Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Cut and paste hewlett doc 4
Intrauterine Devices (IUDs) in Developing Countries:
Assessing Opportunities for Expanding Access and Use
Project Team:
Amy E. Pollack MD, MPH John Ross PhD Gordon Perkin MD
"A deeper commitment to contraception, including the IUD, can ease the burden of many health problems in every country. Foundations can highlight the leverage that contraception offers for lessening those burdens. They can advance advocacy at the highest levels and partner with other donors in a common strategy, thus leveraging the investment of USAID and the private sectors commitment."
IUDs in Developing Countries-Perkin, Pollack and Ross
Table of Contents
Ranking factors influencing programmatic initiatives supporting access and uptake. 40
Successful and unsuccessful programs: lessons learned. 40
Knowledge and acceptance of the IUD . 41
Status of other IUD revitalization program, research, and advocacy efforts. 44
Case-in-point: The USAID Kenya Mission. 46
SUPPLY ISSUES . 48
III.1 Cost/Benefit . 48 III.2 Product patents, intellectual property and licensing . 48 III.3 Public-Private
Partnership . 49
III.5 Timeline . 52
CONCLUSIONS AND RECOMMENDATIONS . 54
IV.1 Conclusions. 54 IV.2 Recommendations. 56 IV.3 Specific
VI.1 APPENDIX 1: NUMBERS OF IUD USERS AND OTHER INDICATORS. 65 VI.2 APPENDIX 2: DISCONTINUATION RATES . 68 VI.3 APPENDIX 3. Numbers with Unmet Need by Region, Spacing/Limiting, and Marital Status, 2000 (in thousands) . 70 VI.4 APPENDIX 4. Projections of IUD Use. 72 VI.5 APPENDIX 5: Preferred method of contraception for future use . 74 VI.6 APPENDIX 6. Percent of Married Women Ever Using the IUD, by Age and Region 76 VI.7 APPENDIX 7. Percent of Currently married Women Who Know Each Method. 79
IUDs in Developing Countries-Perkin, Pollack and Ross
List of Figures:
Figure 1. Images of six IUDs. 12
Figure 2. Advantages of the TCu 380A . 13
Figure 3. Disadvantages of the TCu 380A. 14
Figure 4. Advantages of the LnG IUD. 17
Figure 5. Disadvantages of the LnG IUD . 20
Figure 6. Relation of Total Prevalence to IUD Prevalence . 28
Figure 7. IUD Sources of Supply by Sector . 36
Figure 8. Private involvement and IUD prevalence. 36
Figure 9. Dependence of IUD Prevalence upon IUD Access. 39
Table 1. Principle clinical trials documenting the contraceptive efficacy of the LnG IUD . 18
Table 2. Selected health benefits/disease protection from using the levonorgestrelIUD . 19
Table 3. Cumulative net probabilities of IUD use discontinuation . 23
Table 4. The consequences of additional exposure to pregnancy as impacted by continuation
Table 5. Historical Experience for Increases in IUD Prevalence . 29 Table 6. Projections of IUD prevalence, users, and adopters at a half percent rise per year in IUD
Table 7. Countries Classified by Total Prevalence and IUD Prevalence . 34 Table 8. (DKT) 2004 Social Marketing Program Sales from all sources for IUDs. 37 Table 9. Percent Using the IUD, by Age, No. of Children, Residence, and Education. 43 Table 10. Ever Use of the IUD, by Age and Region . 44 Table 11. Knowledge of IUD and Other Methods, by Region . 44 Table 12. WHO Cost Estimate and a timeline for Phase III Clinical Trial of a New LnG/Mirena
Table 13. Sample matrix of country case studies . 59
IUDs in Developing Countries-Perkin, Pollack and Ross
FORWARD
At the beginning of 2006, the Hewlett Foundation's Population Program sought an assessment of
the potential of the IUD as a contraceptive method in developing countries and ways to improve
IUD availability, acceptance, and effectiveness. A team of three consultants—Amy E. Pollack,
John Ross and Gordon Perkin—conducted an analysis during the first part of 2006 on: (1) public
health benefits of the IUD; (2) programmatic factors influencing IUD use; (3) supply issues,
including analysis of public-private partnerships; and finally (4) options for Foundations
considering support for IUD programs. This report is primarily geared toward the donor
community, especially private foundations, and may also be of interest to program planners,
researchers, and advocates.
IUDs in Developing Countries-Perkin, Pollack and Ross
EXECUTIVE SUMMARY
I. Overview
The intrauterine device (IUD) has long been recognized as an inexpensive, highly effective,
long-acting, reversible method of contraception. Even though it is ideal in so many ways, the
history of its development reflects continual adaptations to minimize the side effects that lead to
early discontinuations, and to maximize both contraceptive and non-contraceptive health
benefits. The IUD should play a greater role than it does today in parts of the world, and
especially sub-Saharan Africa, where fertility rates, unintended pregnancies, and unmet need for
contraception are high. Those same parts of the world struggle against severe health problems
and health system shortcomings that highlight the non-contraceptive (health) advantages of a
hormonal IUD if it could be deployed widely. However, despite past efforts, many parts of the
world in greatest need have surprisingly low rates of IUD use. Understanding the clinical,
setting, and programmatic barriers should inform future investments for the global expansion of
IUD use and new IUD product development.
This report initially reviews the history, clinical aspects, and some of the broad "setting"
characteristics that have most likely influenced IUD use. The second section analyses
programmatic issues that have equal or greater influence on overall use. The third section
analyzes supply issues, examining options for increasing uptake of the TCu 380A or expanding
the marketplace through either a new IUD or greater access to the LnG IUD currently
unavailable in developing countries. Finally, we present an assessment of options for
Foundation support for expanding IUD uptake in the future based on our findings. The report is
meant to be an inclusive review of both published and unpublished findings. Although we were
asked specifically about recommendations as they apply to sub-Saharan Africa, the report is not
limited in that regard. We include our recommendations for development and introduction of a
new IUD for the public sector that would be cost-effective and offer new health benefits. A time
frame for development and clinical assessment of a new IUD is included in the report (see Table
12 on page 54).
II. Current IUD use
WHO estimates that approximately 160 million women worldwide use IUDs today. China has an
estimated two-thirds of these users, or 96 million. Only a small percentage of current users are in
eastern or western Europe or other industrialized countries (10%). The remaining 24% are in
developing countries other than China, concentrated in Vietnam, Egypt, Indonesia, India,
Uzbekistan, and Turkey. These six countries alone contain half of all users in developing
countries excluding China.
A remarkable geographic pattern of IUD users exists. Most developing countries fall into
clusters, which show widely different determinants of use. In the former USSR republics, the
absence of other contraceptive options was paired with strong clinical capacity; in China and
Vietnam it was government control; in North Africa and the Middle East it was an aversion to
sterilization, considerable clinical capacity, and a cultural acceptance of the IUD. In Latin
IUDs in Developing Countries-Perkin, Pollack and Ross
American and most of Asia, however, the pattern is more idiosyncratic. Where clinical services
and trained providers are inaccessible and other method options sometimes exist, IUD uptake is
negligible, as is the case across all of sub-Saharan Africa.
III. Clinical characteristics that influence IUD use
Several types of IUDs exist, however the most widely available IUD is the TCu 380A. It is
highly effective and long acting, easy to insert, and has a low complication rate. No new IUD is
likely to offer a significant improvement over its essential qualities. However, side effects that
lead to early discontinuation, such as increased bleeding or menstrual pain, could be improved
upon, and would likely improve its continuation rate. That would also increase the pool of
satisfied clients (improved provider training and better client counseling should have a similar
effect, on any IUD).
