requires knowledge of the specific test or
with the reported history [111].
reagents used as well as the pharmacokinetics
of the substance or substances being tested [98,
3.4. Assessment of co-existing conditions
111]. Heroin is metabolised to 6-monoacetylmor-
phine (6-MAM), then to morphine and eventually
Physical and biological assessment of the to codeine. Therefore, the presence of 6-MAM is
patient not only confirms dependence, but also usually specific for recent heroin use. Morphine,
provides important information on their overall with or without small amounts of codeine, can
health, fitness and willingness to undertake treat- indicate either heroin or morphine use in the last
ment. A trusting relationship between clinician few days. However, small amounts of morphine
and patient is valuable to establish the free flow in the presence of large amounts of codeine can
of information. A non-judgemental and affirm- suggest intake of high doses of codeine, as co-
ing approach can help to alleviate the sense of deine is also metabolised to morphine [111].
shame and diminished self-esteem many patients
A positive urine test for opioids must be
feel that often leads to the withholding of critical judged cautiously. Although patients are usually
information [38, 111]
required to test positive for opioids in order to
Although important, self-reporting by pa- be offered treatment, the presence of opioids in-
tients often results in questionable validity and dicates recent use, but not necessarily abuse or
reliability [111]. As a result, drug screens, using dependence [111]. On the other hand, the absence
I. Maremmani et al.: Basics on Addiction: a training package for medical practitioners or psychiatrists who treat opioid dependence
of opioid does not exclude either abuse or addic- 3.5. Psychiatric co-morbidities
tion, but merely indicates that the individual has
not used opioids in the previous week. Converse-
In addition to physiological symptoms, as-
ly, positive findings are possible after ingestion of sessment of a patient's behaviour, psychology
large amounts of poppy seeds [111] or for people and cognitive functioning is important in the di-
exposed to prescribed opioids. Urinalysis results agnosis of opioid dependence. Psychological as-
should therefore always be used in the context of sessment includes determining the presence of
a more comprehensive patient assessment to con- co-existing psychological conditions, cognitive
firm a diagnosis of opioid dependence.
impairment, and consideration of the patient's
Further serum testing can detect the presence motivation to treatment and short- and long-term
of other substances of abuse (e.g., alcohol), HIV, goals.
hepatitis C and other common infectious diseases.
Several large-scale epidemiologic studies in-
Voluntary testing for HIV and hepatitis C should dicate approximately 50% of patients with drug
be offered as part of an individual assessment, or alcohol dependency also have psychiatric
with counselling offered before and after the test. distress [82]. Mood and anxiety disorders are
In particular, HIV testing should be routinely of- common in the opioid-dependent population, in
fered to patients in areas with high HIV incidence addition to antisocial behaviour and other per-
rates, particularly if they fall into multiple risk sonality disorders, all of which affect treatment
Table 8. Examples of standardised questionnaires for patient assessment [6,48]
Severity of Opioid Dependence Questionnaire (SODQ) Physical aspects of opioid dependence
Severity of Alcohol Dependence Questionnaire
Physical aspects of alcohol dependence
The Symptom Check List (SCL-90) and General Health Global assessment of mental health
Questionnaire (GHQ)
The Psychiatric Research Interview for Substance and Substance-induced major depression
Mental Disorders (PRISM)
categories. Research suggests opioid-dependent choices and outcomes [33]. It has been estimated
HIV patients have decreased access to quality that up to 16% of opioid dependents suffer from
HIV care and medication, and are more likely to major depression, which is more commonly as-
be non-compliant with treatment [111]. Serology sociated with poly-drug use. Chronic, episodic
testing and vaccination for hepatitis B is recom- low-grade depression or dysthymia can progress
mended for all patients. To offset the risk of pa- to full-blown depression as a result of the stress
tients neglecting to return for repeated treatments and trauma associated with opioid dependence
to complete a hepatitis B vaccination program, [28, 82]. Acute mood disturbances (depressed
vaccination could commence before serology mood, anxiety) are also apparent during opioid
testing, and accelerated vaccination schedules withdrawal [46]. Consequently, when assessing
should be considered [111]. As part of a complete patients, it is important for clinicians to establish
assessment, screening for tuberculosis and sexu- any pre-existing psychological conditions and
ally transmitted diseases should also be consid- recognise that the short- and long-term effects of
ered [38, 111]. A pregnancy test for women with opioids, their withdrawal symptoms and the trau-
reproductive potential should be offered, as early ma of addiction, can all produce symptoms that
as possible in the course of treatment [108, 111]. are similar to those that characterise many mental
disorders [82]. Nevertheless, clinicians should be
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
aware of unusual opioid-related symptoms, such the results should be interpreted in combination
as psychosis or mania, which may require acute with a complete clinical assessment.
The tools for gathering social and cultural in-
The presence of psychiatric conditions also has formation are not as well developed or widely
important implications for treatment choices and available as for physical assessment. Although
medication management. Pharmaceutical agents there is a lack of assessment tools, available re-
such as methadone and buprenorphine have been search suggests that social assessment, e.g., pa-
shown to have a beneficial effect on mental disor- tients' living conditions, occupational situation
ders as well as addiction [82].
and legal issues, needs to be an on-going process,
beyond the scope of a single interview [108].
3.6. Patient assessment tools
Several instruments and questionnaires have
been developed to assist in the patient assessment
A diagnosis of opioid dependence is contin-
process when substance abuse is suspected (Ta- gent on an individualised, comprehensive patient
ble 8). Standard questionnaires can be a useful assessment, which considers the particular risks
adjunct to the assessment process, provided they of this patient population. When considered col-
are delivered in the context of a relaxed patient lectively, the information gained from a complete
interview [48]. The use of structured and semi- physical and psychosocial examination and his-
structured interviews and standardised assess- tory will help to differentiate between substance
ment tools has also improved the reliability of co- use, abuse or dependence, and identify the best
morbid psychiatric diagnoses [82]. In every case, course of treatment.
Key learning points
A comprehensive and individualised patient assessment is critical for the diagnosis of
opioid dependence
The key components for a comprehensive patient assessment include:
Physical/biological evaluation and patient history – drug use, abuse and dependence,
Co-existing somatic and psychiatric conditions
Psychological/social functioning
The potential for tolerance and withdrawal is common to non-pathologic (controlled) use,
abuse and dependence, but is not required to diagnose drug dependence
Documentation of opioid intoxication or withdrawal is important in diagnosis, and should
be made in the context of reported time of last drug usage
Examination of new and old injection sites aids the determination of drug-use chronology
Serum and urine testing is recommended to detect opioids and substances of abuse, as well
as co-existing infectious diseases and conditions
A thorough psychiatric assessment is recommended to detect mental symptoms and to
identify psychiatric co-morbidities, which affect a substantial proportion of the opioid-de-
pendent population
Numerous standardised assessment tools and questionnaires are available to assess de-
pendence, physical and mental health
I. Maremmani et al.: Basics on Addiction: a training package for medical practitioners or psychiatrists who treat opioid dependence
In addition to confirming the presence or ab- practices [111]. Harms to society associated with
sence of opioids and other substances of abuse, opioid dependence include criminal activity and
clinicians should ensure the necessary serum and the economic burden associated with healthcare
urine testing is undertaken to detect co-existing costs (treatment and prevention services, costs
conditions that may affect treatment. Importantly, incurred due to additional health problems), so-
the patient's psychological health must be con- cial welfare and criminal-justice services [111].
sidered, given the high incidence of psychiatric The objectives of treatment for opioid-dependent
co-morbidity and the implications for treatment patients are, therefore, to: reduce dependence on
choice and outcome. A number of standardised abused drugs; reduce the morbidity and mortality
patient assessment tools may aid in the assess- caused by the use of opioids of abuse, or associat-
ment and diagnostic process.
ed with their use, such as infectious diseases; im-
As with other chronic conditions, treatment prove physical and psychological health; reduce
should be structured in such a way as to provide criminal behaviour; facilitate reintegration into
long-term support to patients. Assessment of the the workforce and education system and improve
patient's response to therapy should be undertak- social functioning [113]. Achieving these objec-
en on a regular basis, with a continued focus on tives has clear medical, economic and social ben-
outcome-oriented and individualised treatment.
efits [113]. Accordingly, the World Health Organ-
ization (WHO) has included the opioid agonists
4. Maintenance pharmacotherapies:
methadone and buprenorphine on their model list
treatment principles and clinical
of essential medicines as a result of their strong
evidence base [112]. Essential medicines are de-
fined as those that satisfy the priority healthcare
This section outlines the main principles, needs of the population and they are selected with
goals and strategies underlying medically as- due regard to public-health relevance, evidence
sisted approaches to opioid-dependence treat- on efficacy and safety and comparative cost-ef-
ment, the unique pharmacological profiles of the fectiveness [112]. Access to essential medicines
therapies available to treat opioid dependence, is considered a fulfilment of the human right to
and the safety and efficacy considerations that health according to international law [42].
