Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Capitalallergy.com
Comparison of olopatadine 0.6% nasal spray versus
fluticasone propionate 50
g in the treatment of seasonal
allergic rhinitis
Michael A. Kaliner, M.D.,* William Storms, M.D.,# Stephen Tilles, M.D.,§ Sheldon Spector, M.D.,¶Ricardo Tan, M.D.,¶ Craig LaForce, M.D.,储 Bobby Q. Lanier, M.D.,** and Bradley Chipps, M.D.##
The efficacy of nasal antihistamines (NAHs) for allergic rhinitis (AR) is comparable with or better than second-generation
oral antihistamines, with faster onset of action and greater effect on congestion. Limited data suggest that NAHs may beequivalent to intranasal corticosteroids at reducing the full range of nasal seasonal AR (SAR) symptoms, including congestion.
The efficacy of olopatadine 0.6% nasal spray (2 sprays/nostril b.i.d.) for symptoms of SAR was compared with fluticasone 50microg nasal spray (2 sprays/nostril q.d.) in a double-blind, randomized, parallel-group, 2-week noninferiority trial. A total of130 symptomatic patients were randomized to treatment and they recorded nasal and ocular allergy symptom scores b.i.d.
(morning and evening) in a diary. Both treatments reduced reflective and instantaneous assessments of nasal and ocularsymptoms from baseline throughout the 2-week study period (p ⬍
0.05). The reflective total nasal symptom score (the primaryefficacy variable) decreased by an average of ⫺
45.4% for patients treated with olopatadine 0.6% and by ⫺
47.4% for thosetreated with fluticasone; statistical significance favoring olopatadine was demonstrated at day 1. No significant between-treatment differences were determined for the average 2-week percent changes from baseline for congestion, runny nose,sneezing, itchy nose, and ocular symptoms, although olopatadine had a faster onset of action for reducing all symptoms. Bothtreatments were safe and well tolerated. Olopatadine and fluticasone nasal sprays both reduced nasal and ocular SAR symptomswith no significant between-treatment differences except for a faster and greater onset of action with olopatadine.
(Allergy Asthma Proc 30:255–262, 2009; doi: 10.2500/aap.2009.30.3232)
Key words: Allergic conjunctivitis, allergic rhinitis, allergy, antihistamines, fluticasone, intranasal steroids, nasal
antihistamines, olopatadine, seasonal allergic rhinitis
Copyright (c) Oceanside Publications, Inc. All rights reserved
Allergic rhinitis (AR), one of the most common
The characteristic symptoms of AR are sneezing,
atopic diseases, afflicts an estimated 35–50 mil-
rhinorrhea, nasal itching, nasal congestion, and itchy/
lion people in the United States, up to 30% of the
red/watery eyes. Patients also frequently report head-
general population.1,2 Although often dismissed as a
aches and/or facial pain, snoring, and sleep distur-
"nuisance disorder" by both clinicians and patients,
bance.1,2,4 Although generally not life-threatening, the
the costs are substantial— both in terms of direct ex-
symptoms can be annoying and debilitating—interfer-
penditures and societal costs related to absenteeism
ing with daily activities, performance and concentra-
and presenteeism. AR represents a hyperactive im-
tion, rest, and contributing to absenteeism and presen-
mune system response to otherwise benign, noninfec-
teeism.1,2,4 Rhinitis is often associated with other
tious environmental aeroallergens (
e.g., pollens, mites,
chronic conditions including asthma, eustachian tube
and animal danders).2,3
dysfunction, otitis media, rhinosinusitis, atopic derma-
DO NOT COPY
titis, allergic conjunctivitis, and obstructive sleep ap-nea.1,4,5 Thus, early treatment of rhinitis symptoms can
From the *Institute for Allergy and Asthma, Chevy Chase, Maryland, #The WilliamStorms Allergy Clinic, Colorado Springs, Colorado, §ASTHMA, INC, NW, Asthma
have significant clinical benefit.