The Mirena LnG IUD, its wider distribution limited by cost more than anything else, compares
favorably with the safety and efficacy profile of the TCu 380A, although it is more difficult to
insert and has a shorter approved duration of use. Moreover, studies that compare the two IUDs
suggest that despite a decrease in irregular and menstrual bleeding, continuation rates are lower
than with the TCu 380A, which may reflect user concerns about induced oligomenorrhea or
amenorrhea and a different risk ratio faced by developing country women. A LnG-induced
decrease in uterine blood loss related to either menses or other intrauterine abnormalities
(especially in the peri-menopause) could provide an important therapeutic public health benefit,
especially in sub-Saharan Africa where anemia rates are remarkably high. However, evidence
that the LnG IUD could significantly impact the multifactoral type of anemia present in the
region is very limited to date.
Several newer hormonal IUDs are under development, which include design changes to improve
ease of insertion and possibly alter associated pain or bleeding pattern. Duration of use remains
limited compared to the TCu 380A's 10-year approval (tested for 14 years). Through new
product development, a negotiated public sector price should be possible that would remove the
cost barrier to expanding the IUD market, with a greater diversity of products.
IV. Setting and programmatic characteristics that influence IUD demand and use
Our review of setting and program issues that have influenced IUD use historically points to both
conditions for "success" and barriers for "failure." Invariably, the capacity of the health sector is
key, that is, socioeconomic and infrastructure conditions that constrain access to services.
Equally, policy positions and the vigor with which they are enforced matter greatly, such as, the
shared commitment to the IUD at central, clinic, and provider levels including resources for
ongoing training, information and education, and consumer marketing). The private medical
sector cannot be ignored; it has its own life and can be independently important. Cultural forces
are relevant in some cases. All these factors influence the contraceptive method mix.
"Demand" for a specific method therefore cannot be understood in isolation. While IUD
prevalence is high wherever IUD access is high, "access" is usually a marker for other program
features as well. USAID's IUD revitalization project notes the transition in Kenya's method mix
away from IUD use and toward injectable use, without an increase in total contraceptive
prevalence. Sterilization use also declined in the same period (1998-2003). For these clinical
IUDs in Developing Countries-Perkin, Pollack and Ross
methods, it is impossible to know whether the declines reflect the increasingly stressed health
care delivery setting, the influence of myths about IUD safety, or the appeal of the injectable's
special features. Ultimately, clinic based services that require a trained provider and a medical
procedure (whether a pelvic exam or surgery) rest on a different infrastructure and programmatic
commitment than commodity supply and distribution, whether for pills, condoms, or injectables.
Although some countries in Asia or Latin America neglect the IUD, every country in sub-
Saharan Africa does so, with only very slight differences by education or residence categories. In
addition, although the IUD is considered a safe and effective method for use by HIV infected
women, it provides no STI protection and its promotion has been called into question where
improved barrier methods are badly needed.
In recognition of the underutilization of the IUD, two distinct programmatic efforts are
underway. Certain USAID cooperating agencies have, through various funding streams,
organized to revitalize the IUD through both operations research and program/IEC initiatives,
focused chiefly on sub-Saharan African countries but with exceptions in other regions.
Preliminary results confirm the nature of "macro" level barriers that exist within the health
sector. However, the growing private sector in some countries may present an opportunity for
growth and should be closely followed. While these social marketing, social franchising, or
private clinic services may not reach the lowest socioeconomic quartile, they can, through
consistent commodity supply and trained, less biased providers, expand IUD use within their
own markets. Increasing, the pool of users in that way may quicken interest and familiarity with
the IUD in general.
V. Affordability of a hormonal IUD and its influence on access and supply
In recognition of the significant health benefits of the hormonal IUD, the question was raised as
to whether public sector pricing (removal of the cost barrier) of either the existing Mirena LnG
IUD, a generic version, or a new hormonal IUD could not only provide public health benefits but
also catalyze increased interest in IUD use. Based on current manufacturing limitations a public-
private sector partnership would allow for a public sector price of under USD $5.00 for one
option only- the development of a new IUD. Although this option would require an up front
investment to support certain aspects of the necessary clinical research, it would allow for a re-
design to address design issues associated with the Mirena. The product price differential, in
comparison with the TCu 380A, would be minimized if spread over the first five years of use.
Introduction of this new IUD could be 5 to10 years away, and would be a strategic investment
requiring long-term vision and donor commitment. Such an investment would be highly likely to
succeed in providing a new, highly effective contraceptive option with associated significant
health benefits to women worldwide at a low public sector investment in return for an acceptable
public sector price. In the foreseeable future, however, it is clear that, a new IUD will not change
the landscape of negligible IUD use in sub-Saharan Africa.
VI. Recommendations Development of an affordable hormonal IUD: This should be an area of consideration for
long-term investment, even though there is a lack of evidence regarding the public impact that
would significantly impact use in sub-Saharan Africa. The innovation is one that promises to
change or save lives if deployed on a large enough scale; it offers the potential of expanding IUD
IUDs in Developing Countries-Perkin, Pollack and Ross
use in regions other than sub-Saharan Africa. It should be noted that obtaining a guaranteed public sector price is probably a time-limited option, one that requires a prompt commitment if this objective is to be realized.
If this recommendation is endorsed, key areas of support could include preparation for case
studies in selected countries matched to: 1) WHO clinical trial sites; 2) potential for both private
and public sector expansion; 3) acceptability for ICA donated Mirena LnG. The published case
studies would support advocacy efforts at the national level once trials are completed and
introduction is underway.
Large scale support for promotion or expansion of IUDs in sub-Saharan Africa: Efforts
should focus on continued support for those organizations working with USAID on revitalization
projects. Key areas of support could include: 1) full project evaluation of the revitalization
projects, beyond the possible termination of vulnerable USAID funding; and 2) documentation
and widespread dissemination of evaluation findings from these projects to inform further
investments and program innovation.
Support for Private Sector: Continued or new investments to support expansion of IUD use in
the private sector in selected countries. Again, documentation and widespread dissemination of
program results to inform further investments and program innovation is recommended. A
specific investment in social marketing/franchising projects with the intention of further
describing private sector capacity and replicable models is worth considering.
Identify a grantee to design a collaborative project taking into account currently completed or
proposed case studies of IUD availability, acceptability, and use, and identify 2-4 additional
countries to prepare for the new hormonal IUD introduction.
Research Priorities: Consider supporting the following: Operations research demonstrating
service delivery implementation and demand for the method would be a contribution to the
literature and inform some settings where advocacy requires an evidence base. Research of the
significance of amenorrhea in different populations and how to counsel and communicate about
the advantage of the new IUD to both providers and clients.
IUDs in Developing Countries-Perkin, Pollack and Ross
INTRODUCTION
The intrauterine device (IUD) has long been recognized as a highly effective, long-acting,
reversible method of contraception. Ideal in so many ways, the history of its development
reflects the widespread interest in continual revision of the device concept in an effort to
minimize side effects leading to early discontinuation, and maximize both contraceptive and
non-contraceptive health benefits. In parts of the world where fertility rates, unintended
pregnancy, and unmet need for contraception are high, the IUD should play a greater role in
providing a safe and highly effective contraceptive option than it does today. Despite past efforts,
parts of the world in greatest need have surprisingly low rates of use. This may be because both
clinical aspects of the IUD (foreign body, associated menstrual changes) and programmatic
barriers (the need for the commodity, a procedure, training, and a motivated provider) collide
and dilute the ideal aspects of the contraceptive impact itself.
This paper reviews the history, geographic distribution, and clinical aspects of IUDs that
influence IUD use. The second section analyzes programmatic issues that have equal or greater
influence on overall use. The third section addresses supply issues, examining options for
increasing uptake of the TCu 380A or expanding the marketplace through either a new IUD or
greater access to the LnG IUD currently unavailable in developing countries through lower
pricing. Finally, we will present an assessment of options for Foundation support for expanding
IUD uptake in the future based on our findings. We include our recommendations regarding
development and introduction of a new IUD for the public sector that would be cost effective and
offer new health benefits. A time frame for research and development is included in the report
(see Table 12 on page 54).
