Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Effects of an ultraviolet b radiation absorber on photocarcinogenesis in hairless albino mice
Skin Pharmacol Physiol 2009;22:166–176
Received: September 19, 2008
Accepted after revision: February 20, 2009 Published online: April 8, 2009
Effects of an Ultraviolet B Radiation
Absorber on Photocarcinogenesis in
Hairless Albino Mice
G. Coelho Palermo Cunha a B. van Ravenzwaay a H.A. Tennekes b W. Mellert a
S. Schulte a S. Burkhardt a
a Experimental Toxicology and Ecology, BASF, Ludwigshafen , Germany; b Experimental Toxicology Services,
Nederland BV, Zutphen , The Netherlands
Key Words
Introduction
Photocarcinogenesis ⴢ Ultraviolet radiation ⴢ Skin tumor ⴢ Uvinul 쏐 T 150 ⴢ Hairless mice
Non-melanoma skin cancer (NMSC), the most com-
mon type of human cancer [1, 2] , is mainly caused by so-lar ultraviolet radiation (UVR) exposure [3, 4] . UVR
Abstract
reaching the surface of the earth contains about 5% UVB
Uvinul 쏐 T 150, a UVB absorber, was administered (concentra-
(290–320 nm) and 95% UVA (320–400 nm) radiation [5] .
tion 5%) in a vehicle to the skin of hairless albino mice before
Animal studies have implicated UVB as a predominant
ultraviolet radiation (UVR) exposure for 5 days per week in a
carcinogenic factor in NMSC [6, 7] . In such animal stud-
photocarcinogenicity study. Uvinul T 150 prolonged the la-
ies, UVB carcinogenic efficacy has been reported to peak
tency period to 50% skin tumor incidence (controls: 21–22
at 293 nm, which is very close to the limit of natural sun-
weeks; Uvinul T 150: 36 weeks in males and 31 weeks in fe-
light reaching the earth's surface. At wavelengths 6 340
males). When Uvinul T 150 was applied in an alternating-ex-
nm (UVA), the carcinogenic efficacy was reported be a
posure procedure (3 days/week before and 2 days/week af-
factor of 10 4 lower [8, 9] . Furthermore, UVB has been
ter UVR), the inhibition of photocarcinogenesis was less shown to cause mutations in the p53 tumor suppressor marked (latency period 28–30 weeks). The vehicle formula-
gene, which fosters tumor development by seriously com-
tion had no effect (latency period 20–21 weeks). The sensi-
promising its critical role in the orchestration of the cel-
tivity of the test system was demonstrated by a positive con-
lular responses to genotoxicity and cytotoxicity; over
trol (8-methoxy-psoralene). Although UVB absorption was
90% of human NMSC harbor the p53 mutation [3, 10–
shown to inhibit photocarcinogenesis, the results also sug-
gest that UVA radiation makes a contribution to skin tumor
Primary prevention of UVR exposure is the most ef-
Copyright 2009 S. Karger AG, Basel
fective means of reducing UVR carcinogenesis [15] . Uvi-nul 쏐 T 150 (ethylhexyl triazone) is a highly effective UVB absorber that is approved in Europe, Japan and Australia for use in sunscreens at concentrations of up to 5%. Its absorption spectrum ranges from 250 to 330 nm, with a
2009 S. Karger AG, Basel
Dr. B. van Ravenzwaay
Experimental Toxicology and Ecology
Fax +41 61 306 12 34
E-Mail
[email protected]
Accessible online at:
DE–67056 Ludwigshafen (Germany)
www.karger.com/spp
Tel. +49 621 605 6419, Fax +49 621 605 8134, E-Mail
[email protected]
Table 1. Composition of Uvinul T 150 (5% in vehicle formula-
high-performance liquid chromatography at the beginning and to-
wards the end of the study. No Uvinul T 150 crystals were detected, and the compound was shown to be distributed homogeneously in
the product. The Uvinul T 150 concentrations were found to be in the range of 94.5–104.3% of the nominal concentration.
Phase AEumulgin VL 75
lauryl glucoside, polyglyc-
Animals and Maintenance Conditions
Crl:SKH1-hr mice were supplied by Charles River Laborato-
ries, Sulzfeld, Germany. The age of the animals was approximate-
sodium cetearyl sulfate
ly 6–7 weeks at delivery and 10–11 weeks at the start of treatment.
The animals were singly housed in Makrolon 쏐 cages, supplied by
di-c12-13 alkyl tartrate
Becker & Co., Castrop-Rauxel, Germany. The bedding was type
c12-15 alkyl benzoate
3/4 dust-free, supplied by SSNIFF, Soest, Germany. The animals
were maintained in an air-conditioned room at a temperature of
ethylhexyl triazone
20–24 ° C, a relative humidity of 30–70% and a 12-hour light/12-
hour dark cycle. The animals were maintained on rat/mouse
maintenance ‘GLP' diet, supplied by Provimi Kliba, Kaiseraugst,
Switzerland, and tap water ad libitum. Food was assayed for
chemical as well as for microbiological contaminants. Drinking
magnesium aluminum
water was regularly assayed for chemical contaminants and the
presence of microorganisms. Bedding was regularly assayed for
contaminants (chlorinated hydrocarbons and heavy metals).