are relevant to the use of these pharmacological
interventions throughout the different stages of 4.1.2. Elements of drug-dependence treatment
Treatment of opioid dependence must address
4.1. Principles, goals and strategies for treating the multiple needs of the patient. Pharmacologi-
cal treatments (which are discussed in detail here)
are the critical component of the treatment proc-
4.1.1. Overal aims of drug-dependence treatment
ess but behavioural interventions and/or coun-
selling therapies to address underlying mental
Opioid dependence is a chronic and relaps- disorders and impaired psychosocial functioning
ing medical disorder [62] with consequences also play a key role [77]. Comprehensive pro-
that primarily affect the individual but also have grammes, involving access to psychosocial and
broader effects. Harms to the individual include counselling services and referral to vocational,
an increased risk of mortality as a result of over- financial, housing and family assistance, can help
doses, violence, suicide and smoking- and alco- address the broader aspects of addiction. Indeed,
hol-related diseases; and an increased risk of HIV combining pharmacological treatments with
and hepatitis C infection through unsafe injection counselling aimed at promoting treatment adher-
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
ence and lifestyle change can greatly enhance the is defined as the administration of thoroughly
effectiveness of treatment [92]. Pharmacological evaluated opioid agonists, by accredited profes-
maintenance treatment also helps to initiate and sionals, in the framework of recognised medical
retain contact between patients and substance- practice, to people with opioid dependence, for
abuse specialists, thus enabling these other inter- achieving defined treatment aims [110]. The pri-
ventions to be delivered.
mary aims of maintenance pharmacotherapy are
Due to the complexity of drug dependence, to reduce drug craving and illicit opioid use, and
one treatment approach is not appropriate for eve- where necessary, to prevent withdrawal symp-
ry patient. Pharmacological interventions such toms. By reducing the drive to engage in contin-
as opioid agonist treatments should be initiated ual addictive-drug-seeking and -using behaviour,
according to evidence-based quality standards maintenance treatment can provide an opportu-
to ensure safety and efficacy. Over time, the ap- nity to address the broader ramifications of each
propriate dose and other aspects of treatment can individual's dependence-related problems (e.g.,
be individualised to the patient's needs, without impaired psychosocial functioning and physical
losing the critical factors of success. Treatment health), reduce associated risks (e.g., overdose
plans must be continually assessed and modified mortality, infectious-disease transmission), and
to ensure they meet the patient's changing needs minimise the socio-economic burden imposed on
wider society (e.g., criminality, lost productivity,
healthcare costs of untreated opioid dependence).
4.1.3. Overview of treatment pathways
The medications most frequently used as mainte-
nance therapies are the opioid agonists methadone
The primary pharmacological approach to (typically administered as an oral syrup) and bu-
treating heroin dependence involves opioid ago- prenorphine (administered as a sublingual tablet).
nist maintenance treatment – also known as medi- Buprenorphine is available in two formulations: a
cally assisted treatment, and less appropriately as monotherapy and a buprenorphine–naloxone (4:1
opioid replacement therapy or opioid substitution ratio) combination product designed to reduce the
therapy. Opioid agonist maintenance treatment potential for misuse and diversion. Additional op-
drug to an opioid
agonist for long-
Medically supervised withdrawal
Slow reduction of agonist dose until the
The patient remains on a consistent
patient is completely drug free; typically in
agonist dose level that allows him/her
association with psychosocial support
to function properly
Figure 4. Phases of heroin dependence treatment
I. Maremmani et al.: Basics on Addiction: a training package for medical practitioners or psychiatrists who treat opioid dependence
tions that are used less frequently and have been tion alone cannot be regarded as a viable treat-
less thoroughly evaluated include slow-release ment approach for drug dependence. Rather than
oral morphine and injectable therapies including a first step into long-term treatment, it has been
injectable methadone and diamorphine. The main likened to a ‘revolving door'; many individuals
phases of maintenance treatment are summarised who begin detoxification do not complete it and
in Figure 4. Following induction and stabilisa- many individuals who complete detoxification do
tion, patients typically need to be maintained on not go on to more definitive treatment [70]. Re-
opioid agonist therapy for at least 12 months in sults from a placebo-controlled, randomised trial
order to achieve enduring positive treatment out- of buprenorphine maintenance versus a tapered
comes [41]. Opioid maintenance treatment is as- 6-day regimen of buprenorphine subsequently
sociated with a substantial reduction in the use followed by placebo (individuals in both arms re-
of heroin and other illicit opioids, crime and the ceived cognitive behavioural therapy to prevent
risk of death through overdose. A WHO posi- relapse plus weekly counselling), demonstrated
tion paper on maintenance treatment states it to that buprenorphine maintenance was far superior
be an effective, safe and cost-effective modality to detoxification (1-year retention rates of 75%
for the management of opioid dependence [113]. vs 0% and negative urine screens for illicit opi-
Compared to detoxification or no treatment, both ates, central stimulants, cannabinoids and benzo-
methadone and buprenorphine significantly re- diazepines in 75% of patients remaining in treat-
duce drug use and improve treatment retention ment) [50].
Although maintenance treatment is consid- 4.2. Maintenance treatment of opioid dependence
ered the gold-standard therapeutic strategy (and
is the focus of this article), a popular approach is
There are multiple determinants of the ef-
that of assisting opioid-dependent individuals to fectiveness of maintenance treatment for opioid
medically withdraw from opioids, a process also dependence, including characteristics of the pa-
referred to as opioid detoxification (Figure 4). tient, the medications used and the way treat-
Both methadone and buprenorphine can be used ment is implemented. The primary focus of this
in reducing doses to assist in achieving medical educational supplement will be to highlight the
withdrawal from opioids. Gradual dose reduc- basic pharmacological considerations that are rel-
tions help to minimise the likelihood of signifi- evant in selecting an appropriate medication and
cant withdrawal and allow time for neuronal re- implementing this option to achieve the goals of
adaptation. Alpha-2 adrenergic agonists such as therapy. According to the WHO, the following
clonidine can also be used to reduce the severity attributes are essential for treatments to be used
of opioid withdrawal symptoms. In non-tolerant as maintenance therapy in opioid-dependent pa-
patients, the long-acting opioid antagonist nal- tients [113]:
trexone can be used to prevent relapse to opioids
Opioid properties in order to prevent with-
[111]. Both naltrexone and its active metabolite drawal symptoms and reduce craving
6-β-naltrexol are competitive antagonists at the
Affinity for opioid receptors in the brain in or-
mu and kappa opioid receptors, reversibly block- der to diminish or block the effects of heroin or
ing or attenuating the effects of opioids [91]. As other opioids
a result, a person maintained on naltrexone will
Longer duration of action than abused opioid
not experience any of the sought-after positive drugs to delay the emergence of withdrawal and
effects of heroin. Naltrexone maintenance may reduce the frequency of administration
be effective for selected, mildly ill and highly
Oral administration to reduce the risk of infec-
motivated individuals [90]. However, detoxifica- tions associated with injections
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
The following sections present an overview of 4.2.1.2. Treatment – induction
the basic pharmacological and clinical considera-
tions applicable to the use of the three main main-
Induction describes the initial stage of treat-
tenance pharmacotherapy options: methadone, ment when an individual dependent on street
buprenorphine and buprenorphine–naloxone. heroin or other non-prescribed opioids is initiated
The local manufacturer's prescribing information on maintenance treatment. The primary objec-
should be consulted for comprehensive infor- tives of the induction stage are to ensure safety
mation on dosage, administration, precautions, and to retain patients in treatment by preventing
warnings and contraindications.
or reducing the signs and symptoms of opioid
withdrawal, or preventing relapse in non-tolerant
4.2.1. Methadone treatment
individuals or treatment re-starters in the early
phase of use. It is important to carefully explain
intoxicating effects and withdrawal symptoms to
patients, observe them frequently and ensure safe
Methadone was the first widely used opioid- dosing in seeking to achieve these aims. Once in-
maintenance therapy for the treatment of patients ducted safely, the goal is to achieve an optimal
with opioid dependency [29] and its use assisted dose for longer-term maintenance that prevents
a shift in treatment targets for opioid dependency cravings and addictive opioid use.
from total abstinence to long-term maintenance
A comprehensive assessment of patient drug
[106]. Methadone is a synthetic, lipid-soluble, use, medical, psychological and social conditions,
opioid agonist, which acts with similar affinity previous treatment history and current treatment
to heroin at the mu-receptor [47]. Usually ad- goals should be conducted and documented prior
ministered orally, methadone is readily absorbed to initiating therapy. Corroborative evidence of
via the gastrointestinal tract resulting in a high opioid dependence – observed signs of opioid
but variable bioavailability of 40–100% depend- withdrawal or a history of previous treatment
ing on the individual patient [74]. The onset of for dependence – should be established before
therapeutic benefit with methadone is within 30 initiating treatment. Responses to previous treat-
minutes after ingestion, with an average time to ments can also guide treatment decisions, form-
peak of 2.5 hours [41, 67]. Plasma-methadone ing the basis of the initial treatment plan. Such
concentrations continue to rise for 3–4 hours fol- assessments can also be used to monitor progress
lowing oral ingestion and then decline gradually. during treatment [41, 67].