History of IUD development and use
IUD use was first reported in the 1800's and in 1902 Hallwig designed a pessary that had a stem
extending into the cervical canal that was marketed for self-insertion. This was followed by
Richter's silk and catgut ring covered in nickel and bronze wire and then Pust, who developed
another ring for intrauterine use, utilized during WWI. Problems with infection attributed to the
tail string led to the next innovation, the Graefenburg ring, in 1930. Contraception in Germany
was highly discouraged; Graefenburg was exiled, leaving the problem of high expulsion rates
unaddressed. In the 1960's there was revived interest in the IUD and a plastic device with an
inserter was developed. The first reported international conference on IUDs took place in 1962,
where different products were compared and the Lippes Loop was presented, followed shortly by
the Tatum T with added copper (contraceptiononline 2006). The Dalcon Shield, introduced in
1970 was designed to decrease expulsion, however high rates of infection attributed to its
multifilament tail led to its discontinuation and the stigma associated with IUD use in the US is
still prevalent today.
Trends in IUD use in the rest of the industrialized world are not as dismal but remain
disappointing. Based on 2004 United Nations data, Eastern and Western Europe represent 4-5%
of global IUD use, despite a greater diversity of types of IUDs that have been approved for use
than in the US (Wildemeersch 2003). IUD use in the United States is very low; estimated at 2%
IUDs in Developing Countries-Perkin, Pollack and Ross
in 2002 (Mosher, 2004). Recently, an increase in evidence supporting the use of the LnG IUD
for non-contraceptive benefits such as treatment of menorrhagia in the peri-menopause has lead
to uptake in use preferentially in older women (Hurskainen R 2004). In addition, the
manufacturer reports increased uptake in the US in the same demographic market for treatment
of menorrhagia or for use in menopausal women as hormone replacement therapy (Bronenkant
L., personal communication 2006). This increase in use does not appear to have influenced
provider bias against use in younger contraceptors in the US, at least not yet. Unlike the situation
in the global public sector markets, the cost differential between the TCu 380A and the Mirena
LnG IUD is small in non-subsidized developed country markets.
WHO estimates that approximately 160 million women worldwide use IUDs today. China has an
estimated two-thirds of these users, or 96 million. Only a small percentage of current users are in
eastern or western Europe or other industrialized countries (10%). The remaining 24% are in
developing countries other than China, concentrated in Vietnam, Egypt, Indonesia, India, and
Uzbekistan, and Turkey – those six countries alone contain half of all users in developing
countries excluding China (see
APPENDIX 1).
A remarkable geographic pattern of IUD users exists. All developing countries fall into the
following clusters, which show widely different determinants of use rates. They also help to
identify programmatic reasons for greater or lesser uptake. The percentages below reflect the
population of women using contraception.
1. The former USSR Republics: the five Central Asian Republics have high use (25- 56%),
as does the cluster of Moldova, Belarus, Ukraine (19% - 34%) and most probably Russia. Why? We can speculate that in the absence of hardly any contraception, the IUD entered the scene as fitting the established medical infrastructure, the weakness of the private sector, the unreliable supply lines for pills or injectables, and the pressures to replace abortion with an alternative. Formal IUD targets did not play a role.
2. At the other extreme are China and Vietnam, also with high use (36% - 38%) but for
different reasons. In these countries, intense policy and programmatic pressures for the method (along with sterilization in China) operated. Other options, with the exception of abortion, were less available. Consequently, little is known about what public preferences would have been if a range of contraceptive choices had been available.
3. In the Middle East, including Egypt, Turkey, Jordan, Syria, Lebanon, and Tunisia, use is
moderate to high (15%-36%). Again, formal targets did not play a role. The antipathy towards sterilization left a vacuum for long-term protection, and the medical communities were sufficiently strong to provide services. In Egypt at least, the private medical sector took up provision of the IUD. These countries are also more urbanized than many others, a factor contributing to higher IUD use.
4. The IUD experience in Latin America is mixed. Cuba is an exception with a very high
rate of 44% using the IUD; it has a unique medical system. Eight countries including Mexico, Honduras, Costa Rica, Bolivia, Colombia, Ecuador, Paraguay, and Peru have moderate user rates of 10%-14%. Others are low, especially Brazil, where the IUD is almost unknown at only 1% using it. The reasons for the uneven pattern across Latin America are obscure.
IUDs in Developing Countries-Perkin, Pollack and Ross
5. The Asian pattern is one of low to negligible use, apart from China and Vietnam. Few
countries exceed 4% using IUDs, including the highly populated countries of India, Bangladesh, and Pakistan. The reasons differ greatly, from India's preoccupation with sterilization to Pakistan's program wide weaknesses to Bangladesh's low clinical capacity. Broadly, adoption rates are very low, however there are states in India where IUD prevalence reaches 7% (reasons remain unclear) and in Pakistan a social marketing franchise program (PSI's Green Star program) is slowly expanding IUD use through the private sector. Low user rates of 6%-8% are found in Iran and Indonesia. A decline from 13% to 6% occurred in Indonesia over some years as the injectable rose sharply.
6. Across all of sub-Saharan Africa there is consistently low use: no country exceeds 3%.
Further, the main subgroups according to age, family size, education, and residence are consistently low, so low that use pervades the whole population. Causes of low use are apparently multiple: neglect of the method at the policy/program level, poor infrastructures and low clinical capacity, damaging rumors, female prejudices against an intrauterine foreign body, fear of infection or other complications, and either misinformed or reluctant providers. In any case, the universality of the IUD absence is striking compared to other regions.
In summary, the global pattern of IUD use is a disparate one, and reflects very different causes at work. The overall effect, for total contraceptive use, presents a pattern that is somewhat less puzzling than the odd geographic pattern for each individual method. As a rule, whatever contraceptive option is easiest to obtain, for whatever reasons, tends to dominate.


IUDs in Developing Countries-Perkin, Pollack and Ross
SECTION I
CLINICAL CHARACTERISTICS OF THE MOST
AVAILABLE IUDS
I.1 Types of IUDs
Two highly effective intrauterine devices (IUDs) are available in the global market today. The
TCu 380A ( TCu 380A)(ParaGard, Duramed Pharmaceuticals, Inc., Cincinnati, Ohio) and the
levonorgestrel intrauterine system (LnG IUS or IUD)1 (Mirena, Berlex Inc., Montville, New
Jersey) are currently available in over 100 countries. Figure 1 provides images of these two
IUDs. The TCu200, and multiload Cu 250 and 375 are still listed on the UNFPA commodities
list but because of the proven higher effectiveness of the TCu 380A in comparison, and WHO's
recognition of it as the first choice among the copper bearing devices, these alternatives will not
be specifically addressed in this paper (WHO 1997).
Several other IUDs, also shown in Figure 1, are currently under development or have been
introduced in Europe including the Gynefix IUS and the mini Gynefix IUS (Van Kets H, 1997),
FibroPlant (Wildemeersch 2002), the Femilus™ and Femilus™ Slim (Wildemeersch 2005),
and the Flexi-T (Prosan).
Figure 1. Images of six IUDs
TCu 380A Mirena
Femilis™ Femilis Slim™ Gynefix® IUD In Situ
1 Mirena® is referred to as a levonorgestral-releasing intrauterine system, 20 ug/day. The product monograph specifically indicates that the use of the term "intrauterine system" should differentiate it from the intrauterine contraceptives (IUDs) of the past. In this document we will refer to either Mirena, or the LnG IUD (in this case the same thing unless indicated otherwise).
