Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Tick-borne disease treatment
Vet Times
The website for the veterinary profession
http://www.vettimes.co.uk
TICK-BORNE DISEASE TREATMENT
Categories :
Date : August 24, 2009
Hany M Elsheikha concludes his two-part article on ticks and tick-borne diseases, and explainshow the future of vaccine development looks exciting
IN part one (VT 39.19), the author discussed the biology, ecology, and veterinary
significance of the most prevalent tick species in the UK, Ixodes ricinus.
In this second part, the author focuses on the pathogenic agents transmitted by
I ricinus andpresents treatment options for diseases caused by these pathogens.
Ticks play an indirect role in the transmission of infectious agents, such as viruses, bacteria,protozoa and helminths, which are common to both humans and animals. Their important role indisease transmission is reinforced by ticks having a worldwide distribution and being adaptable toany kind of environment or host.
Transovarian (adult female to egg/larva) and trans-stadial (larva to nymph, nymph to adult)transmission are the mechanisms by which
I ricinus can contribute to the survival and maintenanceof pathogen populations and facilitate transmission to susceptible hosts. Transovarian transmissionof
Babesia divergens from adult
I ricinus ticks to larvae does occur and is believed to be importantin maintaining the life cycle of other tick-borne viral and rickettsial pathogens.
Trans-stadial transmission occurs following the acquisition of other pathogens, such as
Babesia. Intrans-stadial transmission, nymphs and adult ticks infected in a previous life stage emerge infectiveafter moulting, and subsequently transmit disease to other hosts during feeding. The mostimportant disease organisms transmitted to animals by
I ricinus are:
•
Anaplasma phagocytophila (tick-borne fever of ruminants, formerly known as Ehrlichia);
•
Borrelia burgdorferi (causative agent of Lyme disease);
•
B divergens (causes redwater fever in cattle);
•
Staphylococcus aureus (tick pyaemia of sheep); and
•louping-ill virus (causes encephalitis in sheep and grouse).
I ricinus ticks are not host-specific and feed on different host species. They have non-specificfeeding habits-not only feeding on species that are reservoirs for multiple tick-borne pathogens(such as small mammals), but also humans. Therefore, these non-specific feeders are moreimportant as vectors of human disease than host-specific ticks, which are less likely to bitehumans.
Pathogens of human diseases transmitted by
I ricinus include:
•
Anaplasma phagocytophilum (causes granulocytic anaplasmosis in humans);
•
Rickettsia conorii (boutonneuse, or Mediterranean tickbite fever);
•
Coxiella burnetii (Q-fever);
•
Francisella tularensis (tularaemia);
•
B burgdorferi and tick-borne encephalitis; and
•Tettnang, Eyach and Uukuniemi viruses.
Treatment of diseases transmitted by I ricinus
Treatment of human diseases transmitted by
I ricinus is out of the scope of this article; therefore,treatment options are limited to
I ricinus-borne diseases of relevance to animal health and welfarein the UK.
•
Tick-borne fever or anaplasmosis
– Tick-borne fever (TBF) is a febrile disease of domestic and free-living ruminants in the UK andother European countries.
– It is caused by the bacterium
Anaplasma phagocytophilum ().
– The disease affects cattle, sheep and horses; in dogs, anaplasmosis leads to fever, anorexia,joint pain and swelling.
– The disease suppresses the immune system in young lambs, predisposing infected animals toother infections, such as loupingill and tick pyaemia.
– As there is no colostral protection and immunity builds up as animals age, young animals are atthe greatest risk of disease in endemic areas.
– Diagnosis is based on clinical signs, history of exposure to ticks and microscopic examination ofblood smears.
– Short-acting oxytetracyclines are the most effective option.
– If dairy cattle are treated with oxytetracyclines within a few days of infection, the pyrexia isreduced quickly and milk yield restored.
– Other antibiotics, such as penicillin, streptomycin and ampicillin, do not prevent relapses.
– Sulfamethazine has also proved useful.
– Treatment with long-acting tetracyclines may be used as a prophylactic measure in enzooticareas.
•
Borreliosis (Lyme disease)
– This is a common tick-borne zoonotic disease of dogs (it rarely appears in cats).
– Lyme disease is caused by the spirochaete bacterium
B burgdorferi ().
– The disease causes fever, lameness, arthritis, renal failure and meningitis.
– There is no persuasive evidence that
B burgdorferi infection is transmitted in utero in dogs.
– Once an animal contracts Lyme disease, urgent treatment with antibiotics is required.