Water, demineralized water
Experimental Design
The study consisted of 5 groups of 24 male and 24 female hair-
phenoxyethanol, methyl-
less mice. The treatment schedule followed largely the protocol
paraben, ethylparaben,
described by Fourtanier [20] .
butylparaben, propyl-
Group 0 (no UVR) was not exposed to UVR and served as a
paraben isobutylparaben
negative control.
Group 1 (UVR) was exposed 5 days per week (Monday to Fri-
day) for 31 weeks to UVR from a sunlight simulator for 36 min, corresponding to 60% of the minimal erythema dose (MED) that had been established in a pre-study, and served as the positive
high absorption (A 1 1) at approximately 295–320 nm and
a peak at 314 nm. The US Food and Drug Administration
Group 2 (vehicle only before UVR) and group 3 (Uvinul T 150
(FDA), however, suggests testing chemicals which absorb in vehicle, before UVR) received 50 l vehicle and Uvinul T 150 UV light for phototoxicity or photocarcinogenicity [16] .
formulation, respectively, applied to skin on the back and sides
Therefore, the ability of Uvinul T 150 to reduce UVR car-
with a Multipette and dispersed with a glass rod, 30 min prior to the UVR treatment described above.
cinogenesis was investigated in a standard FDA-type pho-
Group 4 (Uvinul T 150 in vehicle, alternating before and after
tocarcinogenesis test using albino hairless mice [17–19] .
UVR) received 50 l Uvinul T 150 formulation on the back and sides 30 min prior to UVR treatment on Monday, Wednesday and Friday, and 30 min after UVR treatment on Tuesday and Thurs-day. The purpose of group 4 was to establish a potential dose-re-
Materials and Methods
sponse relationship concerning the interaction between UVR and Uvinul T 150. In this group, the total UVR dose and the total
Test Substance and Vehicle Formulations
amount of Uvinul T 150 applied was the same as in group 3; how-
Test Substance
. Uvinul T 150 (2,4,6-trianilino-
p -[carbo-2 ⴕ -
ever, there were 3 days of interaction per week (simultaneous
ethylhexyl-1 ⴕ -oxy]-1,3,5-triazine = 2,4,6-tris[
p -([2 ⴕ -ethylhexyl]-
UVR and Uvinul T 150 exposure), rather than 5 times per week
oxycarbonyl)anilino]-1,3,5-triazine; INCI name: ethylhexyl tri-
(as in group 3).
azone, C 48 H 66 N 6 O 6 , CAS No. 88122-99-0, ELINCS No. 402-070-
At the end of the 31-week treatment period, surviving animals
1) at a concentration of 5% was used in a vehicle formulation. The
were maintained for an additional 6 weeks prior to terminal sac-
sun protection factor of the formulation used in the studies re-
ported here was determined to be 14, according to the Colipa II
Moreover, a second positive control group (group 5) was used
(2003) test method.
to establish the sensitivity of the test system and also to detect
Vehicle . The composition of the vehicle formulation is present-
enhanced photocarcinogenesis. To this aim, 8-methoxypsoralen,
ed in table 1 . The vehicle formulation was stored at room tem-
which is a known photocarcinogenic substance, was adminis-
perature prior to use.
tered orally at a dose of 10 mg/kg body weight [21, 22] to hairless
The stability of the Uvinul T 150 formulation was checked albino Crl:SKH1-hr mice. The positive control substance was ad-
monthly. Homogeneity and concentration were determined by ministered 5 times every 2 weeks (according to a repetitive 2-week
Effects of a UVB Absorber on
Skin Pharmacol Physiol 2009;22:166–176
Photocarcinogenesis in Mice
dosing sequence for a maximum of 40 weeks, or until 50% of skin
Group 0 (No UVR, Negative Control) . Untreated hairless
tumor incidence). At 1.5 h after the administration of 8-methoxy-
mice developed no macroscopic skin abnormalities during
psoralen, the animals were exposed to UV radiation for 36 min
the 37-week observation period, and skin microscopy re-
vealed squamous hyperplasia in 1 female only ( table 2 ).
Irradiation Source and UVR Sensor
Group 1 (UVR, Positive Control) . Hairless mice ex-
Four TQ 1000 Z4 doped water cooled lamps, each one covered
posed to UVR developed a high incidence of (multi-)focal
by an optical borosilicate filter (QVF Process Systems, UK), were
ulcerated skin lesions ( table 2 ) and most group 1 animals
vertically suspended within a circular metal frame at the centre
were sacrificed for humane reasons before the end of the
of a special animal room. With the aid of the borosilicate filter, UV emission started at 290 nm with a first peak at 295 nm; thus,
31-week treatment period. The median survival time was
providing a UVR source without UVC. There was significant 26 weeks for both sexes ( table 2; fig. 1 ). The median skin emission of UVA, particularly at wavelengths of 350–380 nm.
tumor induction times (T 50 ), defined as a 50% prevalence
Consequently, the UVB:UVA ratio was 1: 10, which is comparable
of skin lesions of 5–10 mm in planar diameter, were 21
to natural sunlight. During exposure, the racks holding the ani-
and 22 weeks for males and females, respectively ( table 2 ;
mal cages were located approximately 2 m from the UVR source. All racks were irradiated simultaneously, with daily cage rotation
fig. 2 ). Histopathological examination of exposed skin
in the racks. Representative racks were monitored by customized
revealed a high incidence of squamous hyperplasia and
detector systems, which recorded UVA/UVB intensity as well as
squamous cell carcinomas ( table 2 ).