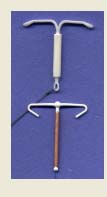
IUDs in Developing Countries-Perkin, Pollack and Ross
Figure 1 (continued).
Mirena (above) and Flexi-T (below) for size comparison
I.2 TCu 380A IUD – Advantages and Disadvantages
The TCu 380A is a polyethylene device with barium sulfate added to allow for visualization by
x-ray. The device measures 36 mm long by 32 mm wide, with a 3 mm bulb at the base of the
stem. Each of the horizontal arms is covered with a copper sleeve and copper wire is wound
around the length of the vertical stem for a total surface area of copper of about 380 mm². A
monofilament double strand polyethylene string is tied to the bulb at the base for use in removal.
The Cu T380A has a 7 year shelf life, and is approved for 10 years of use. Figures 2 and 3
outline advantages and disadvantages of this IUD.
Mechanism of Action
IUDs in general work by preventing effective sperm function and therefore fertilization (Rivera,
1999). The TCu 380A releases copper ions and causes an inflammatory response with associated
local increase of prostaglandins, white blood cells, and change in the normal fluids present in the
uterus and tubes (WHO 1987; Saleem 1996).
Figure 2. Advantages of the TCu 380A
• Highly effective
• Protective against ectopic pregnancy
• Probable protective effect against
endometrial cancer
• Long lasting, convenient and safe
• Cost effective Adapted from Contraceptive technology 2004;
Hubacher and Grimes 2002
Effectiveness The TCu 380A is well known for its high efficacy- the aspect of contraceptive effectiveness attributed to the method itself. Pregnancy assays that are highly sensitive and measure the lowest
IUDs in Developing Countries-Perkin, Pollack and Ross
levels of human chorionic gonadotrophin (HCG) in the blood indicate that fertilization and implantation do not occur in TCu 380A users (Wilcox, 1987). Because the IUD is not user dependent, once placed correctly, effectiveness is high with a failure rate of less than 1 per 100 woman-years and cumulative failure rate at 12 years of 2.2% per 100 women (Sivin 1987). This rate is comparable to the cumulative 10-year failure rate of female sterilization in the US of 1.9 per 100 women.(Peterson 1996). The TCu 380A is FDA approved for 10 years of use however studies indicate that it is effective for 12 years (WHO 1997) and unpublished data indicates the same high efficacy in a small study population after 20 years of use (Sivin 2006 pre-publication communication).
Ectopic Pregnancy
The 12-year cumulative ectopic pregnancy rate of 0.7 makes the TCu 380A protective against ectopic pregnancy compared with non-contraceptive users. (Franks 1990 ).
Non-contraceptive health benefits
The TCu 380A is a non-hormonal method that provides an option to women with contraindications to oral contraception or other hormonal methods, and an equally effective option to women who are not sterilization candidates. There are now two studies that report a statistically significant protective effect against endometrial cancer in copper IUD users (Hubacher D and Grimes DA 2002).
Figure 3. Disadvantages of the TCu 380A
• Provider dependent method • Spontaneous expulsion
• Perforation • String loss
• PID risk with insertion
• Does not protect against STI/HIV Adapted from Contraceptive Technology 2004
Expulsion / Perforation/ String loss Although the IUD is highly effective, proper placement is critical to long-term effectiveness. Spontaneous expulsion of the TCu 380A occurs in 2-10% of users within the first year (Zhang J 1992). Rates were higher in adolescents (and decreased with increased age), women with heavy menstrual flow, and women with a history of cramping pain (dysmenorrhea), with a 30% chance of repeat expulsion (Bahamondes 1995). Expulsion can be "silent" (without any pain) or associated with bleeding and cramping. WHO's RHR 2003 technical report includes interim data from ongoing multicenter IUD safety and effectiveness studies. The cumulative net expulsion rate is reported as 11.2 per 100 women after 10 years and this rate is consistent with their previous 12 year published study of the TCu 380A of 12.5%, with most occurring in the first few years (UNDP/UNFPA Contraception 1997).
IUDs in Developing Countries-Perkin, Pollack and Ross
Perforation2 is rare and occurs at the time of insertion. In experienced hands the estimated perforation rate for copper IUDs is 1 per 1,000 (WHO 1987). There is no evidence that the IUD "migrates" out of the uterus following normal insertion. The myths prevalent in parts of Africa may be the result of outcomes from poor insertion resulting in undetected perforations (Grimes, 2004A). Because copper causes inflammation and can cause scarring in the abdominal cavity near the bowel, bladder and ovaries the copper bearing IUD should be removed. This is most easily accomplished via laparoscopy, however, in places where no skilled provider is available, abdominal surgery may be required.
Lost strings can signal a perforation, undetected expulsion or simply ascension of the strings into the uterine cavity. Where ultrasound is available, identification of the IUD in the uterus and guided removal using local anesthetic at the cervix is possible. When ultrasound is not available the provider must assume an undetected expulsion and either explore the uterus blindly, and/or provide an alternative contraceptive method. There is no published data on rates for lost strings.
All IUDs are provider dependent methods. Both expulsions from improper placement and perforation rates can be minimized by use of standardized training and skilled providers (Chi, 1993; Harrison-Woolrych 2002). Published studies do not report widely on the impact of using less experienced providers on rates of either of these complications. However, provider shortages in many geographic locations, and in particular, in sub-Saharan Africa, make this more of a challenge than simply investing in better guidelines development and training.
PID By far the most controversial issue related to IUD use has been concern that the IUD increases the risk of pelvic inflammatory disease. In the WHO-sponsored IUD trials, the risk of upper genital tract infection was limited to the first 20 days after insertion (Farley TM 1992). A recent systematic review of the literature attempted to assess the added risk of PID following IUD insertion in women with cervical Chlamydia or gonorrhea (Mohllajee 2006). This analysis is complex because of both the nature of the problem and by necessity, the study design; Chlamydia infection is often asymptomatic and it is not possible to randomize infected and uninfected women for IUD placement. Taking into account both cases - the overall increased risk of PID in the modern IUD user has been estimated based on observational studies and appears to be low, even in environments where the background prevalence of STIs (Chlamydia and/or gonorrhea) is high, ranging from 0.6 per 1,000 women (International Collaborative Post-marketing Surveillance of Norplant. 2001) to 1.11% in Nigeria (Sinei 1990) and 1.9% in Kenya at 30 days after insertion of the TCu 380A IUD(Walsh 1994). Because the rate of PID in infected non-IUD users is unknown in these groups, it is impossible to determine the added risk (Shelton 2001).
It is important, however, to consider that almost all other contraceptive methods (condoms as more inclusive barriers, and hormonals and sterilization against PID) do provide some protection against STIs or PID. The copper IUD does not.
2 Perforation is a hole in the wall of an organ; in this case the IUD is accidentally inserted into the wall of the uterine muscle.
IUDs in Developing Countries-Perkin, Pollack and Ross
HIV positive women
Limited research reporting on safety of the copper IUD used in HIV infected women suggests that complication rates are comparable in HIV infected and non infected IUD users.(FHI 2005).
Nulliparous or nulligravid woman Although there is no contraindication to use of the TCu 380A in a women who has never been pregnant or given birth (WHO medical eligibility criteria category 2)3, there may be a higher rate of mechanical problems associated with use. Expulsion rates are higher in adolescents, probably most related to their nulliparous state (Bahamondes 1995). Insertion is also more difficult related to the small diameter of the cervical canal (Grimes 2004). Use of oral non-steroidal anti-inflammatory drugs prior to insertion or even cervical dilators overnight can help prevent vasovagal reactions to insertion but these interventions may not be practical in all low resource settings.
Side Effects - Bleeding and Anemia
The most common medical indication for discontinuation of the TCu 380A is irregular bleeding.