With ongoing dosing, the half-life of methadone
is extended to 13–47 hours, with a mean of 24 4.2.1.2.1. Treatment-naïve patients
Due to its combination of mu-opioid-receptor-
New patients should be dosed with caution
agonist properties, high oral bioavailability and when using methadone in order to safely uptitrate
a prolonged half-life, once-daily oral methadone to a satisfactory dose and achieve steady-state
provides effective long-lasting suppression of plasma concentrations. This approach is neces-
opioid withdrawal symptoms and cravings for sary to mitigate the risks of methadone accumula-
many patients. In addition, as a result of the phe- tion across dosing intervals (due to its prolonged
nomenon of cross tolerance, tolerance to other half-life) and consequent toxicity (including res-
opioids is produced. This means that diminished piratory depression and sedation). The first dose
intensity of opioid effects will be observed, which should be determined for each patient based on
contributes to the reduction in heroin abuse dur- the severity of dependence and level of tolerance
ing methadone maintenance [58].
to opioids, and, if possible, patients should be ob-
I. Maremmani et al.: Basics on Addiction: a training package for medical practitioners or psychiatrists who treat opioid dependence
served for 3–4 hours after the first dose. The first ment. Patients transferring from buprenorphine
2 weeks of treatment are the greatest risk period treatment should be stabilised on 16mg/day or
for methadone toxicity and overdose. During this less for several days prior to transfer. Metha-
time, patients should be observed daily prior to done can be commenced 24 hours after the last
dosing and assessed for signs of intoxication or buprenorphine dose, and the initial methadone
withdrawal. Deaths in the first 2 weeks have been dose should not exceed 40mg. Patients transfer-
associated with methadone doses in the range of ring from naltrexone should be treated as opioid-
25–100mg/day, with most occurring at doses of naïve, as tolerance to opioids is lost after a few
40–60mg/day. Whilst therapeutic maintenance days of naltrexone treatment. Methadone should
doses are typically in the range of 60mg/day or not be administered for at least 72 hours after the
more, the risk of toxicity during methadone in- last naltrexone dose, and the commencing dose
duction requires the use of lower starting doses. should be no more than 20mg [41, 105].
An initial methadone dose of ≤20mg for a 70kg
patient can be presumed safe, even in opioid- 4.2.1.3. Treatment – maintenance
naïve users; this dose will alleviate withdrawal
symptoms in most patients. Caution should be
Typically, effective methadone maintenance
exercised with doses of 30mg or more, and ex- doses are 80–120mg/day. Maintenance doses
treme caution and specialist involvement are higher than 120mg/day may be necessary in some
advisable for doses of 40mg or more [41, 105]. patients, such as those who have a fast metha-
Dose increments of 5–10mg can be considered done metabolism or dual-diagnosis patients,
every 5–7 days as required, with overall weekly while a minority of patients can be maintained
increases no larger than 40mg [41] until a stable effectively on doses less than 60mg/day [15, 32,
maintenance dose is achieved. For individuals 41]. Methadone maintenance doses should be de-
starting treatment who are presumed to have no termined on an individual basis. Patient input to
tolerance, or irregular use at time of treatment treatment decisions, including determination of
initiation, dosages should be low, dose increases dose levels, helps promote a good therapeutic re-
should take not place more often than weekly (at lationship. The optimal maintenance dose should
least until blocking dosages are reached), and reduce opioid cravings and use without produc-
overall increases in daily dose should not be more ing euphoria. Daily administration of methadone
than 10mg. This may be the case for: a) patients is required in order to maintain adequate plasma
who have discontinued treatment recently, and levels and avoid opioid withdrawal. Monitoring
have not yet relapsed into regular drug use; b) pa- drug use can also help assess treatment progress
tients who have just been returned to their natural and may be useful for clinical decision making
environment, with free availability of the opiate [41, 105].
of abuse, without having been started on any ag-
Patients who miss their daily methadone dose
onist treatment while in a protected environment; may be engaging in other drug use or are at risk
c) patients who are not currently tolerant to opi- for leaving treatment. Tolerance to opioids may
ates, but are willing to start some effective treat- be reduced after more than 3 days of missed
ment, or who ask for advice about how to prevent methadone, placing patients at risk of overdose
relapses (diagnostic criteria should be satisfied).
when methadone is reintroduced. If missed for
more than 3 days, methadone should be reintro-
4.2.1.2.2. Patients transferring from other pharmacotherapies duced at half dose, while for more than 5 days
of missed treatment, reintroduction of methadone
When another pharmacotherapy has failed, should be regarded as a new induction [41, 105].
patients may be transferred to methadone treat-
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
4.2.1.4. Cessation of methadone treatment
safety consideration for methadone given its doc-
umented association with QT-interval prolonga-
Patients should be encouraged to remain in tion. On the basis of available evidence, an expert
treatment for at least 12 months to achieve en- panel convened by the United States Center for
during lifestyle changes, with some patients re- Substance Abuse Treatment developed a series of
quiring considerably longer periods. Beyond this safety recommendations for physicians prescrib-
point, no pre-determined treatment-term suits all ing methadone, specifically addressing the need
cases, but benefits are maintained and stability to inform patients about the risk of arrhythmia,
guaranteed by ongoing treatment, while with- assess cardiac history, use electrocardiography
drawal from treatment, no matter how gradual, is for baseline and follow-up assessment, manage
associated with a higher risk of relapse.
risk factors, and be aware of interactions between
Withdrawal from methadone treatment should methadone and other drugs that prolong the QT
be completed slowly and safely, and dose reduc- interval [59].
tions should be made in consultation with pa-
In addition to direct methadone side effects,
tients. Doses should be reduced by 10mg/week some studies have reported that a significant
to a level of 40mg/day, then by 5mg/week. Signs subset of patients (up to a third) may experience
and symptoms of withdrawal may become appar- symptoms of breakthrough withdrawal during
ent as the methadone dose falls below 20mg/day, the 24-hour inter-dosing interval [34]. Failure
with a peak at 2–3 days after cessation of metha- to achieve satisfactory 24-hour withdrawal sup-
done. Supportive care reduces the risk of relapse pression has been linked to individual variation
in the short-term and should be offered for at in methadone pharmacokinetics and the rate of
least 6 months post-methadone treatment [41, decline in plasma concentrations between peak
105]. Clinical monitoring and follow-up is also and trough [32]. Withdrawal symptoms may also
advisable in patients who have been drug-free for indicate that the current dose is inadequate.
a long period and are not receiving treatment.
4.2.1.6. Drug interactions
4.2.1.5. Side effects and symptom complaints
Pharmacodynamic and pharmacokinetic inter-
Many effects of methadone are similar to actions can alter the safety and efficacy of metha-
those of morphine and other opioid agonists. Tol- done for maintenance treatment. Methadone is
erance can develop to some side effects, however metabolised in the liver by CYP450 3A4, 2B6
some side effects (e.g., constipation, increased and 2D6. CYP450-inducing drugs reduce plas-
sweating) can continue to be troubling for some ma methadone levels and can cause withdrawal
patients for the duration of methadone treatment symptoms; these drugs should be avoided in
[3]. The primary hazard of methadone treatment methadone patients if possible. CYP450 3A in-
is the risk of overdose, particularly during induc- hibitors can decrease the metabolism of metha-
tion and when used in combination with other done and cause overdose; specialist advice should
sedative drugs. The relatively slow onset of ac- be sought regarding the use of these drugs [41,
tion and long half-life of methadone mean that 71]. Some psychotropic drugs may increase the
opioid overdose can be deceptive and toxic ef- actions of methadone because they have overlap-
fects may become life threatening many hours ping, additive effects (e.g., benzodiazepines and
after ingestion of methadone. Most deaths during alcohol add to the respiratory depressant effects
the induction period have occurred on the third of methadone) [41]. Similarly, given the associa-
and fourth day of treatment [41].
tion between methadone and QT interval prolon-
Cardiac safety also represents an important gation, there is a need for vigilance in prescribing
I. Maremmani et al.: Basics on Addiction: a training package for medical practitioners or psychiatrists who treat opioid dependence
other agents that have QT prolongation effects in lower intrinsic activity than full-agonist opioids
combination with methadone. For details refer to but a high binding affinity, buprenorphine com-
Pacini et al., 2009 [81].
petes with other agonists, such as methadone,
heroin, morphine and hydromorphone, at the
4.2.2. Buprenorphine treatment
mu-opioid site [10, 49, 103, 107]. As a result, in
the short term, it may not produce sufficient com-
pensatory agonist effects, leading to precipitated
opioid withdrawal. This can largely be avoided
During the initial development of buprenor- by the use of suitable initial dosing and rapid titra-
phine as an analgesic in the 1970s its potential tion to an appropriate maintenance dose [65]. The
utility as a treatment for opioid dependence was lower intrinsic activity of buprenorphine results
recognised [49]. The high-dose sublingual tablet in a lower level of maximum tolerance, which
preparation of buprenorphine was introduced in does not increase over a certain dose threshold
the 1990s and has since been marketed world- (ceiling effect), and its long duration of action
wide for the management of heroin dependence. leads to milder withdrawal symptoms than those
Full agonist
Methadone, heroin
Fatal respiratory
% of maximal effect
Naloxone, naltrexone
Figure 5. Risk of respiratory depression with opioid agonists, partial agonists and antagonists. Reproduced
from Walsh et al., 1994 [104]
Buprenorphine is a highly lipophilic [20] partial seen with morphine or methadone [49].
agonist at mu-opioid receptors and opioid-recep-
Due to its partial agonist action, there is a
tor-like (ORL-1) receptors and has mixed but ‘ceiling' effect to the respiratory depression that
primarily antagonistic actions on kappa and delta occurs with buprenorphine; higher doses do not
opioid receptors [99]. Buprenorphine has a high increase respiratory depression to a significant
affinity for mu-opioid receptors [11] and dissoci- degree [104]. This translates into a lower risk of
ates from the receptor slowly [39], thus produc- fatal overdose by comparison with full agonists
ing powerful opioid agonist effects whilst also such as methadone (Figure 5). However, there is
providing blockade against the effects of other no ceiling effect on buprenorphine's clinical ef-
opiates in a dose-dependent fashion [102].
ficacy, as higher doses have increasing effective-
As a partial mu-opioid-receptor agonist with ness with regard to treatment retention, heroin use
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
and withdrawal suppression [26, 53, 63]. Avail- 6 hours after last opioid use or when objective
ability of mu-opioid receptors is correlated with and clear signs of withdrawal are evident [88].