– Diagnosis is based on history of tick exposure, clinical signs and a demonstration of borrelialinfection, using either direct (isolation, PCR) or indirect (serology) antibody-based tests.
– Doxycycline (10mg/kg) or amoxicillin (20mg/kg) are the drugs of choice.
– Other effective drugs include azithromycin, cephalexin, amoxicillin, tetracycline, penicillin G andchloramphenicol.
– NSAIDs may also be used for symptomatic treatment.
– There is no vaccine against borreliosis for dogs in the UK.
– Doxycycline should not be used in very young animals because it can cause staining of the teeth.
– If the disease is untreated, nervous system, arthritic or other complications may develop weeksor months after the infection.
– Treatment of some later complications of Lyme disease may require antibiotics to be given byinjection for several weeks.
– The disease may recur, with intervals of weeks to months, but should respond to antibiotictreatment each time.
– Full recovery may take some time, as damaged tissue takes time to heal, especially in thechronic stage of the disease.
– Antibiotic therapy does not eliminate the
B burgdorferi bacteria responsible for Lyme disease, butit does suppress the bacteria so that symptoms subside.
•
Babesiosis (redwater fever)
– In the UK, babesiosis is often caused by
Babesia divergens ().
–
B divergens is a serious pathogen for cattle in the UK and northern Europe.
– It is an intraerythrocytic protozoan parasite that is transmitted and spread between cattle by thetick
I ricinus.
– This disease is usually first reported in May or June, when its tick host becomes active.
–
B divergens produces a disease syndrome similar to
B bigemina and
B bovis; however, thecerebral form is rarely seen.
– Redwater fever is often only noticed at the onset of haemoglobinuria, when the disease is at anadvanced stage.
– It not only causes significant losses to the cattle industry, but can also infectimmunocompromised humans, with serious consequences.
– Diagnosis is based on clinical signs, history of exposure to ticks and microscopic examination ofblood smears.
– Mild cases may recover without treatment.
– More severe babesiosis cases require treatment, and imidocarb dipropionate is the drug ofchoice. This is often best combined with a preventive treatment for the unaffected cattle.
– Therapy and blood transfusions will generally save an affected animal, even at an advancedstage of the disease.
– Live vaccines have proven effective and reasonably safe, particularly when vaccination wasrestricted to cattle less than a year old, when they still have natural resistance to the disease.
However, live vaccines have several limitations, the most obvious being that the vaccine itself maycause babesiosis unless its virulence can be controlled.
– A vaccine is not yet available in the UK.
– Imidocarb is most toxic when given intravenously; therefore, intramuscular or subcutaneousadministration is generally recommended.
– Imidocarb is associated with residue problems, especially when used for chemoprophylaxis,which has led to the withdrawal of imidocarb in several European countries.
– Long-acting oxytetracycline against bovine
B divergens infections has no therapeutic effect ifgiven after the parasitaemia has become patent. Despite this, continuous prophylacticadministration allows sufficient numbers of parasites to multiply for antibodies to be produced while
clinical effects are absent.
– The use of continuous oxytetracycline administration is too costly and risky due to the likelihoodof resistance development in bacterial pathogens.
– Trials to control
B divergens infection in calves by stimulating concurrent non-specific immunityfailed.
•
Staphylococcus aureus
– Tick pyaemia in sheep is caused by the bacterium
S aureus, an inhabitant of the normal skin,which enters the bloodstream by the biting action of the blood-sucking nymphal stage of the tick
Iricinus.
– This disease affects lambs born in, or introduced to, a tickinfested area, at the time tick activity isat its greatest (usually in the spring).
– There is evidence that tickborne fever infection, which causes profound leucopaenia, maypredispose tick pyaemia.
– This disease can result in skin abscesses, but in immunosuppressed animals (such as those withtick-borne fever), the bacteria can spread into joints and the spinal column, causing arthritis or evenparalysis.
– Lambs may be affected by both tick pyaemia and joint-ill, especially if the ewes are in poorcondition and have insufficient colostrum.
– Diagnosis is based on history and postmortem, and a bacterial culture of the infected joint.
– Parenteral administration of penicillin or tetracycline can be effective, provided the lesions are nottoo advanced.
– Severe cases should be euthanised.
– Local treatment of affected joints is often disappointing because, by the time lesions are noticed,irreversible damage to the joint may have occurred.
– Long-acting antibiotics, to which particular strains of
S aureus agents are sensitive, can be used
prophylactically, although care should be taken not to underdose to avoid the development ofbacterial resistance.