Irregular bleeding in the first several months of use is common but decreases over time. Women
using the TCu 380A also often have heavier menses than the non-user. If the bleeding is
significant, the health care provider needs to discern whether it is IUD-related or due to an
undiagnosed pregnancy, an infection, or another intrauterine disorder. This in itself is an added
intervention, and in a compromised health service delivery setting where access to providers is
severely limited presents a barrier to IUD selection and continuation.
Iron deficiency anemia can be caused by poor nutrition and/or blood loss in industrialized countries, however it is more broadly multifactoral in developing countries with high prevalence of malaria, hookworm and other chronic disease and infections (HIV included). Actual blood loss associated with TCu 380 use is less than with older model IUDs. The marginal impact on hemoglobin level, studied in populations with normal levels of 12g/dl or greater, is small in the range of -0.5 g/dl occurring during early use may equilibrate over time with longer use (Andrade AT, 1987). The WHO listed Cu IUDs as category 2 –advantage outweighs risk for use in women with anemia. In any event, the TCu 380A does not decrease blood loss associated with menses or other intrauterine disorders. It is unclear what impact the small but steady increased menstrual blood loss associated with use has in a population with a high endemic rate of multifactoral anemia.
I.3 The LnG IUD - Advantages and Disadvantages
The Mirena is a plain plastic T-shaped frame, 32 mm long and wide and impregnated with
barium sulfate making it radiopaque. The steroid reservoir around the stem is a cylindrical
mixture of 52 mg of levonorgestreland polydimethylsiloxane, covered by a membrane that
allows for release of 20ug/day of LNG. Removal strings are attached to the base. MIRENA has a
3. Medical Eligibility Criteria for Contraceptive use (2004)(MEC) is one of the World Health Organization's two evidence based guidelines on contraceptive use, intended for policy-makers, program managers, and the scientific community. A designation of category 2 is "a condition where the advantages of using the method generally outweigh the theoretical or proven risks".
IUDs in Developing Countries-Perkin, Pollack and Ross
3-year shelf life and is effective for at least 5 years. Figures 4 and 5 outline the advantage and disadvantages of this IUD.
Mechanism of Action Mirena is a steroid releasing device that acts locally to cause high levels of levonorgestrelin the endometrial tissue that lines the uterus, but low levels of systemic hormone. It has a minor effect on ovarian function. Both factors of high local impact and low systemic absorption are important in understanding the ultimate advantages of any levonorgestrel-releasing IUD. Local effects include thickened cervical mucus that slows the passage of sperm and a direct suppression of the endometrium causing it to atrophy (dry up) and become unresponsive to estrogen. The progestin effects also cause a decrease in sperm motility in both the uterus and the tubes. This is the result of the same sterile foreign-body reaction noted associated with the T Cu 380A that impedes ovum and sperm transport and fertilization; the LnG IUD also induces production of a glycoprotein that inhibits sperm-egg binding (Mandolin 1997). Together the local effects are responsible for its highly effective contraceptive action (Jensen 2005) and other non-contraceptive effects.
Figure 4. Advantages of the LnG IUD
• Highly Effective
• Protective against ectopic pregnancy
• Possibly protective against PID •
Decreases menstrual/uterine blood loss
• Causes amenorrhea in >20% of users
• Safe, long lasting, convenient
Adapted from Contraceptive Technology 2004
Effectiveness Multiple randomized multicenter studies have confirmed the long-term efficacy of the LnG IUD and report a 1 to 7-year failure rate of 0 to 1.1 in women across all age ranges including those most fertile in the group under 25 years of age (Jensen JT 2005). In several large studies (Table 1) there was no significant difference in contraceptive efficacy between the TCu 380A and the 20 ug per day LnG IUD.
IUDs in Developing Countries-Perkin, Pollack and Ross
Table 1. Principle clinical trials documenting the contraceptive efficacy of the LnG IUD
Taken from Jensen 2005
Ectopic Pregnancy Because of the LnG IUD's high efficacy rate, it is also highly protective against ectopic pregnancy when compared to non-contraceptive users. The US ectopic pregnancy rate is estimated to be 3-4 per 1,000 women-years in non-contraceptors compared to an estimated rate of 0.2 per 1000 women years in LnG IUD users (Sivin 1991)
PID As stated previously, PID rates are low among TCu 380A users and the same is true for LnG IUD users. Rate comparisons against the TCu 380A are shown in Table 2 and are favorable in large trials. In another trial in Scandinavia PID was diagnosed in 0.9% of over 2,000 study subjects using the LnG IUD compared to the overall incidence of PID in the general population of 2.1%.(NDA 21-225; Kani J 1992). This in itself is not enough to determine a protective effect but suggests that there is no added risk. The fact that there is biologic plausibility for protection because of the added barrier provided by progestin induced thickening of cervical mucous is important, an effect also noted in oral contraceptive users (Jonsson 1991). In addition, atrophic changes of the endometrium also caused by the local progestin effect may be protective(Hubacher and Grimes 2002).
IUDs in Developing Countries-Perkin, Pollack and Ross
Table 2. Selected health benefits/disease protection from using the levonorgestrel IUD
Taken from Hubacher and Grimes 2002
HIV positive women The LnG IUD does not offer any known protection against cervical infection from sexually transmitted diseases, or any protection against the acquisition of HIV. Although it is unlikely that the IUD's progestin effects add risk for acquisition or transmission of HIV, the specific impact of locally released progestin (as opposed to oral contraceptives or injectables) has not been studied. The issue of impact of progestin contraceptives more broadly and HIV acquisition was addressed recently at an Africa regional meeting where unpublished data were reviewed and found to be difficult to interpret in general application4. Based on the current WHO Medical Eligibility Criteria (WHO Medical Eligibility Criteria 2004), progestin contraceptives, including the IUD, are not contraindicated in HIV+ women. The LnG was not specifically addressed.
Bleeding and anemia The LnG IUD's local effects caused by the impact of levonorgestrel on the endometrium leads to a measurable decrease in menstrual blood loss (Anderson 1994, Suvisaari J, Lahteenmaki P 1996) and an increase in hemoglobin levels over time. Four studies address actual measures of decreased blood loss as indicated in Table 2. The data from these studies also show a decrease in hemoglobin levels with TCu 380A IUD use. The significance of the measurable increase or decrease is unclear in populations with multifactoral anemia as described above.
The impact of endometrial suppression on menstrual blood loss in LnG IUD users is notable
4 Hormonal Contraception and HIV: Science and Policy; Africa Regional Meeting Nairobi 19-21 September 2005. World Health Organization. Available at: http://www.who.int/reproductive-health/stis/hc_hiv/nairobi_statement.pdf
IUDs in Developing Countries-Perkin, Pollack and Ross
within the first 2 months of use and sustained. In one single-center follow-up study, 25% of 250 women had irregular spotting as opposed to normal menses at 6 months post insertion, and 44% had amenorrhea. At 24 months, 11% of women continued to have irregular spotting and 50% of women reported amenorrhea (Hidalgo M 2002). Two large multicenter randomized trials reported an amenorrhea rate of 17% at one year and 30% at 2 years (Bavega 1989, Sivin 1994).
Quantifying and characterizing menstrual pattern changes is difficult in diverse populations. Although there is an unquestionable decrease in the volume of menstrual blood loss, the irregularity of the resulting bleeding pattern (referred to as "breakthrough bleeding") caused by thin walled blood vessels in the affected endometrium reported in LnG IUD users can be considered a disadvantage. Irregular spotting is most significant during the first few months of use. Discontinuation rates are affected by both initial but also persistent irregular bleeding (WHO ATR 2003) because of both provider and client concern that the bleeding reflects a problem that needs to be diagnosed and resolved (Jacobson 2006, Davie 1996). The changes in menstrual pattern are rapidly reversible with IUD discontinuation (Xiao B 2003).