buprenorphine plasma concentration, withdrawal In contrast with the approach recommended for
symptoms and opioid blockade, with 50–60% methadone induction (‘start low, go slow'), most
receptor occupancy required for adequate with- guidelines recommend buprenorphine induction
drawal symptom suppression [39] and 80–90% should proceed rapidly [16, 23, 65]. It has been
receptor inactivation required for significant re- shown that faster buprenorphine induction im-
ductions in heroin-induced effects [19]. Comer et proves early treatment retention in subsequent
al. reported that 2, 8 and 32 mg of buprenorphine buprenorphine maintenance treatment and higher
(using the buprenorphine–naloxone combina- doses reduce craving [30] (Figure 6).
tion) dose-dependently reduced the available mu-
receptor population by 74, 83, and 91%, respec- 4.2.2.2.2. Patients transferring from other pharmacotherapies
tively [19]. In addition to buprenorphine dose,
receptor blockade also varies with time since ad-
Patients can be inducted onto buprenorphine
ministration. Receptor-binding studies conducted maintenance treatment from either current de-
using PET scanning at 4, 28, 52 and 72 hours post pendent heroin use, or can transfer from metha-
administration of buprenorphine 16mg to heroin- done. As methadone and buprenorphine have
dependent volunteers demonstrated its duration comparable effectiveness in reducing cravings
of action at receptors: 70% of mu-opioid recep- and illicit opioid use, transfer from methadone to
tors were occupied at 4 hours, 46% at 28 hours, buprenorphine may be appropriate when patients
33% at 52 hours and 18% at 72 hours [39].
have not met their treatment goals or have devel-
Buprenorphine is a long-acting drug with an oped intolerable side effects to methadone, or in
elimination half-life of 24–36 hours. The onset patients who wish to change pharmacotherapies,
of effects can be measured within 30–60 minutes e.g. to enable reduced-frequency dosing. Patients
of administration and peak clinical effects occur on low doses of methadone (<40mg) generally
within 1–4 hours. Effects are experienced for up tolerate this transition with minimal discomfort.
to 12 hours at low doses (2mg) and for as long as However, patients on higher methadone doses
48–72 hours at higher doses (16 or 32mg). The may find that buprenorphine precipitates tran-
prolonged duration of effect at high doses ena- sient opiate withdrawal [16, 23, 65]. The first bu-
bles alternate day or three times a week dispens- prenorphine dose should be administered at least
ing regimens [9, 83].
24 hours after the last methadone dose to mini-
mise the likelihood of precipitated withdrawal,
4.2.2.2. Treatment – induction
ideally waiting until patients experience a mild
degree of opioid withdrawal symptoms. Patient
4.2.2.2.1. Treatment-naïve patients
assessment and communication are important
during this phase [65]. The general principle is to
The initial aims of buprenorphine induction cease methadone and delay buprenorphine until
are to control possible physical symptoms quick- patients experience observable withdrawal [16,
ly while avoiding precipitated withdrawal. Suc- 23], generally 2–4 days after the last methadone
cessful induction can be achieved by assessing dose. Symptomatic medication may be used to
patients for opioid tolerance, observable signs of ease withdrawal discomfort.
mild opioid withdrawal, concurrent drug abuse
and concurrent medical conditions. To prevent 4.2.2.3. Treatment – maintenance
precipitated withdrawal with buprenorphine, the
first dose (2–4mg) should be administered at least
The aims and principles of buprenorphine
I. Maremmani et al.: Basics on Addiction: a training package for medical practitioners or psychiatrists who treat opioid dependence
Figure 6. Association of retention rate with intensity of buprenorphine induction. Reproduced from
Bacha, Reast and Pearlstone, 2010 [7]
maintenance treatment are generally equivalent to riod is double the normal daily buprenorphine
those of methadone maintenance treatment and to dose, and the dose for a 72-hour period is three
addiction treatment in general. The optimal main- times the daily dose, up to a maximum of 32mg
tenance dose needs to be individualised according at a time [88].
to the patient's response to buprenorphine. Dur-
Patients who have missed fewer than 5 con-
ing the stabilisation phase, buprenorphine doses secutive days since their last buprenorphine dose
should be titrated according to clinical effect by must be reviewed prior to receiving a further dose
increments of 2–4mg, to reach the recommended to ensure safety (i.e., reduction in tolerance may
target dose of 12–24mg/day by the end of the first have occurred), while patients who have missed
week. Several guidelines recommend aiming to more than 5 days need to recommence treatment
reach doses of 12–16 mg within 2–3 days, subject at a dose no greater than 8mg [65].
to patient response [16, 23]. At each dose review,
patients should be assessed for features of intoxi- 4.2.2.4. Cessation of buprenorphine treatment
cation or withdrawal, craving, additional drug
use, adverse events, adherence to dosing regimen
The decision to withdraw from opioid main-
and satisfaction with buprenorphine treatment tenance treatment should not be made lightly: re-
[65]. Effective maintenance, resulting in reduced lapse to illicit opioid use and treatment dropout is
heroin use and improved treatment retention, high following interruption of a long-term treat-
may be achieved with buprenorphine doses in the ment programme. Patients should be reminded
range of 8–24mg per day, with a maximum daily that the ultimate goal of treatment is to continue
recommended dose of 32mg [88].
not to relapse into addictive use and to achieve,
Alternate-day dosing can be considered in maintain and consolidate other life goals (e.g.
patients who are first stabilised on daily dosing employment, meaningful relationships) and that
[16, 23, 65]. Duration of buprenorphine effects there is no restriction to the length of time they
is dose-dependent, allowing for twice a week can receive maintenance treatment in order to
or three times a week dosing schedules [9, 83]; achieve this goal [97].
however, not all patients can be stabilised on such
A gradual process of treatment withdrawal was
regimens. The dose dispensed for a 48-hour pe- historically believed to result in better outcomes
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
[4]; however, a buprenorphine–naloxone tapering quences [65].
schedule of 7 days was reported to be compara-
Buprenorphine-maintained patients may have
ble to 28 days in terms of opioid-free urine speci- a diminished response to opiates prescribed for
mens at 1- and 3-month assessments in a study analgesia [75]. This can be managed by tempo-
by the National Drug Abuse Treatment Clinical rarily increasing the buprenorphine dose, using
Trials Network (NIDA CTN) [64]. The signs and higher potency opioids such as sufentanil (which
symptoms of buprenorphine withdrawal are qual- is approximately 1000 times more potent than
itatively similar to withdrawal from other opiates morphine) or using non-opioid analgesics [65,
although the withdrawal syndrome experience on 66]. Which option is appropriate depends on the
cessation of buprenorphine is delayed and may severity, onset and duration of pain. In addition,
be milder than withdrawal from heroin, morphine some options may require management in spe-
or methadone [4]. The onset of symptoms is usu- cialist settings [23; 65].
ally around 24–72 hours after the last dose and
the peak is between days 3 and 5 (days 5 and 14 4.2.3. Buprenorphine–naloxone
following long-term maintenance treatment).
Frequent monitoring and review, including 4.2.3.1. Pharmacology
the use of withdrawal scales, counselling and
symptomatic medication should occur regularly
Buprenorphine–naloxone (Suboxone®) is a
during the withdrawal phase. Patients who feel sublingual tablet containing buprenorphine hy-
at risk for relapse should be allowed to return to drochloride and naloxone hydrochloride dihy-
maintenance treatment at any time during taper. drate in a ratio of 4:1. It is available in two dosage
Psychosocial counselling should continue and strengths: 2mg buprenorphine/0.5mg naloxone,
possibly be increased during and after medical and 8mg buprenorphine/2mg naloxone. The phar-
withdrawal [65].
macology of buprenorphine has been described
above. Naloxone is a competitive mu-opioid-
4.2.2.5. Side effects and drug interactions
receptor antagonist, which displaces receptor-
bound opioid molecules and produces a rapid re-
Buprenorphine is principally metabolised by versal of the effects of opioids. The main clinical
CYP450 3A4. Although buprenorphine metabo- use of naloxone is to treat respiratory depression
lism can be influenced by medications that are caused by opioid overdose [85]. Naloxone has
also metabolised by or alter the activity of the cy- low oral bioavailability but has rapid access to
tochrome P450 system, it is less affected by drug mu receptors if administered intravenously. It is
interactions or hepatic disease than other opioids metabolised in the liver, with a short half-life of
such as methadone. Of particular interest in light about 1 hour [85].
of the increased incidence of HIV among inject-
The buprenorphine–naloxone combination
ing drug users, buprenorphine is less likely to be product was developed to decrease the potential
associated with adverse events when given with for diversion and abuse of buprenorphine [73].
efavirenz-containing highly active anti-retroviral The presence of naloxone is intended to deter in-
therapy (HAART) compared with methadone travenous abuse by persons dependent on other
[14]. The combination of buprenorphine with opioids; if administered sublingually, naloxone
benzodiazepines, alcohol or other sedatives has does not cause significant effects due to the poor
been associated with fatal overdoses, due to ad- absorption of naloxone via this route. However,
ditive effects. Appropriate prescription of these if the product is used intravenously or nasally, the
therapeutics, combined with responsible use by antagonistic effect of naloxone elicits an acute
patients, is unlikely to lead to adverse conse- but non-life-threatening withdrawal syndrome
I. Maremmani et al.: Basics on Addiction: a training package for medical practitioners or psychiatrists who treat opioid dependence
in opioid-dependent subjects [73]. Therefore, the oid-dependent volunteers were maintained on a
combination of buprenorphine and naloxone for 40mg dose of hydromorphone and then tested
sublingual administration should diminish the with intramuscular and sublingual buprenor-
parenteral abuse liability of buprenorphine by phine/naloxone (1.0/0.25, 2.0/0.5, 4/1, 8/2 and
opioid-dependent individuals [73]. Notably, bu- 16/4mg); intramuscular hydromorphone (10mg)
prenorphine time to onset, time to peak effect and and naloxone (0.25mg); both intramuscular and
duration of action remain unaltered.