UVR biological effectiveness in MED per hour (biologically
Group 2 (Vehicle before UVR) . The administration of
weighted UVB detector from Solar Light, Glenside, Pa., USA).
vehicle formulation before UVR treatment (group 2) had
Clinical Observations
no effect on photocarcinogenesis. Median survival and
The general state of health of the animals was checked twice
skin tumor induction times were similar to those ob-
daily on working days and once daily during weekends or public
served in group 1.
holidays. A detailed inspection of the treated skin was carried out
Group 3 (Uvinul T 150 before UVR) . The administra-
weekly. Skin lesions were recorded using a mapping sheet. Mice
tion of Uvinul T 150 formulation before UVR treatment
with skin tumors 6 10 mm (planar diameter) or (multi-)focal skin ulcers were sacrificed for humane reasons. Body weights were de-
(group 3) delayed photocarcinogenesis, the median skin
termined at the start of the administration period and at weekly
tumor induction times being markedly higher than in
groups 1 and 2, i.e. 36 and 31 weeks for males and females, respectively ( table 2 ; fig. 2 ). In contrast to the fate of ani-
Histopathology A full necropsy was performed on all animals. The animals
mals in groups 1 and 2, most group 3 animals survived
were anesthetized under CO
the 31-week treatment period ( fig. 1 ), and no group 3 an-
, weighed, killed by decapitation,
exsanguinated and assessed for the presence of gross skin lesions.
imal was sacrificed with ulcerated skin lesions ( table 2 ).
Treated and untreated skin were fixed in 4% formaldehyde solu-
Histopathological examination of UVR exposed skin re-
tion and processed. Sections of the skin and gross lesions of the
vealed a lower incidence of squamous hyperplasia and
skin were prepared, stained with HE and examined by light mi-croscopy.
squamous cell carcinomas in group 3 than in groups 1 and 2 ( table 2 ).
Statistics
Group 4 (Uvinul T 150 before or after UVR). When
Body weights were analyzed by comparison of the each group
Uvinul T 150 formulation was delivered before irradia-
with the control group using the Dunnett's test (two-sided) for the
tion on 3 days and after irradiation on 2 days of the week,
hypothesis of equal means [23, 24] . Tumor incidences and time to 50% tumor incidence were analyzed using the Peto logrank test
the number of animals sacrificed with ulcerated skin le-
sions was reduced, whereas the number of animals sacri-ficed with skin tumors (10 mm) was increased in com-parison to groups 1 and 2 ( table 2 ). The median survival
times in group 4 were 34 and 36 weeks for males and fe-males, respectively ( table 2 ; fig. 1 ). The median skin tu-
Irradiation intensities at the site of the cages were 3.16 mor induction time in group 4 was higher than that seen
8 0.25 and 0.36 8 0.02 W/m 2 for UVA and UVB, respec-
in groups 1 and 2, but lower than that seen in group 3,
tively. On a weekly basis, these levels are equivalent to i.e. 28 and 30 weeks for males and females, respectively 341.3 and 38.9 KJ/m 2 . There were no treatment-related ( table 2 ; fig. 2 ). Histopathological examination of ex-effects on body weight development (data not shown). posed skin revealed an incidence of squamous hyperpla-Other clinical and microscopic observations are de-
sia and squamous cell carcinomas in group 4 similar to
scribed as follows:
that seen in groups 1 and 2 ( table 2 ).
Skin Pharmacol Physiol 2009;22:166–176
Coelho Palermo Cunha et al.
Table 2. Clinical and microscopic observations in a photocarcinogenesis study with Uvinul T 150 in hairless mice
negative control)
positive controla)
before or after UVRd)
Median survival time, weeks
Median time to tumor T50, weeks
Early deaths, ne 0
Due to skin ulcers
Due to skin tumors ≥10 mm
Animals with skin pathology, n
Squamous hyperplasia
Squamous cell papilloma
Squamous cell carcinoma
50 = Median skin tumor induction time, defined by a 50%
50 l Uvinul T 150 formulation applied to skin of the back
prevalence of skin lesions of 5–10 mm in planar diameter; n.d. =
and sides 30 min before UVR treatment.
not determinable within experimental observation period (37
d 50 l Uvinul T 150 formulation on the skin of the back and
sides 30 min before (Monday, Wednesday and Friday) or after
a Exposed daily, 5 days per week (Monday to Friday) for 31 (Tuesday and Thursday) UVR treatment.
weeks, to UVR from a sunlight simulator for 36 min, correspond-
e Mice that developed skin tumors ≥10 mm (planar diameter)
ing to 60% of the MED.
or (multi-)focal skin ulcers were sacrificed for humane reasons.
b 50 l vehicle formulation applied to skin of the back and sides
30 min before UVR treatment.