The LNG IUD has most recently been successfully marketed and used to treat menorrhagia. The above studies address the issue of menstrual disruption in the normally menstruating woman. However, local application and impact of progestin on the endometrium is an important intervention for women who bleed too much due to age related changes of the endometrium or certain benign but problematic pathologic changes. Most of recent publications on LnG IUD use point to the almost perfect therapeutic match for women approaching or in menopause. Several studies indicate that the LNG IUD provides a non-surgical alternative to ablative therapy or hysterectomy for many women with dysfunctional uterine bleeding, uterine myomas, and adenomyosis (Stewart 200, Fedele 1997), These are problems that a percentage of all women suffer from regardless of ethnic background or socioeconomic class. All surgical procedures carry associated risk, and this is especially true in developing countries.
Figure 5. Disadvantages of the LnG IUD
• Provider dependent method
• Spontaneous expulsion
• PID risk with insertion (unknown)
• Does not protect against STI/ HIV
Adapted from Contraceptive Technology 2004
Expulsion/Perforation/String loss It is difficult to estimate ease or difficulty of IUD insertion. That said, many providers acknowledge the greater level of insertion difficulty related to the insertion method design associated with the LnG IUD compared with the TCu 380A. Data do indicate that experience with insertion (numbers of insertion per provider) affects rates of perforation for all IUDs. This inverse relationship (less experience/higher perforation rate) is independent of whether the
IUDs in Developing Countries-Perkin, Pollack and Ross
provider is a physician versus mid-level provider (Harrison-Woolrych 2003) but points to the importance of ease of insertion by design. Until recently most data regarding perforation and expulsion of the IUD were collected in expert centers and were specific to the copper IUDs. With increasing introduction and uptake, new data demonstrate not only the risk of both with the LnG IUD but also the risk of complications associated with the resulting intra-abdominal location, once thought to be of much less concern than with the copper IUDs. Other non-medicated, non-copper bearing IUDs used in the past (that had higher failure rates) were not considered a risk if left inside the abdomen following perforation. However, more like the copper bearing IUDs, the LnG IUD continues to release progestin following perforation and presents a problem. Although a perforation can go unrecognized for months or even years, intra- abdominal scarring or adhesion formation can occur and is considered an indication for removal (Van Houdenhoven 2006).
A recent retrospective study from the Netherlands reported a perforation rate of 2.6/1,000 LnG IUD insertions. Previous studies of uterine perforation related to several of the copper bearing IUDs estimated the incidence of perforation of 1 per 1,000. Risk factors for perforation include the experience of the provider however it seems that perforation is less likely to occur if a withdrawal rather than a push out technique (as is the case with the Mirena LnG IUD) is used (Van Houdenhoven 2006). This is important when considering investment in a generic Mirena vs. the development of a new device that addresses this concern.
PID As reviewed above, there is a small increased risk of PID for the 20 days following IUD insertion. Studies documenting this did not include the LnG IUD, however, because this risk is believed to be related to vaginal contamination during insertion there is no reason to believe that the risk would be different for the LnG IUD.
I.4 Additional clinical considerations
IUD failure and intrauterine pregnancy
Although both the TCu 380A and the LnG IUD are very highly effective they still have failures.
As mentioned, all failures should raise alarm about the possibility of ectopic pregnancy, a life
threatening circumstance in environments where access to health care is minimal (such as rural
parts of SSA, where, as an indicator of access to surgery, obstetric fistula rates are very high). In
addition, an intrauterine pregnancy with an IUD in place requires removal of the IUD, adding
some additional risk of miscarriage with removal. If the IUD is not removed, severe infection has
been reported in the second trimester.
Postpartum and post-abortion IUD use Both the TCu 380A and the LnG IUD are considered safe for placement immediately post partum and post abortion when no intrauterine infection is present. Although higher expulsion rates have been observed following postpartum and post second trimester abortion placement of
IUDs in Developing Countries-Perkin, Pollack and Ross
copper IUDs, expulsion rates are not higher following first trimester abortion (Grimes D. 2004). Data for the LnG IUD are not available, however because expulsion is probably the result of increased uterine irritability and contractility related to the delivery or the procedure one should expect the same.
For several decades the promise of expanding IUD uptake through immediate postpartum IUD services has lead to targeted interventions. Experience shows that the added burden during hospital based deliveries (particularly for good counseling and informed consent) predicts an increase during the introductory phase but a lack of sustainability. This appears to be true across all strata of maternity care infrastructures. Given the complexity of public sector maternity service delivery in poorer settings (in Africa the need to introduce PMTCT and the severe provider shortage), it is unlikely that postpartum IUD services will take hold broadly (Jacobson R- EngenderHealth 2006, Chirenge M- Zimbabwe 2006 , Somelela N- IPPF, 2006 personal communications). Postpartum IUD use is common in parts of China and Egypt where IUD use is high, and Mexico where it is moderate and obstetric care is institutionalized. Overall, adding counseling and IUD provision to a weak obstetric delivery setting is a formidable task and should only be attempted where quality monitoring can be implemented to assure informed consent.
Abortion services are provided on a random basis in almost all developing countries, influenced by laws for the most part. In those countries where abortion is legal, and therefore more available and organized in the public and private sectors post-abortion IUD placement is a safe option. Although described in the literature in the study setting, these publications describe outcomes of clinical trials that examine safety and efficacy (Grimes DA 2004). Published literature on programmatic implementation is scant, probably because model programs are rare.
Discontinuation of IUD use; clinical considerations
On a population basis, side effects are identified as the primary cause for discontinuation of use
of the IUD (See APPENDIX 2 for one-year discontinuation rates for 19 countries). These side
effects include irregular bleeding with both the copper bearing IUDs and the LnG IUD, and
increased menstrual bleeding with the copper bearing IUDs and amenorrhea with the LnG IUD.
However, removal rates due to bleeding pattern changes vary greatly among study populations.
A recent study from Scandinavia reported very low rates of discontinuation at 5 year follow-up
(2.1) (Pakarinen 2003) however other older studies that compare discontinuation with copper
IUDs report 5 year rates of 20% (Sivin I 1990, Andersson 1994). This may be a reflection of
different counseling practice rather than an inherent cultural problem.
One can assume that a certain amount of discontinuation is due to provider concern when these
normal side effects occur, and the lack of good training that would enable them to discern the
true need for removal, or adequate pre-insertion counseling to help the user discern in some
cases. Either way, this is an attendant risk with both the TCu 380A and the LnG IUD. Where
ultrasound or other diagnostics are unavailable the provider cannot differentiate between a lost
IUD vs. lost strings, bleeding from infection vs. a normally irregular bleeding pattern, or in the
case of a LnG IUD, method induced amenorrhea vs. intrauterine pregnancy or ectopic
pregnancy. In the case of Depo-Provera induced amenorrhea, which occurs in roughly 60 % of
users at 1 year, the short-term nature of the method and inability to "discontinue" at will has not
appeared to hamper uptake. Discontinuation rates at one year are high but reasons for this are not
IUDs in Developing Countries-Perkin, Pollack and Ross
clearly method related and could be access related. For long-term Depo-Provera users, it can
take up to a year to return to a regular menstrual pattern following discontinuation.
Table 3 represents six year interim results from a WHO sponsored multicenter trial designed to
compare the TCu 380A and the LnG IUD5.
Table 3. Cumulative net probabilities of IUD use discontinuation
(Standard error) per 100 women after six years of use (interim data to 30 September 2003)
Taken from WHO Annual Technical report 2003 Importance of the Continuation Rate The significance of discontinuation rates should not be underestimated. A simple calculation provides an idea of the impact of early discontinuation of a method. Note that most users are past postpartum amenorrhea and so are fully fecund, so unless users quickly adopt an alternative method, which many or most do not, they are exposed.