and Allergy Center, Seattle, Washington, ¶California Allergy and Asthma Medical
Topical nasal antihistamines (NAHs) represent the
Group, Los Angeles, California, 储
Carolina Allergy and Asthma Consultants, Raleigh,
latest addition to the armamentarium for treating
North Carolina, **North Texas Institute for Clinical Trials, Fort Worth, Texas, and##Capital Allergy and Respiratory Disease Center, Sacramento, California
AR. The efficacy of these topical agents is compara-
Funding for this research was supported by Alcon Laboratories, Inc., Fort Worth, Texas
ble with or better than second-generation oral anti-
W. Storms, M.A. Kaliner, and B. Chipps are consultants/speakers and receive grantsupport from Alcon Research Ltd. In addition, S. Tilles is a consultant/speaker for
histamines, with a much faster onset of action; and,
Alcon and C. LaForce and M. Kaliner are on the advisory board
unlike oral antihistamines, the NAHs also reduce
Address correspondence and reprint requests to Michael A. Kaliner, M.D., Institute
nasal congestion. Limited data suggest that NAHs
for Allergy and Asthma, 5454 Wisconsin Avenue, Suite 1700, Chevy Chase, MD20817
may be equivalent to intranasal corticosteroids
E-mail address: [email protected]
(INSs) at reducing the full range of nasal AR symp-
Copyright 2009, OceanSide Publications, Inc., U.S.A.
toms, including congestion.6
Allergy and Asthma Proceedings
Copyright @ Oceanside Publications, Inc. All rights reserved.
For permission to copy, go to www.copyright.com
Olopatadine 0.6% nasal spray is the most recent
NAH to reach the U.S. market. This mast cell stabiliz-ing agent is also a potent topical H1-antagonist and hasbeen available since 1996 as an ophthalmic solution totreat the signs and symptoms associated with allergicconjunctivitis.7 In 2008 the nasal formulation was ap-proved for treatment of the symptoms of seasonal AR(SAR) in patients ⱖ12 years of age.
In patients with SAR, olopatadine nasal spray has
established an onset of action within 30 minutes and,when administered for 2 weeks, has shown signifi-
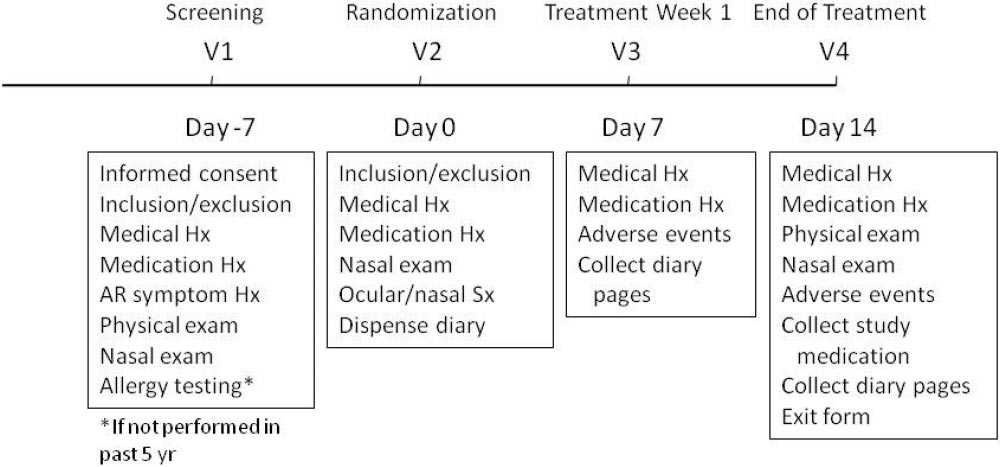
Figure 1. Study protocol.
cant efficacy in reducing nasal allergy symptoms,including congestion, when compared with place-bo.8 –10 Evaluations using the Rhinoconjunctivitis
tion. Patients who had abnormal nasal anatomy, severe
Quality of Life Questionnaire and the Work Produc-
congestion, recent upper or lower respiratory infection
tivity and Activity Impairment Questionnaire–
or chronic sinusitis, or cardiovascular disease were
Allergy Specific, have also shown significant posi-
excluded. Also excluded were smokers, known nonre-
tive health outcomes with treatment.9,11,12
sponders to antihistamines, and patients with concur-
Antihistamines, including NAHs, are recommended
rent upper or lower airway disease that could interfere
by current guidelines and practice parameters as first-
with successful nasal drug administration/absorption
line therapy for SAR.1,4 However, INSs are considered
(e.g., rhinitis medicamentosa and asthma).