Characteristic pictures of normal skin, skin papillo-
Uvinul T 150 markedly prolonged the latency period to
mas and squamous cell carcinomas are shown in figures skin tumor development relative to unprotected or vehi-3–5 .
cle-only-treated UVR-exposed mice. When Uvinul T 150
Group 5 (8-Methoxypsoralen). The substance was ad-
was applied before or after UVR exposure on alternate
ministered orally at a dose of 10 mg/kg body weight 5 days of the week (group 4), the inhibition of photocar-times every 2 weeks (according to a repetitive 2-week dos-
cinogenesis was less marked. This finding was not en-
ing sequence for a maximum of 40 weeks, or until 50% tirely unexpected. The purpose of group 4 was to estab-skin tumor incidence) 36 min prior to UV radiation, and lish a potential dose-response relationship concerning resulted in a clear reduction in the median skin tumor the interaction between UVR and Uvinul T 150. In this induction time. The results of this study are shown in group, the total UVR dose and the total amount of Uvinul figure 6 . For reasons of comparison with the control T 150 applied was the same as in group 3 (5 times treat-group, the figures show the incidence of skin-tumor-
ment before UVR), however, there were 3 periods of in-
bearing animals related to the cumulative UV dose.
teraction (simultaneous UVR and Uvinul T 150 expo-sure) per week, rather than 5 (as in group 3). Thus, if there is an interaction, this should be less pronounced com-
Discussion
pared to a 5-day (full) protection. The observation of a dose-response relationship consequently strengthens the
The effect of Uvinul T 150, a highly effective UVB ab-
conclusion of an interaction – in the present case, a pro-
sorber, on photocarcinogenesis was investigated. When tective effect of the test substance. In principle, such a administered at a concentration of 5% in a vehicle formu-
dose-response assessment could also have been achieved
lation to the skin of hairless albino mice before UVR ex-
by omitting the Uvinul T 150 treatment after the UVR
posure at 60% of the MED on 5 days per week (group 3), exposure; however, with the present treatment scheme,
Effects of a UVB Absorber on
Skin Pharmacol Physiol 2009;22:166–176
Photocarcinogenesis in Mice
Mortality prevalence
Fig. 1. Mortality rates for male (
a ) and fe-
male (
b ) mice. Mice with skin tumors 6 10
Mortality prevalence
mm (planar diameter) or (multi-)focal
skin ulcers were sacrificed for humane
reasons. Group 1: exposed daily, 5 days per
week (Monday to Friday) for 31 weeks, to
UVR from a sunlight simulator for 36 min,
60% of the MED. Group 2: 50
formulation applied to skin of the back and sides 30 min prior to UVR treatment.
Group 3: 50 l Uvinul T 150 formulation
applied to skin of the back and sides 30 min prior to UVR treatment. Group 4: 50
l Uvinul T 150 formulation on the skin of
the back and sides 30 min prior to (Mon-
day, Wednesday and Friday) or after (Tues-day and Thursday) UVR treatment.
the total dose of the chemical applied to the animal re-
both in male and female albino hairless mice. The latency
mained constant, which enhances comparability with periods of 21 and 22 weeks in male and female UVR con-group 3.
trols and 20 and 21 weeks for the UVR vehicle groups are
As the total UVR dose and the total amount of Uvinul
similar to those reported by others. Sambuco et al. [18]
T 150 was equal in both studies, the difference in latency reported, for a group exposed to 1,200 RBU (Robertson-period is only related to the time of administration (5 ad-
Berger units), T 50 values of approximately 25 and 24
ministrations/week prior to UVR vs. 2 days/week prior +
weeks for males and females, respectively. Ananthaswa-
3 days/week after UVR treatment). These results indicate
my [14] reported a T 50 value of 25 weeks for female C3H
that the protective effect is only achieved if Uvinul T 150 mice [20] and a T 50 value of 20 weeks when exposed to 2.7 is administered prior to UVR exposure. This is not sur-
MED/week. A shorter latency period (of approximately
prising given the fact that Uvinul T 150 absorbs the harm-
16–17 weeks) was reported by van Kranen et al. [26] in
ful UVB radiation and protects against sunburn (as a cell
hairless mice. These differences in T 50 values can be part-
proliferation stimulus) and consequently against UVB-
ly explained by the strain of mouse used, the nature of
induced mutations.
UVR treatment, as well as by the criteria used to diagnose
The vehicle formulation had no effect on photocar-
skin tumors (i.e. the size of non-melanoma skin tumors).
cinogenesis. The absence of a vehicle effect on photocar-
The effect of skin tumor size on positive diagnosis was
cinogenesis is not simply a matter of course [18] . Sam-
elegantly demonstrated by Sambuco et al. [18] showed
buco et al. [18] reported vehicle-enhanced skin tumor de-
skin tumor prevalence curves over time for skin tumor
velopment in 3 out of 8 photocarcinogenicity studies, sizes of 1 1, 1 2 and 1 4 mm run virtually parallel, but were
Skin Pharmacol Physiol 2009;22:166–176
Coelho Palermo Cunha et al.
Skin tumor prevalence
Skin tumor prevalence
Fig. 2. Tumor rates for male (
a ) and female
(
b ) mice (tumors 6 5 to 10 mm in planar
diameter). Group 1: exposed daily, 5 days
per week (Monday to Friday) for 31 weeks,
to UVR from a sunlight simulator for 36
min, 60% of the MED. Group 2: 50 l ve-hicle formulation applied to skin of back
and sides 30 min prior to UVR treatment.