The available evidence shows the TCu 380A having a better continuation rate than that of the current LnG. That is not the only criterion for preferring one device over another, but it is an important one and we think points to the need to invest heavily in counseling and clinical training with the introduction of a hormonal IUD. When one device returns women to an exposed state for unwanted pregnancy sooner than another (and surely all IUD users wish to avoid pregnancy), there will be more unfortunate events than with the inferior device. Here is a simple exercise to illustrate that.
Assume a population of 100,000 women subject to an annual pregnancy rate of 300/1000 women. If the difference in mean continuation between the TCu 380A and the LnG were 3.5
5 We are still trying to confirm where the trial is ongoing but we don't believe that there is an African site,
IUDs in Developing Countries-Perkin, Pollack and Ross
years vs. 3.25 years, the results of the 3 months of extra exposure to pregnancy are shown in the
Table 4. If instead the difference were 3.5 years vs. 3.0 years, i.e. 6 months, the numbers would
double. The truth may be somewhere between, but the point is that the continuation rate is very
important. We do not know what the final, improved LnG device would do; hopefully it would
compete.
Table 4. The consequences of additional exposure to pregnancy as impacted by
continuation rates
difference difference
15,000 Extra pregnancies
5,000 Extra abortions if 1/3 of pregnancies are aborted
10,000 Extra births
100 Extra maternal deaths if the MMR is 1000/100,000 births
2,000 Extra infant deaths if the IMR is 200, or
2,500 Extra child deaths if the U5MR is 250
Plus Extra orphans in a high-HIV environment
Adapted from Ross 2006 Anemia: Could the LnG IUD have a public health impact?
Anemia is defined as a reduction below normal in the concentration of erythrocytes or hemoglobin in the blood; a normal hemoglobin (Hb) level in women is greater than 12 g/dl . Anemia occurs when the equilibrium is disturbed between blood loss (through bleeding or destruction) and blood production, which can be affected by a large number of factors. Iron deficiency anemia, one type of anemia, is caused by a lack of iron stores. Other nutritional deficiencies contribute to the range of anemias found in developing countries, but anemia is multifactoral and significant contributors include hookworm, malaria and other chronic diseases that affect either red blood cell survival or production. Blood loss, as a cause of anemia, is not listed in the developing country literature. Post-hemorrhagic anemia, caused by large amounts of blood loss due to an accident or an obstetric event is not listed because if the patient survives, the body should be able to replenish stores. Although hemorrhage is listed as the greatest cause of maternal death, it is the underlying anemia that tips the scale and leaves women without capacity to survive the loss of even normal blood loss associated with delivery, causing more than 60,000 deaths per year (UNICEF 2004).
Iron deficiency anemia is listed by WHO as one of the top ten most serious health problems. In adults iron deficiency affects the workforce with an estimated productivity loss of up to 2% of GDP in the worst countries. It not only increases the risk of a bad obstetrical outcome but contributes to the risk of prematurity, and low birth weight. Children born to iron deficient mothers start life with lower stores inherited from the mother and have difficulty catching up. They enter the critical period from age 6-24 months, when breast feeding supplementation begins, without ability to recover to build adequate iron supplies for health and normal cognitive and psycho-motor development, affecting approximately 40-60% of the developing world's children.
Documentation of anemia prevalence across Africa is poor and derived from small studies. The distribution of up to 80% in coastal regions and 30-40% inland reflects the multifactoral nature
IUDs in Developing Countries-Perkin, Pollack and Ross
of the problem given the impact of malaria, hookworm, and socioeconomic factors. Although about half of all cases are probably attributed to iron deficiency anemia a report in 2001 that looked at strategies to address iron deficiency anemia suggested that iron supplementation and micronutrient replacement is likely to have less impact than expected unless coupled with treatment for malaria and hookworm. It is also likely that the HIV epidemic is contributing more to this picture than ever before. (MacPhail 2001; OMNI 2006).
Why is all of this important? It is true that a medicated IUD such as the LnG IUD would decrease menstrual blood loss. In the woman who is child spacing one might argue that this saving would help to build iron stores that could make a next pregnancy safer for both mother and newborn. However, in light of the complexity of the anemia ‘puzzle" it remains unclear whether a decrease of menstrual blood loss and a potential marginal increase in hemoglobin in women who are child spacing would lead to lives saved.
In some countries where the uptake of Depo-Provera has been documented, the resulting amenorrhea provides a real time opportunity to look at any impact this might have on hemoglobin levels in select settings and should be further examined.
IUDs in Developing Countries-Perkin, Pollack and Ross
SECTION II
PROGRAMMATIC ISSUES
II.1 Estimating demand and potential demand in an expanded market
If, by asking about demand, we are asking whether there is some untapped interest out there in
adopting contraception, the answer is always yes. In every population there are at least some
women, and men, who do not want the next pregnancy. That is evidenced by the unmet need
figures, even though many women included in the "unmet need" category disagree and say they
will not use a method. However, they are roughly matched by other women who say they intend
to use a method, even though the surveys classify them as not having unmet need (this group is
mainly spacers who want a birth within two years but not right now).
In any case, unmet need does not serve well as a gauge of likely contraceptive adoption over the
next few years. In most countries, it is so large as to constitute an unrealistic outer limit of the
market. Prevalence of contraceptive use seldom rises more than one or two percent of couples
per year, and within that an individual method usually rises less. The global figure of 17% of
married women with unmet need (APPENDIX 3) will fall only gradually as prevalence rises,
and the grand total of 123 million women in need may even grow as populations increase.
As to "demand," in no country are women vocally "demanding" the IUD, but in all countries
there is at least some unexpressed potential for IUD use, and there is always a subgroup that
would use the method if it were readily available and presented effectively. "Market" is a more
useful concept than demand since it combines the element of programmatic push with the likely
response by the client. A stronger program combined with a more favorable setting makes for a
larger market. So to get a handle on the "potential demand", or likely increase in IUD use, we
need to consider the impact of strengthening a program along with the constraints of the setting.
That is, equally strong programs applied in all regions will yield less in sub-Saharan Africa than
elsewhere, but they will still yield something, and the yield will be greater where the program is
stronger.
Demand is heavily affected by the quality and reliability of the program as a whole over time,
which reflects the lack of resources in the setting. These lacking resources include: the absence
of providers altogether, not just that of trained providers; poor stability in the entire commodity
supply chain, not just that for IUD supply; and migration of public commodities into the private
sector (theft) for all re-salable commodities, etc.
Historical Experience for Increases in IUD Use
One fix on the probable market for increased uptake of the IUD is to look at the past record in
countries that have multiple surveys, to show the trends. Table 5 (page 29) details historical
experience for increases in IUD prevalence for 26 countries. These are countries where a clear
rise occurred, though the levels vary greatly, from very low in Nepal and Sri Lanka to very high
in Egypt and Uzbekistan. The annual pace of increase also varies, from lows of around 0.100
point to highs of around 1.000. That is, from a tenth of a point rise per year to a full point rise per
IUDs in Developing Countries-Perkin, Pollack and Ross
year. For example, in Iran, the rise over five years was from 7.0% to 8.3% of married women using the IUD, for an annual rise of 0.260 point per year. Across all countries the median rise is 0.506, a half point per year. Egypt is an outlier at 1.422 points per year over a long period. This gives rough bounds on what might be achieved in new national campaigns. The past IUD increases have of course occurred in the context of what total prevalence has done, which across all countries averages about 1.5 points per year, with an upper limit of around 2.5 points. A rise in IUD use can be partly due to substitution, with another method declining. For instance, in Egypt, pill use fell from 20% of women to only 9% during the IUD's steep climb. Or, the IUD rise can be accompanied by other increases, as in Tunisia, where pill use doubled from 5% to 11% while IUD use also rose. Figure 6 below shows the relationship between total prevalence and IUD prevalence. Figure 6a provides data based on 248 surveys taken since 1980, giving the two prevalence rates, and Figure 6b is based just on 114 surveys in the 26 countries with increases. In Figure 6a, there is a cluster at the extreme lower left for near zero values on both prevalence rates (as in sub-Saharan Africa), showing a direct relation for numerous countries. On the right side of the chart are the outliers, for which both figures are very high –IUD prevalence and total prevalence averaging around 65%. Figure 6b is similar, but without the cluster of near zero values at the lower left and with fewer points. IUD use has been an important determinant of total contraceptive use in many countries, and IUD use can be expected to rise at no more than about a point a year at the national level. Therefore, an optimistic scenario for selected sub-Saharan Africa countries would be a rise from about 3% now to 13% over 10 years. That would be a substantial contribution but it would require an intensive campaign with providers, resting upon the appeal of a new IUD device that promises health benefits. To be acceptable in a policy sense, it would have to be folded into the context of multiple method offerings. For a comparison, the injectable rose very rapidly in a few cases, but only moderately or not at all in other cases, as with the IUD. Peak cases are Indonesia (1.48 points/year from 1994 to 2002), and Namibia (1.37 points/year from 1992 to 2000).