"the gold standard" by some clinicians. On the other
Medication washout times were 30 days for systemic
hand, many patients are concerned about potential INS
corticosteroids and inhaled or ocular corticosteroids; 7
side effects and also desire a product that works
days for INSs, leukotriene inhibitors, anticholinergic
quickly to relieve symptoms.3
agents, systemic antifungal agents, and systemic anti-
A double-blinded parallel-group environmental ex-
biotics; 3 days for ocular and nasal antiallergy agents,
posure chamber study in 425 patients with SAR
oral antihistamines, nonsteroidal anti-inflammatory
showed that a single dose of olopatadine nasal spray
drugs, decongestants, and over-the-counter cough/
induced a significant reduction of allergy DO NOT COPY
cold and sleep aids; and 1 day for nasal and ocular
Copyright (c) Oceanside Publications, Inc. All rights reserved
0.05) within 30 minutes and lasting for 12 hours
saline. Patients who were receiving immunotherapy
when compared with an INS, mometasone furoate.8
were required to be stable for 30 days before and
However, the relative efficacies of the NAHs and INSs
throughout the trial. Use of any prescription or over-
beyond 12 hours were not evaluated. The current study
the-counter nasal spray was not allowed.
was undertaken to evaluate the comparative efficacy ofolopatadine 0.6% nasal spray with that of an INS,
Study Design
fluticasone propionate, 50 g/puff, in a 2-week SAR
This was a 2-week, multicenter (seven sites in the
United States), double-blind, randomized, two-armparallel-group clinical trial of olopatadine 0.6% nasal
MATERIALS AND METHODS
spray and fluticasone propionate 50 g nasal spray.
Beginning with the screening visit and continuing to
end of treatment, patients recorded in a diary the
Patients were ⱖ12 years of age with a ⱖ2-year his-
symptom severity of their itchy nose, runny nose,
DO NOT COPY
tory of spring/summer AR. All patients showed aller-
stuffy nose, sneezing, itchy/burning eyes, tearing/wa-
gic sensitivity to a currently prevalent (at time of
tery eyes, and ocular redness using a 4-point scale (0 ⫽
study) seasonal allergen within the past 5 years, de-
absent, 1 ⫽ mild, 2 ⫽ moderate, and 3 ⫽ severe). The
fined by a positive reaction on skin-prick testing (a
sum of scores for the four nasal symptoms was defined
wheal size of ⱖ3 mm greater than the diluent) or
as the total nasal symptom score (TNSS), and the sum
intradermal testing (a wheal size of ⱖ7 mm greater
of the scores for the three ocular symptoms was de-
than the diluent) within the past 5 years, and were
fined as the total ocular symptom score (TOSS). Pa-
symptomatic on trial entry. The study protocol was
tients evaluated their symptoms as experienced at that
approved by an institutional review board, and an
moment (instantaneous) and in the hours since the last
informed consent document was signed by all of the
dose of study medication (reflective), in the morning
patients or by the parent or legal guardian for patients
before any other activity, and at bedtime.
⬍18 years old.
The study design is shown in Fig. 1. For patients who
Women of childbearing potential were enrolled if
did not require a medication washout period, the
they agreed to use an acceptable method of contracep-
screening and randomization visits were combined. At
May–June 2009, Vol. 30, No. 3
Copyright @ Oceanside Publications, Inc. All rights reserved.
For permission to copy, go to www.copyright.com
screening, patients had to have a minimum reflective
analysis with respect to baseline patient demographic
TNSS of ⱖ4 with a maximum score of 10 and an
and clinical characteristics was first performed to val-
individual score for congestion of ⱕ2. The patients
idate the between-group comparability. Between-
were randomized to dose fluticasone propionate (50
group comparisons were conducted using the Stu-
g) nasal spray q.d. and olopatadine 0.6% nasal spray
dent's t-test for numeric variables or Pearson's chi-
b.i.d., 2 sprays of each per nostril for the 2-week treat-
square test for categorical variables.
ment period. Because the olopatadine and fluticasone
Paired t-test was used for within-subject before–after
bottles were distinctly different, as were the treatment
comparisons. Analysis of covariance using age as the
regimens, foil-wrapped bottles with appropriate dos-
covariant and the repeated measures analysis of vari-
ing instructions were distributed to the patients in
ance were further performed to adjust the potential
nontransparent envelopes. In this manner, the study
impact of age difference between the treatment groups
staff, investigator, sponsor, monitors, and patients
and the time effect on the primary outcome measure-
were unaware of any individual patient's assigned
ment (TNSS). Statistical analysis was performed using
SAS (PC-9.1.2; SAS Institute, Cary, NC) by an indepen-
Diary scores, protocol compliance, medication changes,
dent biostatistician. A 95% confidence level was set to
and reported adverse events were reviewed after each
week of treatment. The exit visit (day 14) included phys-ical and nasal examinations and measurement of vital
Of 132 patients screened at 7 U.S. centers, 130 met the
study criteria and were randomized to treatment. All
enrolled patients completed the study. There were 63male and 64 female patients with ages ranging from 12
Efficacy. The primary efficacy variable was the 2-week
to 73 years (mean, 35.3 years; SD, 13.48). Fifty-six per-
average percent change in reflective TNSS. Secondary
cent were white, 21% were African American, 13%
efficacy variables included the percent changes in instan-
were Hispanic/Latino, and 10% were Asian. The treat-
taneous TNSS and reflective/instantaneous TOSS. Indi-
ment groups were similar in terms of demographic
vidual symptoms (i.e., runny nose, itchy nose, sneezing,
characteristics (Table 1) except that the mean age was 5
stuffy nose, watery/tearing eyes, itchy/burning eyes,
years older in the olopatadine group. All patients had
and ocular redness) were also analyzed to
seasonal allergies to tree, grass, and/or weed allergens,
Copyright (c) Oceanside Publications, Inc. All rights reserved
documented by positive skin tests.