Group 3: 50 l Uvinul T 150 formulation applied to back and sides 30 min prior to UVR treatment. Group 4: 50 l Uvinul
T 150 formulation on back and sides 30
min prior to (Monday, Wednesday and
Friday) or after (Tuesday and Thursday) UVR treatment.
delayed by about 4 weeks (i.e. longer latency period) with is the evaluation of the interaction between UVR and the each larger size of tumors used to determine the T 50 val-
sunscreen. This interaction can be protective, which may
ue. Consequently, tumor size would not appear to be crit-
be expected of a sunscreen; however, the modification of
ical for diagnosis of treatment-related effects within a the sunscreen by the absorption of UVR could, in prin-study. However, standardization of animal strain, UVR ciple, also result in an activation of the UV-absorbing dose and tumor size would greatly facilitate inter-study chemical, resulting in an enhancement of skin tumor for-comparisons.
mation. Some compounds, such as the psoralenes, are
The sensitivity and ability of this photocarcinogenic-
known for such a reaction. Thus, to demonstrate that the
ity study to detect an enhancement in skin tumor forma-
test system (the mouse strain and treatment conditions)
tion was demonstrated by the positive control 8-me-
used in these investigations was sensitive enough to de-
thoxypsoralen. In both males and females, there was a tect a reduction in the latency time, 8-methoxypsoralen clear and statistically significant reduction in the tumor was used as a positive control. As the UVR system em-latency period. The regulatory background of our study ployed in this study has an emission spectrum that also
Effects of a UVB Absorber on
Skin Pharmacol Physiol 2009;22:166–176
Photocarcinogenesis in Mice
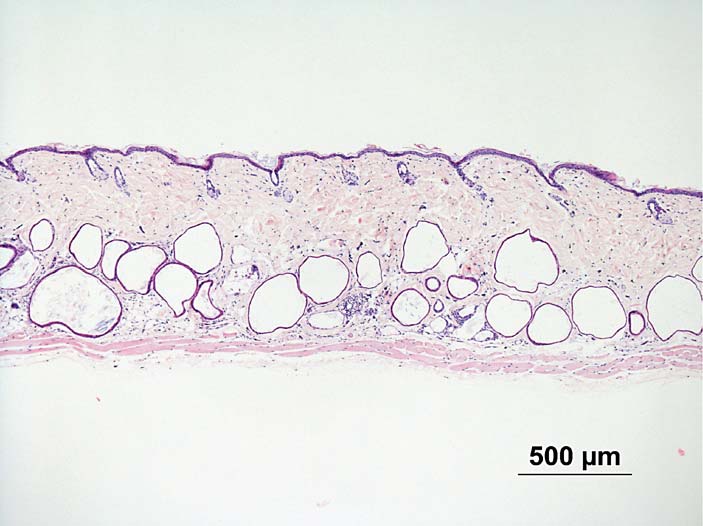
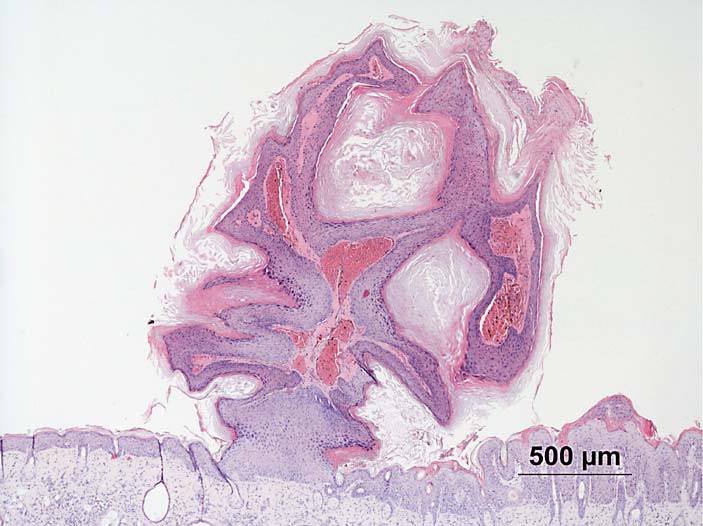
Fig. 3. Skin of male control hairless albino
mouse, without UV radiation. HE. ! 40.
Fig. 4. Skin of female hairless albino mouse
after UV radiation; squamous cell papil-
loma. HE. ! 40.
covers UVA, 8-methoxypsoralen (which enhanced skin could thus be argued that this extreme part of the UVB tumor formation upon exposure to UVA) was a suitable spectrum may not have been adequately tested. However, compound for this purpose.
as the absorption of UVR by Uvinul T 150 already starts
The emission spectrum of the UVR system used in at 250 nm, and is quite pronounced at 290 nm (A 1 0.75)
these studies has a first peak at 295 nm, with minor it is unlikely that the minor UVR exposure at 290 nm amounts of UVR emitted at a wavelength of 290 nm. It would have affected the study. Increased UVR exposure
Skin Pharmacol Physiol 2009;22:166–176
Coelho Palermo Cunha et al.
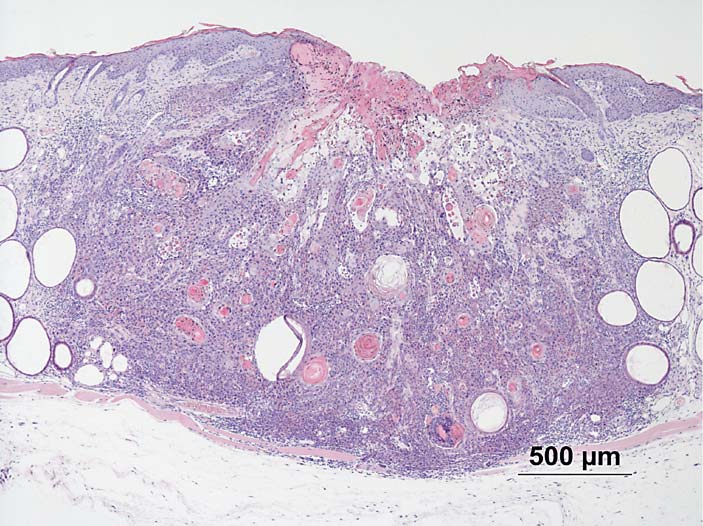
Fig. 5. Skin of female hairless albino mouse
after UV radiation; squamous cell carci-
noma. HE. ! 40.