– Both benzathine penicillin and long-acting tetracyclines are effective against
S aureus, while thelatter are also effective against the rickettsial agent of tickborne fever.
– Louping-ill (ovine encephalomyelitis, infectious encephalomyelitis of sheep, trembling-ill) is anacute viral disease, primarily of sheep, caused by a neurotropic single-strand RNA flavivirus.
– It is characterised by a biphasic fever, depression, ataxia, muscular incoordination, tremors,posterior paralysis, coma and death.
– Louping-ill is a tick-transmitted disease, the occurrence of which is closely related to thedistribution of the sheep tick
I ricinus, which requires an environment with a high relative humidity.
– Louping-ill can affect sheep of all ages and can infect a range of hosts (it is an important diseasein grouse).
– It is endemic in rough, upland areas in Scotland, northern England, Wales and Ireland.
– Colostrum will protect lambs in areas where ewes have been exposed to the virus and, therefore,clinical signs will generally be seen in weaned lambs. Young lambs that have not receivedsufficient colostrum are also susceptible to disease, as are naïve sheep of any age brought into anendemic area.
– Although cases of louping-ill can occur at any time of year, the disease is most prevalent duringperiods of maximal tick activity
– between April and June, and again in September.
– Diagnosis is based on clinical grounds, history of an introduction to tick-infested pastures in anendemic area, serological assays and reverse transcription PCR.
– Louping-ill virus is transmissible to humans.
– No specific treatment for louping-ill virus infection is effective. Unlike sheep, cattle affected bylouping-ill may respond favourably to good nursing and symptomatic treatment.
– If there is evidence that susceptible animals have been exposed to the disease, administration ofthe louping-ill antiserum within 48 hours of exposure to the infection is recommended.
– Recumbent animals must be euthanised.
– A formalin-inactivated commercial vaccine is available that has been used successfully for manyyears in endemic areas.
– Two doses of vaccine, with an interval of two to eight weeks between injections, is recommendedto achieve optimal protection against natural infection.
– Vaccination of pregnant ewes during the last trimester is advocated to ensure lambs receivemaximal levels of passively acquired antibodies and are protected during the initial critical monthsof life.
Despite the veterinary and zoonotic importance of tickborne diseases (TBDs), relatively littleresearch has been carried out on the ticks and diseases associated with them, and many questionsregarding tick epidemiology and the host's response remain unanswered.
A better understanding of the species' biology and hostparasite interactions may lead to improvedcontrol mechanisms and new trends in TBD management. Development of acaricide resistance isa serious problem in the control of several TBDs, although the real extent of the problem isunknown.
Characterisation of the tick genomes will have a great impact on the discovery of new protectiveantigens. The future of tick vaccine development research is exciting because of new andemerging technologies for gene discovery, vaccine formulations and delivery methods. Any controlstrategy should be based on knowledge of the local tick ecology, which would improve theefficiency of the control programme, reduce the risk of acaricide resistance and environmentalpollution, and cut costs considerably.
• Ticks are distributed worldwide and impact on human and animal health, as well as food animalproduction.
• Ticks can transmit various diseases to several animal species and humans.
•
I ricinus is a good example of a tick vector with multiple pathogens affecting companion animals,livestock and humans.
• Apart from the use of antimicrobials against TBD, prevention is based mainly on adequate tickcontrol.
• For economic and animal welfare reasons, the best option is to take preventive measures, ratherthan treat TBD.
• Ideal control strategies should be directed towards both tick control and the blocking of pathogentransmission.
• Integrated control (identification of risk areas, tick control, chemoprophylaxis and vaccination)should be considered in endemic TBD areas.
Brocklesby D W and Purnell R E (1977). Failure of BCG to protect calves against Babesia
divergens infection,
Nature (London) 265: 343.