Safety. Safety evaluation included nasal examination
Primary Efficacy: Reflective TNSS
for significant anatomic abnormalities, evidence of in-
Pretreatment values for reflective TNSS were similar
fection, bleeding, and ulcerations of the mucosa; and
for both treatment groups (olopatadine 0.6%, 6.72 ⫾
physical examination of the head/eyes; ears, nose,
1.88 SD; fluticasone, 6.49 ⫾ 1.66 SD; p ⫽ 0.4599). The
throat, and neck; skin and extremities; cardiovascular
mean 2-week average reflective TNSS was 3.52 (⫾2.01
and pulmonary systems; abdomen, lymph nodes and
SD) for olopatadine and 3.37 (⫾2.18 SD) for fluticasone,
neurological signs. Unsolicited patient-reported ad-
a 45.4 and 47.4% reduction from baseline, respectively
verse events were also recorded, regardless of relation-
(Fig. 2). Per t-test, the observed difference (flutica-
ship to treatment.
sone ⫺ olopatadine ⫽ ⫺0.154) was not statisticallysignificant (p ⫽ 0.6771). The 95% CI for treatment dif-
Sample Size Estimation. The study was powered
ference in mean 2-week average score was ⫺0.886 to
DO NOT COPY
based on the hypothesis that the differences in mean
0.577, which is within the defined noninferiority mar-
2-week average reflective TNSS between the olopata-
gin of 2. An analysis of covariance using age as the
dine 0.6% nasal spray and the fluticasone propionate
covariant indicated that the between-group age differ-
50 g nasal spray would be within 2 points. Per sample
ence was not a significant factor in treatment outcome;
size calculation, when the standard deviation is within
the between-groups difference remained statistically
3 points and the nonevaluable rate is not ⬎10%, a
insignificant after adjusting to age difference. The re-
sample size of 65 patients/group would be sufficient to
peated measures analysis of variance with adjustments
detect a 2-point (noninferiority margin) between-group
for time effect and time-by-treatment interaction con-
difference with a 90% statistical power at 95% confi-
firmed the noninferiority conclusion (p ⫽ 0.7551).
dence level.
Data Analysis. All enrolled patients completed the
study. Therefore, the intent-to-treat population was the
TNSS during the 2-Week Period by Day. Per the within-
same as the per-protocol population. Comparability
subject before–after comparison using paired t-test, the
Allergy and Asthma Proceedings
Copyright @ Oceanside Publications, Inc. All rights reserved.
For permission to copy, go to www.copyright.com

Table 1 Comparison in patient baseline characteristics
(n ⴝ 130)
(n ⴝ 65)
(n ⴝ 65)
*The p values of between-group comparisons using Student's t-test for numerical variables and Pearson 2-test for categoricalvariables.
#Gender information not available for three patients.
§Other races are Indian in the olopatadine group, East Indian in the fluticasone group.
large variance observed, only the reflective sneezingscore reached statistical significance (29.2% versus8.76%; p ⫽ 0.0378; Fig. 4).
Reflective TOSS and Individual Ocular Symptoms
Scores. Pretreatment values for reflective TOSS were
Copyright (c) Oceanside Publications, Inc. All rights reserved
similar for both treatment groups (olopatadine 0.6%,4.25 ⫾ 2.05 SD; fluticasone propionate, 4.18 ⫾ 1.84SD; p ⫽ 0.8491), and both groups reported similarreductions over the course of the study: 38.5 and
Figure 2. Mean average reflective total nasal symptom scores
40.6% for the olopatadine and fluticasone groups,
(TNSSs) at baseline and end of treatment (2 weeks).
respectively (p ⫽ 0.8402; Fig. 5). The mean 2-weekaverage reflective individual ocular symptom scoresfor itching/burning eyes, tearing/watery eyes, and
mean daily reflective TNSS decreased throughout the
eye redness were also significantly reduced based on
2-week treatment period in both groups. No significant
the within-patients analysis, with no between-treat-
differences between treatments were noted using the
ment differences in the magnitude of reduction.
magnitude of decrease per two-sample Student's t-test.