at 290 nm, however, would certainly have resulted in a
reduced latency period for unprotected control animals.
where the exponent n reflects time-associated accelera-
Our results indicate that Uvinul T 150 delayed photo-
tion of the carcinogenic process, and is always greater
carcinogenesis by UVB absorption. However, despite the than 1.
significant increase in time T 50 , Uvinul T 150 did not ful-
UVR carcinogenic efficacy has been reported to peak
ly prevent UVR-induced skin tumor development. Con-
at 293 nm (UVB) and to be a factor of 10 4 lower with
sequently, the results of the present study suggest that UVA1 at wavelengths 6 340 nm ( [8, 9] , but the Druckrey
UVA also could contribute to skin cancer induction. Al-
equation (2) indicates much stronger time-associated ac-
though most studies conducted so far have focused on celeration of the carcinogenic process with UVA1 (where
UVB as inducer of NMSC, there is experimental evidence
n = 2.9) than with UVB (where n = 1.6). Some reports in-
that UVA1 may also cause DNA damage (possibly via re-
dicate that the contribution of solar UVA to human
active oxygen species) as well as squamous cell carcino-
NMSC may have been underestimated [34] . Based on se-
mas in albino hairless mice [8, 9, 27–30] . The quantitative
quencing of the p53 gene in keratinocytes from solar ker-
aspects of UVA1- and UVB-induced skin carcinogenesis atoses and squamous cell carcinomas, it was shown that
are of interest in this context. The relationship between UVA and UVB caused similar numbers of p53 gene mu-
the daily dose (D) and the median skin tumor induction tations in both benign and malignant human skin tu-
time (T) for both UVA1 [9] and UVB [27] has been dem-
mors, with UVB-induced mutations being restricted to
the upper areas of the tumors and UVA-induced muta-tions predominating at the basal germinal layer [35, 36] .
Penetration of UVA to the dividing basal/stem cell layer
may be important to fix acute DNA damage as heritable
genomic mutations. Hence, the UVA waveband of sun-light is likely to contribute to skin tumor development in
where r = 0.62 for UVB and 0.35 for UVA1 and 1/r = 1.6 humans. Several lines of research also point in this direc-for UVB and 2.9 for UVA1. Equation 2 is identical to that
tion; UVA radiation is associated with the intracellular
established for chemical carcinogens in a single dose and
formation of oxygen radicals resulting in both cellular as
chronic experiments by Druckrey et al. [32–34] :
well as genetic damage, e.g. DNA strand breaks and T–G
Effects of a UVB Absorber on
Skin Pharmacol Physiol 2009;22:166–176
Photocarcinogenesis in Mice
umor-bearing animals (%)T
Cumulative irradiation (J/m2)
mor-bearing animals (%)u
Fig. 6. Impact of 8-methoxypsoralen on
skin tumors in hairless male ( a ) and female
Cumulative irradiation (J/m2)
( b ) mice. d = Control + UV; * = 8-me-
thoxypsoralen + UV.
transitions in the p53 gene [5, 37] . An additional aspect, erties of UVA radiation (tumor promotion – induced by which is likely to be involved in UVR enhancement of cellular damage and protein kinase C induction [39] , im-skin carcinogenesis, and particularly melanoma develop-
munosuppression and a potentially weak genotoxic ef-
ment, is its induction of immunosuppression [38] . Con-
fect, most likely related to oxidative DNA damage) is the
sequently, it is likely that the combination of several prop-
cause of skin tumor development. The particular impor-
Skin Pharmacol Physiol 2009;22:166–176
Coelho Palermo Cunha et al.
tance of oxidative stress in UVR-induced skin carcino-
formulations with UVB absorbers only, as well as those
genesis has been demonstrated [40] . In this study, UVR-
containing UVA and UVB absorbers. These authors in-
treated hairless mice were given either control or lutein/
ferred that blocking out UVA did not provide increased
zeaxanthin-enriched diets. The carotenoids lutein and photoprotection against p53 mutations or skin cancer de-zeaxanthin are structurally related to  -carotene and
velopment. However, their data showed that it may have
have superior antioxidant properties.  -Carotene and vi-
been difficult to estimate the added protective effect of
tamin C have demonstrated their antioxidative proper-
UVA sunscreens because of the small number of affected
ties in chemoprevention trials. The results of the UVR animals.
studies showed a increased tumor-free survival time, an
In conclusion, the results of the present study indicate
increased T 50 value (approximately 17 vs. 20 weeks) and that the UVB filter Uvinul T 150 provides protection reduced tumor multiplicity and tumor volume in ani-
against UVR-induced skin cancer, but also that a combi-
mals receiving the lutein/zeaxanthin-enriched diets [41] .