sublingual buprenorphine alone (8mg); and pla-
cebo found that the combination produced dose-
4.2.3.2. Reduced abuse liability
related opioid antagonist effects when admin-
istered intramuscularly but that the same doses
Numerous controlled and observational stud- produced neither significant agonist or antago-
Figure 7. Mean ‘willing to take the drug again' responses across all buprenorphine maintenance
doses in the study by Comer et al., 2010 [18]
ies have confirmed the reduced abuse liability of nist effects when administered by the sublingual
buprenorphine–naloxone relative to buprenor- route [96]. Results from a study of 12 intravenous
phine. The findings of a study in which 12 opi- heroin users maintained on each of three different
oid-dependent volunteers were stabilised on a sublingual buprenorphine levels (2, 8 and 24mg)
60mg daily dose of morphine and then received showed that the subjective ratings of ‘drug liking'
a series of challenges with buprenorphine alone and ‘desire to take the drug again' were signifi-
(2mg intravenous dose), or in combination with cantly lower for buprenorphine–naloxone than
naloxone (ratios of 2:1, 4:1 and 8:1) were that bu- for buprenorphine or heroin (Figure 7). Similar
prenorphine alone did not precipitate withdrawal results were found for the amount of money that
and had similar agonist effects to those of mor- participants were willing to pay for each drug.
phine; buprenorphine plus naloxone at ratios of Subjects were most likely to self-administer drug
2:1 and 4:1 produced moderate to high increases when maintained on the lowest sublingual bu-
in global opiate withdrawal, bad drug effect and prenorphine dose [18]. Retrospective, real-world
sickness; while the 8:1 ratio produced only mild data collected from interviews with injecting drug
withdrawal symptoms [73]. A study in which opi- users from the Australian Illicit Drug Reporting
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
System (IDRS) indicated that buprenorphine– centage of opioid-free urine samples over time
naloxone was less likely to be injected than either did not differ by drug or dosage. The percentage
methadone or buprenorphine [22].
of patients with ≥12 consecutive opioid-negative
urine samples did not differ by drug and was sig-
4.2.3.3. Treatment – induction
nificantly greater for patients receiving higher
doses of either agent. Induction success, compli-
The rationale for induction onto buprenor- ance, non-opioid drug use, retention and Addic-
phine–naloxone is similar to that for buprenor- tion Severity Index scores did not differ among
phine. To avoid precipitated opioid withdrawal, the groups [53].
the first dose of buprenorphine–naloxone is de-
layed by 12–24 hours from the last opioid use, 4.2.3.5. Buprenorphine–naloxone and take-home dos-
upon presentation of observable withdrawal ing
signs. Induction on buprenorphine–naloxone
from illicit opioid use has been shown to be ef-
Due to its favourable safety profile and reduced
fective and well tolerated in a NIDA CTN trial of abuse liability, buprenorphine–naloxone may
234 opioid-dependent subjects. The study found hold particular value for patients in whom unsu-
that 90% of participants successfully completed pervised ‘take-home' dosing is used. In an Aus-
the 3-day induction period, reaching the target tralian 3-month trial of 119 subjects randomised
dose of 16mg buprenorphine/4mg naloxone, and to observed or unobserved (weekly take-home)
68% completed the 13-day taper program [5].
administration of buprenorphine–naloxone, re-
tention and heroin use were not significantly dif-
4.2.3.4. Treatment – maintenance
ferent between the two dosing groups. Treatment
with close clinical monitoring, but no observation
EU prescribing information recommends of dosing, was significantly cheaper (AU$ 1663
that the dose of buprenorphine/naloxone be in- compared with AU$ 2138) and therefore signifi-
creased progressively according to the clinical cantly more cost-effective [8]. Buprenorphine–
effect and should not exceed a maximum single naloxone might therefore help to alleviate pres-
daily dose of 24mg/6mg [89], although adopting sure on resources by allowing safe ‘take-home'
a best-practice approach by titrating individu- dosing.
al doses according to clinical effect means that
some patients may require higher or lower dos- 5. Conclusion
age for optimum response. As for buprenorphine,
patients should be assessed at least weekly dur-
Opioid dependence is a chronic metabolic
ing the stabilisation phase to allow assessment brain disease manifesting with several biological,
of patient response to therapy and appropriate sociological and individual effects. Treatment
dose adjustment. EU prescribing information for opioid dependence aims to improve the well-
states that following satisfactory stabilisation, being and social functioning of individuals and
buprenorphine/naloxone may be administered on to reduce the associated health and social conse-
alternate days or thrice weekly in some patients quences. Given the complexity of this condition,
(the buprenorphine/naloxone dose given on any no single treatment approach is effective for all
1 day should not exceed 24mg/6mg) [89]. In a individuals, and people with opioid dependence
17-week, double-blind, double-dummy trial, dai- should therefore be offered access to a range of
ly dosing of buprenorphine–naloxone (8mg/2mg high-quality treatments to respond to their vary-
and 16mg/4mg) was compared with methadone ing retention and response-related needs [113].
(45mg and 90mg) in 268 participants. The per-
Opioid agonist maintenance treatment has be-
I. Maremmani et al.: Basics on Addiction: a training package for medical practitioners or psychiatrists who treat opioid dependence
come the first-line treatment for opioid depend- Decisions should be informed by a sound under-
ence. Agonist maintenance treatment benefits standing of the basics of addiction, the principles
individuals with opioid dependence through re- of opioid maintenance treatment, and the clinical
ductions in addictive drug use and associated application of available options. Whilst treatment
mortality risks, increased stability, improved with methadone, buprenorphine and buprenor-
well-being and social functioning; benefits to phine–naloxone has the same therapeutic aims,
society include reductions in the incidence of these products/compounds have unique pharma-
criminal behaviour, reduced health and criminal- cological properties and safety profiles that need
justice costs and increased productivity [113].
to be considered when formulating treatment
In order to realise the full benefits of opioid plans. This supplement provides an overview of
maintenance treatment, it is necessary that clini- the basic knowledge required to deliver mainte-
cians deliver treatment in a manner that meets nance treatment in a safe and effective manner.
certain quality standards, as derived from the
available scientific evidence base, while tailoring
the treatment to the individual in order to meet the
complex and unique needs of different patients.
Key learning points
Opioid dependence is a chronic metabolic brain disease and several biological, sociologi-
cal and individual factors are implicated in its development
Effective treatment
Is accessible for as many people as possible
Involves a set of pharmacological and psychosocial interventions
Aims to reduce or cease addictive opioid use, prevent harms associated with opioid
use, improve quality of life for the patient and benefit the wider community
Opioid agonist maintenance treatment is the most cost-effective form of treatment
Primary options are methadone, buprenorphine and buprenorphine–naloxone
Access to psychosocial interventions can significantly enhance success
The benefits of maintenance programmes increase the longer the person remains in treat-
Many people do need to receive treatment for a number of years
Methadone, buprenorphine and buprenorphine/naloxone:
Are broadly comparable in terms of retention and effectiveness in reducing addictive
opioid use or any opioid use
Buprenorphine is preferred for detoxification/short-term programmes
Methadone is associated with specific side effects
Respiratory depression and QT prolongation
Fewer drug interactions with buprenorphine and HAART
Methadone is associated with greater overdose risk with benzodiazepines
Buprenorphine–naloxone is associated with the lowest abuse potential
Regular monitoring allows the clinician to evaluate and adapt therapy to meet the needs
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
effectiveness of observed versus unobserved
administration of buprenorphine-naloxone
for heroin dependence. Addiction. 102:(12)
1. A.P.A. (2000): Desk Reference to the Diagnostic
Criteria from DSM-IV-TR. American 9. Bickel W. K., Amass L., Crean J. P., Badger
Psychiatric Association, Washington, DC.
G. J. (1999): Buprenorphine dosing every
2. A.P.A. (2000): DSM-IV-TR. Diagnostic
1, 2, or 3 days in opioid-dependent patients.
and Statistical Manual of Mental Disorders.
Psychopharmacology (Berl). 146:(2) 111-118.
American Psichiatric Association, Washington. 10. Bickel W. K., Stitzer M. L., Begelow G. E.,
3. A.P.A. (2006): Opioid-related disorders.
Liebson I. A., Jasinski D. R., Johnson R. E.
American Psychiatric Association Practice
(1988): Buprenorphine: dose-related blockade
Guidelines for the Treatment of Psychiatric
of opioid challenge in opioid dependent
Disorders compendium. American Psychiatric
humans. J Psychopharmacol Exper Ther. 247
Association, Arlington, VA. pp. 452-465.
4. Amass L., Bickel W. K., Higgins S. T. (1994): 11. Boas R. A., Villiger J. W. (1985): Clinical
A preliminary investigation of outcome
actions of fentanyl and buprenorphine. The
following gradual or rapid buprenorphine
significance of receptor binding. Br J Anaesth.
detoxification. In: Magura S., Rosenblum A.
57:(2) 192-196.
(Eds.): Experimental therapeutics in addiction 12. Branstetter S. A., Low S. (2010): Natural
medicine. Haworth Press, Binghampton, N.Y.
history of addictive diseases. In: Miller N.