However, again, a difference in onset of action was
On day 1, the mean percent reduction from baseline
evident, with faster and greater relief for patients
DO NOT COPY
was 26.7% for patients who received olopatadine com-
treated with olopatadine in the first 3 days (Fig. 6).
pared with 13.6% for fluticasone (p ⫽ 0.0432; Fig. 3 A).
Individual Reflective Nasal Symptoms Scores. Both ol-
Instantaneous TNSS, TOSS, and Individual Symptoms
opatadine and fluticasone groups had significant re-
Scores. Similar trends were observed for the instanta-
ductions in the reflective scores for runny nose, itchy
neous nasal and ocular symptom scores. Both olopata-
nose, sneezing, and stuffiness over the 2 weeks of
dine 0.6% and fluticasone propionate nasal sprays
treatment per paired t-test. No significant between-
showed significant reductions in the mean instanta-
treatment differences in a specific mean 2-week aver-
neous scores over the 2 weeks of treatment, with no
age were detected (Fig. 4). A difference in onset of
significant between-treatment differences detected for
action was observed for all nasal symptoms, with
any measurement (Table 2). Instantaneous TNSS de-
greater percent reductions in patients treated with ol-
creased in similar manner to reflective TNSS on a
opatadine in the first 72 hours, although because of the
day-to-day basis (Fig. 3 B). Significant between-group
May–June 2009, Vol. 30, No. 3
Copyright @ Oceanside Publications, Inc. All rights reserved.
For permission to copy, go to www.copyright.com

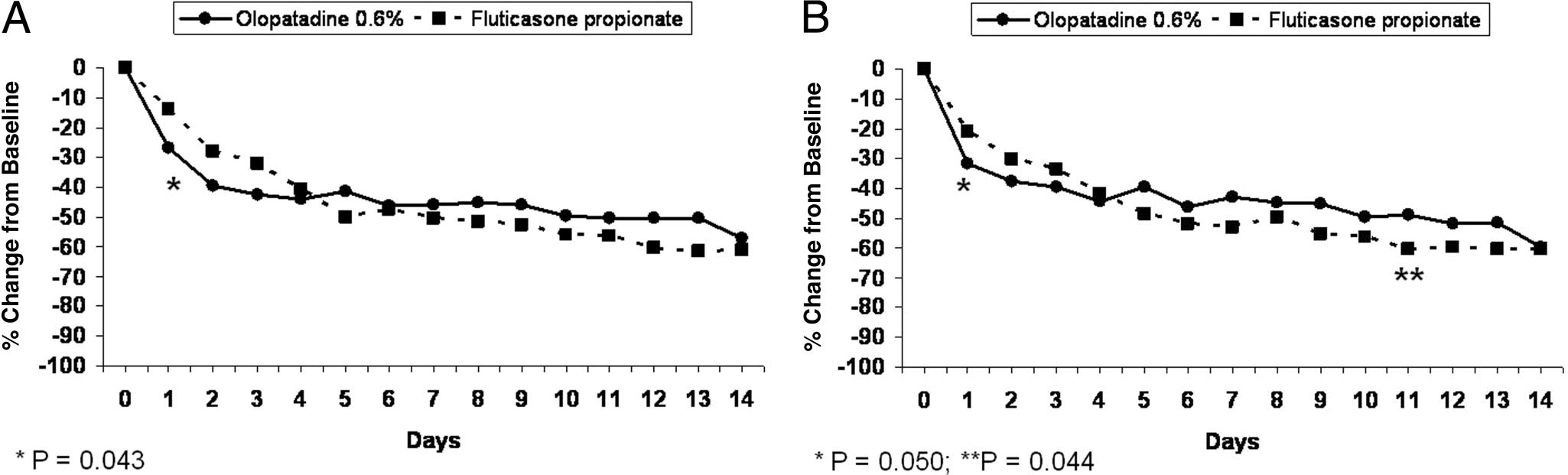

Figure 3. (A) Mean daily percent change in reflective total nasal symptom scores (TNSSs) from baseline during the 2-week treatment period.