nation of UVA and UVB absorbers merits further inves-
Moreover, Fourtanier [20] reported inhibition of photo-
carcinogenesis in hairless mice by topical application of the broad UVA absorber Mexoryl SX, whereas the inhib-itory effects of the UVB absorber 2-EHMC (2-ethylhexyl-
Acknowledgments
p-methoxycinnamate) were found to be less marked. An-anthaswamy and colleagues [42] reported inhibition of
We would like to gratefully acknowledge the excellent techni-
cal assistance of Peschl UV Consulting, and Gunther Rank and
solar simulation-induced p53 mutations and protection Martina Schalber of BASF Experimental Toxicology and Ecol-
against skin cancer development in C3H mice by sun-
screens with human sun protection factors 15–22 using
References
1 Miller DL, Weinstock MA: Nonmelanoma
8 De Gruijl FR, Sterenborg HJ, Forbes PD, Da-
15 Molho-Pessach V, Lotem M: Ultraviolet ra-
skin cancer in the United States: incidence. J
vies RE, Cole C, Kelfkens G, van Weelden H,
diation and cutaneous carcinogenesis: en-
Am Acad Dermatol 1994; 30: 774–778.
Slaper H, van der Leun JC: Wavelength de-
vironmental factors in skin diseases. Curr
2 Strom S: Epidemiology of basal and squa-
pendence of skin cancer induction by ultra-
Probl Dermatol 2007; 35: 14–27.
mous cell carcinomas of the skin; in Weber
violet irradiation of albino hairless mice.
16 Howard PC, Sams RL, Bucher JR, Allaben
R, Miller M, Goepfert H (eds): Basal and
Cancer Res 1993; 53: 53–60.
WT: Phototoxicology and photocarcinogen-
Squamous Cell Skin Cancers of the Head and
9 De Laat A, van der Leun JC, de Gruijl FR:
esis at the US Food and Drug Administra-
Neck. Baltimore, Williams and Wilkins,
Carcinogenesis induced by UVA (365 nm)
tion's National Center for Toxicological Re-
radiation: the dose-time dependence of tu-
search. J Food Drug Anal 2002; 10: 252–257.
3 Brash DE, Rudolph JA, Simon JA, Lin A,
mor formation in hairless mice. Carcinogen-
17 Sambuco CP, Davies RE, Forbes PD, Hober-
McKenna GJ, Baden HP, Halperin AJ, Pon-
esis 1997; 18: 1013–1020.
man AM: Photocarcinogenesis and consum-
ten J: A role for sunlight in skin cancer: UV-
10 Pfeifer GP, You YH, Besaratinia A: Muta-
er product testing: technical aspects. Toxicol
induced p53 mutations in squamous cell
tions induced by ultraviolet light. Mutat Res
Mech Methods 1991; 1: 75–83.
carcinoma. Proc Natl Acad Sci 1991;
2005; 571: 19–31.
18 Sambuco CP, Forbes PD, Davies RE, Learn
11 Gervin CM, McCulla A, Williams M, Ouhtit
DB, D'Aloisio LC, Arocena M, Hoberman
4 Parrish JA, Jaenicke KF, Anderson RR: Ery-
A: Dysfunction of p53 in photocarcinogen-
AM: Photocarcinogenesis: measuring the re-
thema and melanogenesis action spectra of
esis. Front Biosci 2003; 8: 715–717.
producibility of a biologic response to ultra-
normal human skin. Photochem Photobiol
12 De Gruijl FR: p53 mutations as a marker of
violet radiation exposure in mice. Front
1982; 36: 187–191.
skin cancer risk: comparison of UVA and
Biosci 2003; 8: 26–33.
5 Maier T, Korting HC: Sunscreens – which
UVB effects. Exp Dermatol 2002; 11(suppl
19 Forbes DF, Beer JZ, Black HS, Cesarini JP,
and what for? Skin Pharmacol Physiol 2005;
Cole CA, Davies RE, Davitt JM, de Gruijl F,
13 Ouhtit A, Muller K, Davis DW, Ullrich SE,
Epstein J, Fourtanier A, Green A, Koval T,
6 Kligman LH, Kligman AM: The nature of
McConkey D, Ananthaswamy HN: Tempo-
Ley RD, Mascotto R, Morison W, Osterberg
photoaging: its prevention and repair. Pho-
ral events in skin injury and the early adap-
R, Sliney D, Urbach F, van der Leun JC,
todermatol 1986; 3: 215–227.
tive responses in ultraviolet-irradiated
Young AR: Standardized protocols for pho-
7 Forbes PD: Experimental photocarcinogen-
mouse skin. Am J Pathol 2000; 156: 201–207.
tocarcinogenesis safety testing. Front Biosci
esis: an overview. J Invest Dermatol 1981; 77:
14 Ananthaswamy HN, Loughlin SM, Cox P,
2003; 8: 848–854.
Evans RL, Ullrich E, Kripke MI: Sunlight
20 Fourtanier A: Mexoryl SX protects against
and skin cancer: inhibition of p53 mutations
solar-simulated UVR-induced photocarc
in UV-irradiated mouse skin by sunscreens.
nogenesis in mice. Photochem Photobiol
Nature Med 1997; 3: 510–514.
1996; 64: 688–693.
Effects of a UVB Absorber on
Skin Pharmacol Physiol 2009;22:166–176
Photocarcinogenesis in Mice
21 Klecak G, Urbach F, Urwyler H: Fluoroqui-
29 Kelfkens G, de Gruijl FR, van der Leun JC:
37 Halliday GM, Agar NS, Barnetson RS, Anan-
nolone antibacterials enhance UVA-induced
Tumourigenesis by short wave ultraviolet-A:
thaswamy HN, Jones AM: UV-A fingerprint
skin tumors. J Photochem Photobiol B 1997;
papillomas versus squamous cell carcino-
mutations in human skin cancer. Photochem
mas. Carcinogenesis 1991; 12: 1377–1382.