S., Gold M. S. (Eds.): Addictive disorders
5. Amass L., Ling W., Freese T. E., Reiber C.,
in medical populations. Wiley-Blackwell,
Annon J. J., Cohen A. J., Mccarty D., Reid
Chichester. pp. 53-72.
M. S., Brown L. S., Clark C., Ziedonis D. M., 13. Bryant B., Knights K. (2011): Analgesics.
Krejci J., Stine S., Winhusen T., Brigham G.,
Pharmacology for healthcare professionals.
Babcock D., Muir J. A., Buchan B. J., Horton
Mosby, Chatswood, NSW. pp. 277-307.
T. (2004): Bringing buprenorphine-naloxone 14. Cance-Katz E. F. (2005): Treatment of opioid
detoxification to community treatment
dependence and coinfection with HIV and
providers: the NIDA Clinical Trials Network
hepatitis C virus in opioid-dependent patients:
field experience. Am J Addict. 13:(Suppl 1)
the importance of drug interactions between
opioids and antiretroviral agents. Clin Infect
6. Arbabzadeh-Bouchez S., Lepine J. P. (2003):
Dis. 41 Suppl 1 S89-S95.
Measurements of depression and anxiety 15. Caplehorn J. R. M., Dalton M. S., Cluff M. C.,
disorder. In: Kasper S. D. B. J. A., Ad Sitsen
Petrenas A. (1994): Retention in methadone
J. M. (Eds.): Handbook of Depression and
maintenance and heroin addicts' risk of death.
Anxiety. Marcel Dekker, Inc., New York, NY.
Addiction. 89:(2) 203-207.
pp. 127-150.
16. Center for Substance Abusetreatment,
7. Bacha J., Reast S., Pearlstone A. (2010):
Substance Abuse and Mental Health Services
Treatment practices and perceived challenges
Administration (2004): Clinical Guidelines
for European physicians treating opioid
for the Use of Buprenorphine in the
dependence. Heroin Addict Relat Clin Probl.
Treatment of Opioid Addiction. Treatment
12:(3) 9-19.
Improvement Protocol (TIP) Series 40. DHHS
8. Bell J., Shanahan M., Mutch C., Rea F., Ryan
Publication No. (SMA) 04-3939. Available
A., Batey R., Dunlop A., Winstock A. (2007):
A randomized trial of effectiveness and cost-
indexhtm. Accessed on March 8, 2011.
I. Maremmani et al.: Basics on Addiction: a training package for medical practitioners or psychiatrists who treat opioid dependence
17. Chahl L. A. (1996): Opioids - mechanisms of
Probl. 7:(3) 21-26.
action. Aust Presc. 19 63-65.
27. Dietis N., Guerrini R., Calo G., Salvadori
18. Comer S. D., Sullivan M. A., Vosburg S. K.,
S., Rowbotham D. J., Lambert D. G. (2009):
Manubay J., Amass L., Cooper Z. D., Saccone
Simultaneous targeting of multiple opioid
P., Kleber H. D. (2010): Abuse liability of
receptors: a strategy to improve side-effect
intravenous buprenorphine/naloxone and
profile. BrJ Anaesth. 103:(1) 38-49.
buprenorphine alone in buprenorphine- 28. Dilts Jr S. L., Dilts S. L. (2005): Opioids. In:
maintained intravenous heroin abusers.
Frances R. J., Miller S. I., Mack A. H. (Eds.):
Addiction. 105:(4) 709-718.
Clinical textbook of addictive disorders. The
19. Comer S. D., Walker E. A., Collins E. D.
Guilford Press, New York, NY. pp. 130-156.
(2005): Buprenorphine/naloxone reduces 29. Dole V. P., Nyswander M. E. (1965): A medical
the reinforcing and subjective effects of
treatment for diacetylmorphine (heroin)
heroin in heroin-dependent volunteers.
addiction: A clinical trial with methadone
Psychopharmacology (Berl). 181:(4) 664-675.
hydrocloride. JAMA. 193 80-84.
20. Cowan A., Lewis J. W., Macfarlane I. R. 30. Doran C., Holmes J., Ladewig D., Ling
(1977): Agonist and antagonist properties of
W. (2005): Buprenorphine induction and
buprenorphine, a new antinociceptive agent.
stabilisation in the treatment of opiate
Br J Pharmacol. 60:(4) 537-545.
dependence. Heroin Addict Relat Clin Probl.
21. De L. A., Coupar I. M. (1996): Insights into
opioid action in the intestinal tract. Pharmacol 31. Doweiko H. E. (2009): Opioid abuse and
Ther. 69:(2) 103-115.
addiction. Concepts of chemical dependency.
22. Degenhardt L., Larance B. K., Bell J. R.,
Brooks/Cole Cengage Learning, Belmont, CA.
Winstock A. R., Lintzeris N., Ali R. L.,
pp. 158-178.
Scheuer N., Mattick R. P. (2009): Injection 32. Dyer K., Foster D., White J., Somogyi A.,
of medications used in opioid substitution
Menelaou A., Bochner F. (1999): Steady-state
treatment in Australia after the introduction of
pharmacokinetics and pharmacodynamics in
a mixed partial agonist-antagonist formulation.
methadone maintenance patients: comparison
Med J Aust. 191:(3) 161-165.
of those who do and do not experience
23. Department of Health Administration
withdrawal and concentration-effect
(2007): Drug Misuse and Dependence: UK
relationships. Clin Pharmacol Ther. 65:(6)
Guidelines on Clinical Management. Available
at: Available at: wwwmerckmanualscom. 33. Dyer K. R. (2005): Methadone maintenance
Accessed on March 30, 2011.
treatment and mood disturbances:
24. Dewick P. M. (2009): Medicinal natural
Pharmacological and psychological
products: a biosynthetic approach. John Wiley
implications. Heroin Addict Relat Clin Probl.
& Sons Ltd, Chichester.
25. Dhawan B. N., Cesselin F., Raghubir R., 34. Dyer K. R., White J. M. (1997): Patterns of
Reisine T., Bradley P. B., Portoghese P. S.,
symptom complaints in methadone maintained
Hamon M. (1996): International Union of
patients. Addiction. 92:(11) 1445-1455.
Pharmacology. XII. Classification of opioid 35. Feltenstein M. W., See R. E. (2008): The
receptors. Pharmacol Rev. 48:(4) 567-592.
neurocircuitry of addiction: an overview. Br
26. Di Petta G., Leonardi C. (2005): Buprenorphine
J Pharmacol. 154:(2) 261-274.
high-dose, broad spectrum, long-term 36. Finkel R., Clark M. A., Cubeddu L. X. (2009):
treatment: A new clinical approach to opiate
Opioids. Lippincott's Illustrated Reviews:
alkaloid dependency. Heroin Addict Relat Clin
Pharmacology. Lippincott, Williams &
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
Wilkins, Baltimore, MD. pp. 159-170.
and the brain: the neurobiology of compulsion
37. Gold M. S., Pottash A. L., Extein I., Martin D.
and its persistence. Nat Rev Neurosci. 2:(10)
A., Finn L. B., Sweeney D. R., Kleber H. D.
(1981): Evidence for an endorphin dysfunction 46. Jaffe J. H., Jaffe A. B. (2000): Opioid related
in methadone addicts: lack of ACTH response
disorders. In: Sadock B. J., Sadock V. A.
to naloxone. Drug Alcohol Depend. 8:(3) 257-
(Eds.): Comprehensive textbook of psychiatry.
Lippincott Williams & Wilkins, 1038-1062.
38. Gourevitch M. N., Arnsten J. H. (2005): 47. Jaffe J. H., Martin W. R. (1992): Opioid analgesics
Medical complications of drug use. In:
and antagonists. The pharmacological basis
Lowinson J. H., Ruiz P., Millman R. B.,
of therapeutics. McGraw Hill,Inc., 485-521.
Langrod M. (Eds.): Substance abuse: a 48. Jarvis T. J., Tebbutt J., Mattick R. P., Shand
comprehensive textbook. Lippincott Williams
F. (2005): Assessment. Treatment Approaches
& Wilkins, Philadelphia, PA. pp. 840-862.
for Alcohol and Drug Dependence: An
39. Greenwald M., Johanson C. E., Bueller J.,
Introductory Guide. John Wiley & Sons, Ltd,
Chang Y., Moody D. E., Kilbourn M., Koeppe
R., Zubieta J. K. (2007): Buprenorphine 49. Jasinski D. R., Pevnick J. S., Griffith J. D.
duration of action: mu-opioid receptor
(1978): Human pharmacology and abuse
availability and pharmacokinetic and
potential of the analgesic buprenorphine: a
behavioral indices. Biol Psychiatry. 61:(1)
potential agent for treating narcotic addiction.
Arch Gen Psychiatry. 35:(4) 501-516.
40. Grimm J. W., Hope B. T., Wise R. A., Shaham 50. Kakko J., Svanborg K. D., Kreek M. J.,
Y. (2001): Neuroadaptation. Incubation of
Heilig M. (2003): High 1-year retention and
cocaine craving after withdrawal. Nature.
improved social function in a buprenorphine-
412:(6843) 141-142.
assisted relapse prevention treatment for
41. Henry-Edwards S., Gowing L., White J., Ali
heroin dependence: A randomized, placebo-
R., Bell J., Brough R., Lintzeris N., Ritter A.,
controlled Swedish trial. Lancet. 361 662-668.
Quigley A. (2003): Clinical guidelines and 51. Kalivas P. W., O'brien C. (2008): Drug addiction
procedures for the use of methadone in the
as a pathology of staged neuroplasticity.
maintenance treatment of opioid dependence.