(B) Mean daily percent change in instantaneous TNSSs from baseline during the 2-week treatment period.
Copyright (c) Oceanside Publications, Inc. All rights reserved
Figure 4. Mean percent changes from baseline in the reflective nasal symptom scores for days 1, 2, and 3 of treatment and the specific 2-week
averages.
DO NOT COPY
differences favored olopatadine on day 1 (p ⫽ 0.0501)and fluticasone on day 11 (p ⫽ 0.0437).
Both treatments were well tolerated. There also were
no treatment-related changes in physical (includingnasal) examination findings for either group. Eighteenpatients (olopatadine, 11; fluticasone, 7) reported a to-tal of 29 adverse events; 9 were determined to berelated to treatment: epistaxis/nasal blood (3), bad/bitter taste (2), sore throat (1), cough (1), sleepiness (1),with olopatadine, and eyes mildly injected (1) with
Figure 5. Mean average reflective total ocular symptom scores
fluticasone. Adverse events were nonserious, did not
(TOSSs) at baseline and end of treatment (2 weeks).
Allergy and Asthma Proceedings
Copyright @ Oceanside Publications, Inc. All rights reserved.
For permission to copy, go to www.copyright.com

Figure 6. Mean percent changes from baseline in the reflective ocular symptom scores for days 1, 2, and 3 of treatment and the specific 2-week
averages.
Table 2 Comparisons in 2-wk average percent changes from baseline in instantaneous assessments of
symptoms
( DO NOT COPY
(n ⴝ 65)
Copyright (c) Oceanside Publications, Inc. All rights reserved
Itching/burning eyes
Tearing/watering eyes
*The p values of between-group comparison using Student's t-test.
TNSS ⫽ total nasal symptom score; TOSS ⫽ total ocular symptom score.
DO NOT COPY
interrupt treatment continuation in the study, and
duction for specific parameters. The only difference
were resolved with or without treatment.
observed was a faster and greater onset of action witholopatadine 0.6%. This finding was not surprising be-
cause the usual earliest onset of action for INSs is
In this 2-week study, treatment with either olopata-
between 2 and 24 hours (for mometasone furoate, flu-
dine 0.6% nasal spray (2 sprays/nostril b.i.d.) or fluti-
casone propionate 50 g nasal spray (2 sprays/nostril
dipropionate, and triamcinolone acetonide).13–17 An
q.d.) provided relief from symptoms of SAR. Reduc-
environmental chamber study comparing olopatadine
tions in both nasal and ocular allergy symptoms were
0.6% nasal spray with the INS, mometasone furoate,
observed for both treatments with no significant be-
reported an onset of action within 30 minutes for ol-
tween-treatment differences in the magnitude of re-
opatadine versus 2.5 hours with mometasone.8
May–June 2009, Vol. 30, No. 3
Copyright @ Oceanside Publications, Inc. All rights reserved.
For permission to copy, go to www.copyright.com
In this study, symptom reductions with olopatadine
lergy symptoms is to target the allergic eyes directly
exceeded those with fluticasone by at least 10% for all
reflective measures evaluated during one or more of
In conclusion, in patients with active SAR, olopata-
the first 3 days of treatment. Statistical significance was
dine 0.6% nasal spray (2 sprays/nostril b.i.d.) and flu-
attained on the 1st day of treatment for the primary
ticasone propionate 50 g (2 sprays/ nostril q.d.) given
efficacy variable, TNSS (olopatadine, ⫺26.69%; flutica-
over 2 weeks provided comparable clinical benefit for
sone, ⫺13.64%; p ⫽ 0.043), and also for sneezing (ol-
nasal and ocular allergy symptoms. The study findings
opatadine, ⫺29.17%; fluticasone, ⫺8.76%; p ⫽ 0.038).
support olopatadine nasal spray as an effective first-
This is the first study directly comparing the efficacy
line treatment for the rapid and sustained relief of the
of olopatadine 0.6% nasal spray to an INS, and it is of
symptoms of SAR. More comparative data on the effi-
interest that both agents reduced nasal stuffiness to a
cacy of INSs and NAHs are warranted in this patient
similar degree. In this population of patients with ac-
population because both medications are considered to
tive SAR, olopatadine reduced nasal congestion score
be first-line therapies for relieving the symptoms of
by a 2-week average of 22.2% compared with 29.5%
with fluticasone (p ⫽ 0.4035). Current guidelines andpractice parameters for AR note that NAHs do reduce
nasal congestion, but suggest that INSs are more po-
The editorial and technical expertise of Judith Farrar, Ph.D., is
tent.1,4 Additional studies may be required to confirm
greatly appreciated. The authors also acknowledge the staff of the
the observation of equal efficacy between olopatadine
various offices in which data were collected. Dr. Chipps would like
to specifically thank his study coordinator, Bryce Autret.