Photobiol 2005; 81: 3–8.
22 Chignell CF, Haseman JK, Sik RH, Tennant
30 De Gruijl FR: Photocarcinogenesis: UVA vs.
38 Peak JG, Peak MJ: Ultraviolet light induces
RW, Trempus CS: Photocarcinogenesis in
UVB radiation. Skin Pharmacol Appl Skin
double-strand breaks in DNA of cultured
the Tg.AC mouse: lomefloxacin and 8-me-
Physiol 2002; 15: 316–320.
human P3 cells as measured by neutral filter
thoxypsoralen. Photochem Photobiol 2003;
31 Dunnett CW: A multiple comparison proce-
elution. Photochem Photobiol 1990; 52: 387–
dure for comparing several treatments with
23 Dunnett CW: A multiple comparison proce-
a control. J Am Stat Assoc 1955; 50: 1096–
39 Kornhauser A, Wamer WG, Lambert LA:
dure for comparing several treatments with
Light-induced dermal toxicity: effects on
a control. J Am Stat Assoc 1955; 50: 1096–
32 Druckrey H: Quantitative aspects of chemi-
cellular and molecular levels; in Marzulli
cal carcinogenesis. UICC Monograph 1967;
FN, Maibach HI: Dermatotoxicology, ed 7.
24 Dunnett CW: New tables for multiple com-
Abington, Taylor & Francis, 2007.
parisons with a control. Biometrics 1964; 20:
33 Druckrey H, Preussmann R, Ivankovic S,
40 Matsui MS, Deleo VA: Induction of protein
Schmähl D: Organotropic carcinogenic ef-
kinase C activity by ultraviolet radiation.
25 Kalbfleisch J, Prentice RL: The Statistical
fects of 65 different N-nitroso compounds in
Carcinogenesis 1990; 11: 229–234.
Analysis of Failure Time Data, ed 2 (Wiley
BD rats. Z Krebsforsch 1967; 69: 103–210.
41 Astner S, Wu A, Chen J, Philips N, Rius-Diaz
Series in Probabilities and Mathematical
34 Druckrey H, Schagen B, Ivankovic S: Erzeu-
F, Parrado C, Mihm MC, Goukassian DA,
Statistics). Hoboken, John Wiley and Sons,
gung neurogener Malignome durch einma-
Pathak MA, González S: Dietary lutein/zea-
lige Gabe von Äthyl-nitrosoharnstoff (ÄNH)
xanthin partially reduces photoaging and
26 van Kranen HJ, Westerman A, Berg RJW,
an neugeborene und junge BD-IX Ratten. Z
photocarcinogenesis in chronically UVB-ir-
Kram N, van Kreijl CF, Wester PW, de Gruijl
Krebsforsch 1970; 74: 141–161.
radiated Skh-1 hairless mice. Skin Pharma-
FR: Dose-dependent effects of UVB-induced
35 Berg RJW, de Gruijl FR, van der Leun JC: In-
col Physiol 2007; 20: 283–291.
skin carcinogenesis in hairless p53 knockout
teraction between ultraviolet A and ultravio-
42 Ananthaswamy HN, Ullrich SE, Mascotto
mice. Mutat Res 2005; 571: 81–90.
let B radiations in skin cancer induction in
RE, Fourtanier A, Loughlin SM, Khaskina P,
27 Sterenborg HJCM, van Weelden H, van der
hairless mice. Cancer Res 1993;
Bucana CD, Kripke ML: Inhibition of solar
Leun JC: The dose-response relationship for
simulator-induced p53 mutations and pro-
tumourigenesis by UV radiation in the re-
36 Agar NS, Halliday GM, Barnetson RS, Anan-
tection against skin cancer development in
gion 311–312 nm. J Photochem Photobiol B
thaswamy HN, Wheeler M, Jones AM: The
mice by sunscreens. J Invest Dermatol 1999;
1988; 2: 179–194.
basal layer in human squamous tumors har-
112: 763–768.
28 Sterenborg HJCM, van der Leun JC: Tumor-
bors more UVA than UVB fingerprint mu-
igenesis by a long wave UV-A source. Photo-
tations: a role for UVA in human skin carci-
chem Photobiol 1990; 51: 325–330.
nogenesis. Proc Natl Acad Sci 2004;
Skin Pharmacol Physiol 2009;22:166–176
Coelho Palermo Cunha et al.
Source: http://www.toxicology.nl/images/publications/UV_CARCINOGENESIS_PAPER_2009.pdf
Thank you for purchasing IMAX B8 charger. This is h i g h - s p e e d r e c h a r g e / d i s c h a r g e e q u i p m e n t manufactured with high technology & professional control software. It enables you maintain your battery with optimal status with high level of safety. Optimized user interface When charging or discharging, B8 has an function that sets the feeding
Serving People with Developmental Disabilities since 1978 on a World-renowned saxophonist Branford Marsalis made a very special appearance at the newly renovated Kodak Hall at Eastman Theatre to receive the 13th Annual Lifetime Inspiration Award. The award, bestowed annually by the Lifetime Assistance Foundation, was presented to Branford Marsalis by WXXI's Thomas Hampson and Patrick Burke, Regional President of First Niagara Financial Group and Chairman of the Foundation Board.