Available at: wwwhealthvicgovau. Accessed 52. Kalivas P. W., Volkow N. D. (2005): The neural
on Feb 16, 2011.
basis of addiction: a pathology of motivation
42. Hogerzeil H. V. (2006): Essential medicines
and choice. Am J Psychiatry. 162 1403-1413.
and human rights: what can they learn from 53. Kamien J. B., Branstetter S. A., Amass L.
each other? Bull World Health Organ. 84:(5)
(2008): Buprenorphine-naloxone versus
methadone maintenance therapy: a randomised
43. Hughes J., Smith T. W., Kosterlitz H. W.,
double-blind trial with opioid-dependent
Fothergill L. A., Morgan B. A., Morris H.
patients. Heroin Addict Relat Clin Probl.
R. (1975): Identification of two related
10:(4) 5-18.
pentapeptides from the brain with potent opiate 54. Karan L. D., Benowitz N. L. (2000):
agonist activity. Nature. 258:(5536) 577-580.
Substance abuse: dependence and treatment.
44. Hulse G. K., English D. R., Milne E., Holman
In: Carruthers S. G., Hoffman B. B., Melmon
C. D. (1999): The quantification of mortality
K. L., Nierenberg D. W. (Eds.): Melmon and
resulting from the regular use of illicit opiates.
Morrelli's Clinical Pharmacology. McGraw
Addiction. 94:(2) 221-229.
Hill, 1053-1091.
45. Hyman S. E., Malenka R. C. (2001): Addiction 55. Kelley A. E., Berridge K. C. (2002): The
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
neuroscience of natural rewards: relevance to
B. (2010): From research to the real world:
addictive drugs. J Neurosci. 22 3306-3311.
buprenorphine in the decade of the Clinical
56. Koneru A., Satyanarayana S., Rizwan
Trials Network. J SubstAbuse Treat. 38 Suppl
S. (2009): Endogenous opioids: their
physiological role and receptors. Global 65. Lintzeris N., Clark N., Winstock A., Dunlop
Journal of Pharmacology. 3:(3) 149-153.
A., Muhleisen P., Gowing L., Ali R., Ritter A.,
57. Kosten T. R., George T. P. (2002): The
Bell J., Quigley A., Mattick R. P., Monheit B.,
Neurobiology of Opioid Dependence:
White J. (2006): National Clinical Guidelines
Implications for Treatment. Research Reviews
and Procedures for the Use of Buprenorphine
- Science & Practice perspectives.
in the Treatment of Opioid Dependence. http://
58. Kosten T. R., Mccance-Katz E. (1995): New
pharmacotherapies. In: Oldham J. M., Riba
M. B. (Eds.): American Psychiatric Press 66. Macres S. M., Moore P. G., Fishman S. M.
Review of Psychiatry. American Psychiatric
(2009): Acute pain management. In: Barash
Press, Washington. pp. 105-127.
P. G. (Ed.) Clinical anesthesia. Lippincott
59. Krantz M. J., Martin J., Stimmel B., Mehta D.,
Williams & Wilkins, Philadelphia, PA. pp.
Haigney M. C. (2008): QTc Interval Screening
in Methadone Treatment: the CSAT Consensus 67. Maremmani I. (2009): The Principles and
Guideline. Ann Intern Med Epub.
Practice of Methadone Treatment. Pacini
60. Kreek M. J., Nielsen D. A., Butelman E. R.,
Editore Medicina & AU-CNS, Pisa.
Laforge K. S. (2005): Genetic influences on 68. Martin W. R., Eades C. G., Thompson J. A.,
impulsivity, risk taking, stress responsivity
Huppler R. E., Gilbert P. E. (1976): The effects
and vulnerability to drug abuse and addiction.
of morphine- and nalorphine- like drugs in
Nat Neurosci. 8:(11) 1450-1457.
the nondependent and morphine-dependent
61. Laudet A. B. (2008): The road to recovery:
chronic spinal dog. J PharmacolExpTher.
where are we going and how do we get
197:(3) 517-532.
there? Empirically driven conclusions and 69. Martin W. R., Jasinski D. R. (1969):
future directions for service development
Physiological parameters of morphine
and research. Subst Use Misuse. 43:(12-13)
dependence in man, early abstinence,
protracted abstinence. J Psychiatr Res. 7 9-17.
62. Leshner A. I. (1997): Addiction is a brain 70. Mattick R. P., Breen C., Kimber J., Davoli
disease, and it matters. Science. 278:(5335)
M. (2009): Methadone maintenance therapy
versus no opioid replacement therapy for opioid
63. Ling W., Charuvastra C., Collins J. F., Batki
dependence. CochraneDatabaseSystRev:(3)
S., Brown L. S. J. R., Kintaudi P., Wesson D.
R., Mcnicholas L., Tusel D. J., Malkerneker 71. Mccance-Katz E. F., Sullivan L. E., Nallani
U., Renner J. A. J., Santos E., Casadonte P.,
S. (2010): Drug Interactions of Clinical
Fye C., Stine S., Wang R. I., Segal D. (1998):
Importance among the Opioids, Methadone
Buprenorphine mainteinance treatment of
and Buprenorphine, and Other Frequently
opiate dependence: a multicenter randomized
Prescribed Medications: A Review. Am J
clinical trial. Addiction. 93 (4) 475-486.
Addict. 19:(1) 4-16.
64. Ling W., Jacobs P., Hillhouse M., Hasson A., 72. Mclellan A. T., Lewis D. C., O'brien C. P.,
Thomas C., Freese T., Sparenborg S., Mccarty
Kleber H. D. (2000): Drug dependence, a
D., Weiss R., Saxon A., Cohen A., Straus
chronic medical illness: implications for
M., Brigham G., Liu D., Mclaughlin P., Tai
treatment, insurance, and outcomes evaluation.
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
Jama. 284:(13) 1689-1695.
82. Pani P. P., Maremmani I., Trogu E., Gessa G.
73. Mendelson J., Jones R. T. (2003): Clinical and
L., Ruiz P., Akiskal H. S. (2010): Delineating
pharmacological evaluation of buprenorphine
the psychic structure of substance abuse and
and naloxone combinations: why the 4:1 ratio
addictions: Should anxiety, mood and impulse-
for treatment? Drug Alcohol Depend. 70:(2
control dysregulation be included? J Affect
Suppl) S29-S37.
Disord. 122 185-197.
74. Meresaar U., Nilsson M. I., Holmstrand 83. Perez De Los C. J., Martin S., Etcheberrigaray
J., Anggard E. (1981): Single dose
A., Trujols J., Batlle F., Tejero A., Queralto
pharmacokinetics and bioavailability of
J. M., Casas M. (2000): A controlled trial of
methadone in man studied with a stable isotope
daily versus thrice-weekly buprenorphine
method. Eur J Clin Pharmacol. 20:(6) 473-
administration for the treatment of opioid
dependence. Drug Alcohol Depend. 59:(3)
75. Mitra S., Sinatra R. S. (2004): Perioperative
management of acute pain in the opioid- 84. Purves D., Augustine G. J., Fitzpatrick
dependent patient. Anesthesiology. 101:(1)
D., Katz L. C., Lamantia A. S., Mcnamara
J. O., Williams S. M. (2003): Glossary.
76. National Institutes of Health, U. S. Department
Neuroscience. Sinauer Associates, Sunderland
of Health Human Services (2010): Drugs,
brains and behavior: the science of addiction. 85. Rang H. P., Dale M. M., Ritter J. M. (2003):
Available at: wwwdrugabusegov. accessed on
Analgesic drugs. In: Rang H. P., Dale M. M.,
Jan 26, 2011.
Ritter J. M. (Eds.): Pharmacology. Churchill
77. National Institutes of Health, U. S. Department
Livingstone, Edinburgh. pp. 562-584.
of Health Human Services (2010): Principles 86. Rang H. P., Dale M. M., Ritter J. M. (2003):
of Drug Addiction Treatment. Available at:
Drug dependence and abuse. In: Rang H. P.,
wwwnidanihgov. Accessed on Jan 26, 2011.
Dale M. M., Ritter J. M. (Eds.): Pharmacology.
78. Newman R. G. (1995): The Pharmacological
Churchill Livingstone, Edinburgh. pp. 594-
Rationale for Methadone Treatment of
Narcotic Addiction. In: Tagliamonte A., 87. Rang H. P., Dale M. M., Ritter J. M. (2003):
Maremmani I. (Eds.): Drug Addiction and
How drugs act: general principles. In: Rang
Related Clinical Problems. Springer-Verlag,
H. P., Dale M. M., Ritter J. M. (Eds.):
Wien New York. pp. 109-118.
Pharmacology. Churchill Livingstone,
79. Noble M., Treadwell J. R., Tregear S. J.,
Edinburgh. pp. 7-21.
Coates V. H., Wiffen P. J., Akafomo C., 88. Rb Pharmaceuticals Limited (2010): Subutex
Schoelles K. M. (2010): Long-term opioid
summary of product characteristics. Available
management for chronic noncancer pain.
at: wwwmedicinesorguk. Accessed on March
80. O'connor P. G. (2008): The Merck Manual: 89. Rb Pharmaceuticals Limited (2011): Suboxone
Psychiatric Disorders: Drug Use & Dependence.
summary of product characteristics. Available
Available at: wwwmerckmanualscom.
at: wwwemaeuropaeu. Accessed on 16
Accessed on Jan 12, 2011.
February 2011.