The mean reduction in nasal congestion with ol-
opatadine nasal spray observed here is comparable
with that reported in other 2-week trials in patients
1. Wallace DV, Dykewicz MS, Bernstein DI, et al. The diagnosis
and management of rhinitis: An updated practice parameter. J
with SAR.9,10 Individual studies with INSs (budes-
Allergy Clin Immunol 122:S1–S84, 2008.
onide, triamcinolone acetonide, and fluticasone propi-
2. Marple BF, Fornadley JA, Patel AA, et al. Keys to successful
onate) in patients with SAR have generally shown
management of patients with allergic rhinitis: Focus on patient
percentage reductions from baseline for nasal conges-
confidence, compliance, and satisfaction. Otolaryngol Head
tion of ⬎30%, but it is difficult to compare studies
Neck Surg 136:S107–S124, 2007.
3. Asthma and Allergy Foundation of America (AAFA). Con-
because of differences in treatments and
sumer survey, 2005. Available online at www.aafa.org; last
cols.18–22Copyright (c) Oceanside Publications, Inc. All rights reserved
Fluticasone propionate nasal spray (200 g
accessed May 2008.
daily) reduced nasal congestion by ⬃40% when given
4. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic rhinitis and its
to ⬎20 patients with SAR for 15 days in two separate
impact on asthma (ARIA) 2008 update (in collaboration with
clinical trials. However, no screening data are available
the World Health Organization, GA2LEN and AllerGen). Al-lergy 63:S8 –S160, 2008.
for congestion in those studies, and scores were re-
5. Settipane RA. Rhinitis: A dose of epidemiological reality. Al-
ported on a visual analog scale (0 –100).19,20
lergy Asthma Proc 24:147–154, 2003.
Both olopatadine and fluticasone reduced ocular al-
6. Kaliner MA. A novel and effective approach to treating rhinitis
lergy symptoms to a similar degree. This might be
with nasal antihistamines. Ann Allergy Asthma Immunol 99:
expected because all allergy medications reduce ocular
383–391, 2007.
7. Berger WE. Once-daily olopatadine ophthalmic solution 0.2% in
allergy symptoms to some extent.1,4 However, re-
the treatment of allergic conjunctivitis and rhinoconjunctivitis.
cently, there has been interest specifically in the effect
Expert Rev Pharmacoecon Outcomes Res 7:221–226, 2007.
of INSs on ocular allergy. The authors of a 2-week
8. Patel D, Garadi R, Burbaker M, et al. Onset and duration of
study of fluticasone furoate (110 g q.d.) in patients
action of nasal sprays in seasonal allergic rhinitis patients: Ol-
DO NOT COPY
with SAR suggested that this INS "might present a
opatadine hydrochloride versus mometasone furoate monohy-drate. Allergy Asthma Proc 28:592–599, 2007.
single treatment option for the nasal and ocular symp-
9. Meltzer EO, Hampel FC, Ratner PH, et al. Safety and efficacy of
toms of SAR."23 Over the 2-week treatment period, the
olopatadine hydrochloride spray for the treatment of seasonal
reflective TOSS decreased by 33.8% in that study,
allergic rhinitis. Ann Allergy Asthma Immunol 95:600 – 606,
which is in the range of the reductions observed in
TOSS in this study (olopatadine, 38.5%; fluticasone,
10. Ratner PH, Hampel FC, Amar N, et al. Safety and efficacy of
olopatadine hydrochloride nasal spray for the treatment of
40.6%). Obviously, the earlier article discounts the fact
seasonal allergic rhinitis to mountain cedar. Ann Allergy
that most allergy medications provide some level of
Asthma Immunol 95:474 – 479, 2005.
ocular protection. Of greater concern and as noted in
11. Fairchild CJ, Meltzer EO, Roland PS, et al. Comprehensive
the package inserts, is the potential for adverse ocular
report of the efficacy, safety, quality of life, and work impact of
effects with INSs. In the present study, the only treat-
olopatadine 0.6% and olopatadine 0.4% treatment in patientswith seasonal allergic rhinitis. Allergy Asthma Proc 28:1– 8,
ment-related ocular adverse event, mild eye injection,
was reported by a fluticasone-treated patient. The best
12. Hampel FC, Ratner PH, Amar NJ, et al. Improved quality of life
treatment for patients with more than mild ocular al-
among seasonal allergic rhinitis patients treated with olopata-
Allergy and Asthma Proceedings
Copyright @ Oceanside Publications, Inc. All rights reserved.