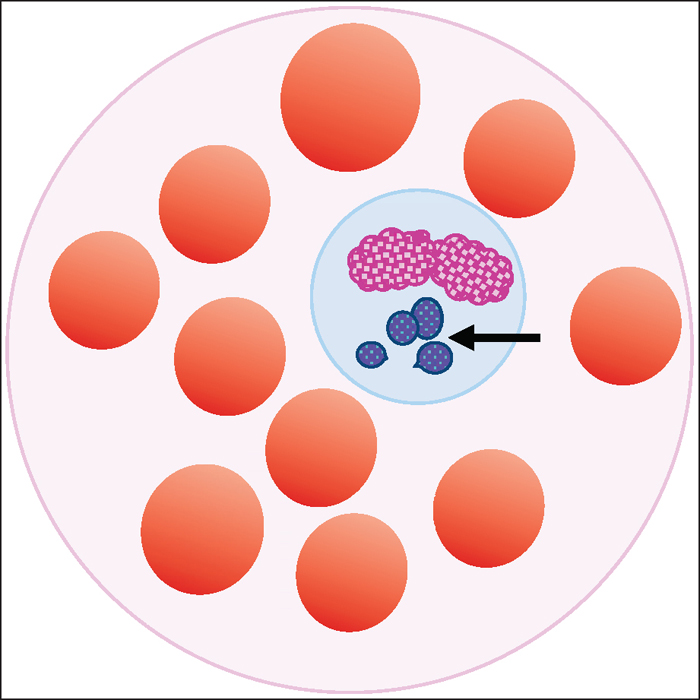
Figure 1. Illustration of a peripheral blood smear demonstrating variably sized basophilic
inclusions, representing a cluster of Anaplasma (Ehrlichia) phagocytophilum (arrowed)
contained within vacuoles in the cytoplasm of a leukocyte.

Figure 2. Spriochete bacteria Borrelia burgdorferi, the causative agent of Lyme disease.
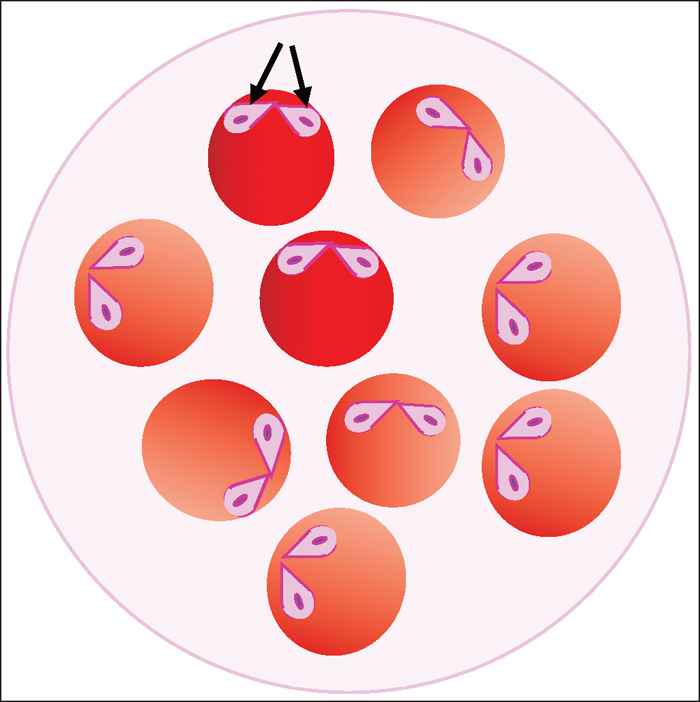
Figure 3 (right). Illustration showing the intraerythrocytic protozoan Babesia divergens in a
blood smear. Merozoites are pear shaped (arrows) and often occur in pairs, joined at the tip,
which is a result of binary schizogony.
Source: https://www.vettimes.co.uk/article/tick-borne-disease-treatment/?format=pdf
The Power of NaTure aquaPresÉN® The Power of NaTure There are two main ingredients that define our AQUAPRESÉN products. The power of activated water and whey give our products the effects that make them so WHAT IS ACTIVATED WATER? Activated water is water that has been The AQUAPRESÉN SWISS product line with electrochemically altered and has the ability to activated water as its main ingredient is one of destroy microbiological creatures like bacteria, the most innovative developments in the field virus and fungus. Because this effect is achieved of natural cosmetic of the last years. Activated through an electrochemical process activated water has a broad application field: Through water is 100% natural and lacks any chemicals. the use of this highly effective ingredient our This has been scientifically proven.
CATHOLIC DISTRICT SCHOOL BOARD OF EASTERN ONTARIO Active Teachers Great-West Life is a leading Canadian life and health insurer. Great-West Life's financial security advisors work with our clients from coast to coast to help them secure their financial future. We provide a wide range of retirement savings and income plans; as well as life, disability and critical illness insurance for individuals and families. As a leading provider of employee benefits in Canada, we offer effective benefit solutions for large and small employee groups. Great-West Life Online Information and details on Great-West Life's corporate profile, our products and services, investor information, news releases and contact information can all be found at our website www.greatwestlife.com. Great-West Life's Toll-Free Number To contact a customer service representative at Great-West Life for assistance with your medical and dental coverage, please call 1-800-957-9777.