81. Pacini M., Maremmani A. G. I., Dell' Osso 90. Ries R., Fiellin D., Miller S., Saitz R. (2009):
L., Maremmani I. (2009): Opioid Treatment
Principles of Addiction Medicine. Lippincott
and "Long-QT Syndrome (LQTS)": a Critical
Williams & Wilkins, Philadelphia, PA.
Review of the Literature Heroin Addict Relat 91. Schifano F. (2011): Drug abuse: treatment
Clin Probl. 11:(4) 21-28.
and management. In: Ghodse H., Herrman
I. Maremmani et al.: Basics on Addiction: a training package for medical practitioners or psychiatrists who treat opioid dependence
H., Maj M., Sartorius N. (Eds.): Substance
of the orbitofrontal cortex. Cereb Cortex.
abuse disorders: evidence and experience.
10:(3) 318-325.
Wiley-Blackwell, Chichester. pp. 53-74.
101. Volkow N. D., Fowler J. S., Wang G. J.
92. Schottenfeld R. S. (2008): Opioid maintenance
(2003): The addicted human brain: insights
treatment. In: Galanter M., Kleber H. D.
from imaging studies. J Clin Invest. 111:(10)
(Eds.): The American Psychiatric Publishing
Textbook of substance abuse treatment. 102. Walsh S. L., Eissenberg T. (2003): The
American Psychiatric Publishing, Inc.,
clinical pharmacology of buprenorphine:
Arlington, VA. pp. 289-308.
extrapolating from the laboratory to the clinic.
93. Shaham Y., Shalev U., Lu L., De W. H.,
Drug Alcohol Depend. 70:(2 Suppl) S13-S27.
Stewart J. (2003): The reinstatement model 103. Walsh S. L., June H. L., Schuh K. J.,
of drug relapse: history, methodology and
Preston K. L., Bigelow G. E., Stitzer M.
major findings. Psychopharmacology (Berl).
L. (1995): Effects of buprenorphine and
168:(1-2) 3-20.
methadone in methadone-maintained subjects.
94. Simon E. J. (1991): Opioid receptors and
Psychopharmacology (Berl). 119:(3) 268-276.
endogenous opioid peptides. Med Res Rev. 104. Walsh S. L., Preston K. L., Stitzer M. L.,
11:(4) 357-374.
Cone E. J., Bigelow G. E. (1994): Clinical
95. Sneader W. (2005): Plant product analogues
pharmacology of buprenorphine: ceiling
and compounds derived from them. Drug
effects at high doses. Clin Pharmacol Ther.
discovery: a history. John Wiley & Sons Ltd,
55:(5) 569-580.
Chichester. pp. 115-150.
105. Ward J., Mattick R., Hall W. (1998):
96. Stoller K. B., Bigelow G. E., Walsh S. L.,
Methadone Maintenance Treatment and other
Strain E. C. (2001): Effects of buprenorphine/
Opioid Replacement Therapies. Harwood
naloxone in opioid-dependent humans.
Academic Publishers, Amsterdam.
Psychopharmacology (Berl). 154:(3) 230-242. 106. Ward J., Mattick R. P., Hall W. (1998): The
97. Strain E. C., Lofwall M. R. (2008):
effectiveness of methadone maintenance
Buprenorphine maintenance. In: Galanter M.,
treatment. Methadone Maintenance Treatment
Kleber H. D. (Eds.): The American Psychiatric
and Other Opioid Replacement Therapies.
Publishing Textbook of substance abuse
Harwood Academic Publishers, Amsterdam.
treatment. American Psychiatric Publishing,
Inc., Arlington, VA. pp. 309-324.
107. Wesson D. R. (2004): Buprenorphine in
98. Verebey K. G., Meenan G., Buchan B. J.
the treatment of opiate dependence: its
(2005): Diagnostic laboratory: screening
pharmacology and social context of use in the
for drug abuse. In: Lowinson J. H., Ruiz P.,
U.S. J Psychoactive Drugs. Suppl 2 119-128.
Millman R. B., Langrod M. (Eds.): Substance 108. Westphal J., Wasserman D. A., Masson C. L.,
abuse: a comprehensive textbook. Lippincott
Sorensen J. L. (2005): Assessment of opioid
Williams & Wilkins, Philadelphia, PA. pp.
use. In: Donovan D. M., Marlatt G. A. (Eds.):
Assessment of Addictive Behaviors. The
99. Virk M. S., Arttamangkul S., Birdsong W.
Guilford Press, New York, NY. pp. 215-247.
T., Williams J. T. (2009): Buprenorphine is 109. Who (1992): The ICD-10 Classification of
a weak partial agonist that inhibits opioid
Mental and Behavioural Disorders. Clinical
receptor desensitization. J Neurosci. 29:(22)
descriptions and diagnostic guidelines. World
Health Organization, Geneva.
100. Volkow N. D., Fowler J. S. (2000): Addiction, a 110. Who (2004): Neuroscience of psychoactive
disease of compulsion and drive: involvement
substance use and dependence. Available
Heroin Addiction and Related Clinical Problems 13 (2): 5-40
Accessed May 28, 2010.
111. Who (2009): Guidelines for the Psychosocially
Icro Maremmani, Matteo Pacini and Pier Pao-
Assisted Pharmacological Treatment of Opioid lo Pani contributed equally to this work.
Dependence. Available at: wwwwhoint.
Accessed on May 28, 2010.
Conflict of interest
112. Who (2010): WHO Model List of Essential
Medicines. Available at: http://wwwwhoint/
The authors disclose the following relevant fi-
medicines. Accessed on Febr 10, 2011.
nancial relationships: Icro Maremmani: [None],
113. Who, Unodc, Unaids (2004): WHO/UNODC/ Matteo Pacini [None], Pier Paolo Pani: [None].
UNAIDS position paper: Substitution Acknowledgements
maintenance therapy in the management
of opioid dependence and HIV/AIDS
This seminar summarises a training resource
prevention. Available at: wwwwhoint/ developed by Professor Icro Maremmani (Presi-
substance_abuse/publications/treatment/en/ dent of EUROPAD and AU-CNS) and Professor
indexhtml. Accessed on May 28, 2010.
Pier Paolo Pani (President of the Italian Society
114. Yahyavi-Firouz-Abadi N., See R. E. (2009): of Addiction Medicine) on behalf of the Basics
Anti-relapse medications: preclinical models on Addiction (BoA) Group: Francesco Auriem-
for drug addiction treatment. Pharmacol Ther. ma (Napoli), Jacopo Bizzarri (Bolzano), Pietro
124:(2) 235-247.
Casella (Roma), Lucia D'Ambrosio (Matera),
115. Yuferov V., Levran O., Proudnikov D., Nielsen Giovanna De Cerce (Campobasso), Stefano
D. A., Kreek M. J. (2010): Search for genetic Dell'Aera (Enna), Fernando Fantini (Lanciano),
markers and functional variants involved in the Paola Fasciani (Chieti), Michele Ferdici (Agri-
development of opiate and cocaine addiction gento), Giuseppe Filippone (Palermo), Piero Fun-
and treatment. Ann N Y Acad Sci. 1187 184- done (Melfi), Riccardo Gionfriddo (Siracusa),
Guido Intaschi (Viareggio), Francesco Lamanna
116. Zahm D. S. (2010): Pharmacotherapeutic (Pisa), Claudio Leonardi (Roma), Angelo Gio-
approach to the treatment of addiction: vanni Icro Maremmani (Pisa), Icro Maremmani
persistent challenges. MoMed. 107:(4) 276- (Pisa), Andrea Michelazzi (Trieste), Carlo Mine-
strini (Città di Castello), Franco Montesano (Ca-
tanzaro), Matteo Pacini (Pisa), Pier Paolo Pani
Role of the funding source
(Cagliari), Maria Chiara Pieri (Bologna), Vico
This initiative was supported by an unre- Rosolino Ricci (La Spezia), Francesco Ruffa
stricted educational grant from Reckitt Benckiser (Firenze), Alberto Santa Maria (Bari), Carmelo
Pharmaceuticals to the European Opiate Addic- Siragusa (Caserta), Lorenzo Somaini (Biella),
tion Treatment Association (EUROPAD) and the Luigi Stella (Napoli), Enrico Teta (Torino), And-
Association for the Application of Neuroscien- rea Vendramin (Padova). Editorial assistance was
tific Knowledge to Social Aims (AUCNS).
provided by Real Science Communications.
Received April 2, 2011 - Accepted May 3, 2011
Source: http://www.psichiatriaedipendenze.it/wp-content/uploads/2016/02/Maremmani_et_al_2011.pdf
Earth and Planetary Science Letters 287 (2009) 434–441 Contents lists available at Earth and Planetary Science Letters The relationship between riverine lithium isotope composition and silicateweathering rates in Iceland N. Vigier , S.R. Gislason K.W. Burton , R. Millot , F. Mokadem a CRPG-CNRS, Nancy-Université, 15 rue ND des Pauvres, 54501 Vandoeuvre les Nancy Cedex, Franceb Univ. of Iceland, Icelandc The Open University, Milton Keynes, UKd BRGM, Metrology, Monitoring, Analysis Division, 3 Av. Claude Guillemin, BP 6009, 45060 Orleans Cedex 2, France
Volume 5, Issue 2Marie's Story .page 1 Executive Director .page 4 Agents in my Brain .page 1 Day on the Hill .page 5 Legislative Update .page 2 Mediterranean Diet .page 5 Mental Health News .page 3 MHAM Stories .page 7 repeatedly that they don't take Section 8, even from properties that accepted Section 8 at one time. After hearing this message over and