For permission to copy, go to www.copyright.com
dine HCl nasal spray 0.4% and olopatadine 0.6% compared
treatment of ragweed-induced allergic rhinitis. J Allergy Clin
with vehicle placebo. Allergy Asthma Proc 27:202–207, 2006.
Immunol 97:749 –755, 2003.
13. Berkowitz RB, Bernstein DI, LaForce C, et al. Onset of action of
19. Martin BG, Andrews CP, van Bavel JH, et al. Comparison of
mometasone furoate nasal spray (NASONEX) in seasonal aller-
fluticasone propionate aqueous nasal spray and oral monte-
gic rhinitis. Allergy 54:64 – 69, 1999.
lukast for the treatment of seasonal allergic rhinitis symptoms.
14. Lumry WR. A review of the preclinical and clinical data of
Ann Allergy Asthma Immunol 96:851– 857, 2006.
newer intranasal steroid use in the treatment of allergic rhinitis.
20. Ratner PH, Howland WC III, Arastu R, et al. Fluticasone pro-
J Allergy Clin Immunol 104:S150 –S158, 1999.
pionate aqueous nasal spray provided significantly greater im-
15. Selner JC, Weber RW, Richmond GW, et al. Onset of action of
provement in daytime and nighttime nasal symptoms of sea-
aqueous beclomethasone dipropionate nasal spray in seasonal
sonal allergic rhinitis compared with montelukast. Ann Allergy
allergic rhinitis. Clin Ther 17:1099 –1109, 1999.
Asthma Immunol 90:536 –542, 2003.
16. Day JH, Briscoe MP, Rafeiro E, et al. Onset of action of intra-
21. Tinkelman D, Falliers C, Gross G, et al. Multicenter evaluation
nasal budesonide (Rhinocort Aqua) in seasonal allergy rhinitisstudied in a controlled exposure model. J Allergy Clin Immunol
of triamcinolone acetonide nasal aerosol in the treatment of
105:489 – 494, 2000.
adult patients with seasonal allergic rhinitis. Ann Allergy 64:
17. Day JH, Buckeridge DL, Clark RH, et al. A randomized, double-
234 –239, 1990.
blind, placebo-controlled, controlled antigen delivery study of
22. Bjerrum P, and Illum P. Treatment of seasonal allergic rhinitis
the onset of action of aerosolized triamcinolone acetonide nasal
with budesonide and disodium cromoglycate. Allergy 40:65–
spray in subjects with ragweed-induced allergic rhinitis. J Al-
lergy Clin Immunol 97:1050 –1057, 1996.
23. Kaiser HB, Naclerio RM, Given J, et al. Fluticasone furoate nasal
18. Bernstein DI, Creticos PS, Busse WW, et al. Comparison of
spray: A single treatment option for the symptoms of seasonal
triamcinolone acetonide nasal inhaler with astemizole in the
allergic rhintis. J Allergy Clin Immunol 119:1430 –1437, 2007. e
Copyright (c) Oceanside Publications, Inc. All rights reserved
DO NOT COPY
May–June 2009, Vol. 30, No. 3
Copyright @ Oceanside Publications, Inc. All rights reserved.
For permission to copy, go to www.copyright.com
Source: http://www.capitalallergy.com/pdfs/chipps/62.pdf
Computerised anticoagulation The historical perspective of monitoring vitamin K antagonists Australia - Human Brain (low ISI) Canada - Rabbit (high ISI) Hong Kong - Human Brain (low ISI) South Africa - Human Brain (low ISI) Sweden - Rabbit (high ISI) U.K - Human Brain (low ISI) Zimbabwe - Rabbit (high ISI) USA - Rabbit (high ISI) 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0 7.5 8.0 8.5 9.0
Trouble obsessionnel compulsif Trouble obsessionnel compulsif Trouble obsessionnel-compulsif (TOC) Classification et ressources externes Un lavage de main répétitif est un symptôme du trouble obsessionnel-compulsif. Le trouble obsessionnel compulsif (abrégé TOCcaractérisé par l'apparition répétée de - produisant de l'inconfort, de l'inquiétude, de l'appréhension et/ou de la peur;