IUDs in Developing Countries-Perkin, Pollack and Ross
Figure 6. Relation of Total Prevalence to IUD Prevalence
Figure 6a. Total prevalence follows IUD prevalence
(248 national surveys)
Prevalen
Figure 6b. Total prevalence follows IUD prevalence in countries having past IUD increases
(114 national surveys)
Prevalen
(Source: Macro DHS StatCompiler)
IUDs in Developing Countries-Perkin, Pollack and Ross
Table 5. Historical Experience for Increases in IUD Prevalence
LATIN
AMERICA
MIDDLE EAST
SUB-SAHARAN
AFRICA
CENTRAL
ASIAN REP.
*Percent of married women aged 15-49 using the IUD **Read: Iran's IUD prevalence rose by 0.260 point per year From 1992 to 1997, that is, from 7.0% to 8.3%. (Source: Macro DHS StatCompiler)
IUDs in Developing Countries-Perkin, Pollack and Ross
Projections for Users and Adopters: Examining the numbers
The historical experience just outlined affords one gauge of the IUD market; repeat surveys show
that a working maximum is an increase of 1% per year in the proportion of married women using
the IUD. If we take the median value, which happens to be half of that (0.5%), as a basis for
illustrative projections, we can estimate the number of new adoptions per year per country, as in
Table 6 below. A range around each number is readily obtained -- simply take half of each
number to represent one-fourth percent rise per year (0.25%), or add half to the number to
represent three-fourths percent rise per year (0.75%). If there is an interest in exploring a full one
percent rise per year, double the number of adopters. (All this could be shown as alternative
projections but the table below would become cumbersome.)
Table 6 shows first the IUD prevalence in the latest survey, with regional averages based upon
aggregated numbers of users and women in the countries. The second column shows what IUD
prevalence would be five years later, by adding a half percent per year (2.5% in 5 years). The
next two columns give the numbers of married women and users, with users calculated as
prevalence multiplied by women. The next to last column gives the estimated numbers of IUD
adoptions needed to match the prevalence increase. There are three components to the adopters
needed: (a) a half percent of married women to raise prevalence by the assumed amount, (b)
adoptions to replace last year's dropouts from terminations, aging out, etc. (33% of last year's
users), and (c) a 2% increase to allow for population growth. The final column compares
adoptions to women to get the annual acceptance rate. (The top figure, for all less developed
regions, is 6%, but that is for annual adoptions, not the net annual rise in prevalence of one-half
percent per year. The difference is due to the three components just explained.)
Countries and regions differ sharply in their adoption rates because adoptions depend heavily
upon the numbers required to replace last year's IUD dropouts. The assumed mean continuation
period of 3.5 years (for the TCu 380A) implies the reciprocal, an annual loss from the using pool
of 28%, and about 5% more who age out or end their marriage, for 33% total. Where users are
numerous, as in high prevalence areas, dropouts are also numerous, driving up the adopters
needed to maintain prevalence, quite apart from raising it. So adoption rates must be high where
IUD prevalence is high to maintain this equilibrium. Both are maintained at a very low level in
sub-Saharan Africa.
Each regional figure reflects the dominance of particular countries. East Asia's figures reflect
China's high IUD use, whereas South America's figures reflect Brazil's very low use. Applying
the assumed prevalence increase of a half percent to every country is crude of course, but the
exercise yields the aggregate picture, with proper country weights by population, and it gives a
useful order of magnitude. As noted above any total can be lowered or raised to assume an
annual increase of a fourth percent or three-fourths percent, etc. Higher adoption rates have
occurred in special projects, but national changes usually come about slowly. (The full table is in
APPENDIX 4).
IUDs in Developing Countries-Perkin, Pollack and Ross
Table 6. Projections of IUD prevalence, users, and adopters at a half percent rise per year
in IUD prevalence
Adoption
Rate (% of
Prevalence
after 5 yrs at
Adoptions
latest survey
1/2% rise/yr
MWRA in 2005
Users in 2005
during 2006
LESS DEVELOPED
REGIONS
941,040,280
145,843,807
56,625,597
Sub-Saharan Africa
Central Asia Rep.
N. Africa/Middle East
Caucasus
Latin American & Caribbean
(Source: Appendix 4).
Intention to Use the IUD
Respondents in DHS surveys who are not using a method are asked whether they intend to do so,
and if so what method they would prefer. Rather dramatic patterns appear across some 140
surveys when preference for the IUD is compared to the preference for the competing methods
of pill and injectable (re-supply) or sterilization (long-term), all by region. (APPENDIX 5 shows
only the most recent survey for each country.)
Briefly, the patterns exactly parallel the use patterns. Hardly anyone in sub-Saharan Africa
names the IUD, but many do so in the Middle East. The injectable is popular in sub-Saharan
Africa, with a remarkable uniformity across the many countries. The injectable is decidedly
unpopular (or less known) in the Middle East. In Latin America, it and the IUD are sometimes
high and sometimes low. Sterilization is unpopular in the Middle East but very popular in Latin
America. The pill is popular everywhere.
Geographically, the highly disparate pattern of method choice is as notable for future intentions
as it is for current use, and it might remain so if nothing were to change. However, both use and
intentions are amenable to change, as evidenced by the increased uptake of the injectable,
although that is a much different kind of method and it reflected major exertions by public or
private sector forces. In much of sub-Saharan Africa the injectable has been on the rise. From
1990-1994, UNFPA shipments of progestin-only injectables increased from 4.5 million to 16.7
million doses. USAID began providing supplies in 1992 to other countries. In some countries,
such as Nepal, the injectable is supplied in pharmacies (Finger WR 1995). Myths about the
injectable's impact on fertility were barriers at first; however it appears that efforts directed to
IUDs in Developing Countries-Perkin, Pollack and Ross
overcome those have succeeded in many places and the ease of administration has probably aided the continued rise in uptake.
II.2 Current strategies for an IUD revitalization
A critical question is whether a new IUD would be additive or a partial replacement? To address
this question it is important to think through a strategy of expanded IUD use beyond the above
calculations for a gradual increase in uptake balanced by annual discontinuations. First, consider
a favorable opportunity for expansion, where the setting has a proven track record for delivery of
services and the program, weak or strong, has demonstrated some commitment to the IUD. In
that case, we can assume that there would be both an addition and some replacement to use of
the TCu 380A, assuming that all other programmatic conditions were equal, such as cost and
supply of the new IUD. But in cases where the setting is weak and hard to impact through a
"vertical" introduction of a new IUD, any contribution of a new IUD would seem to be additive,
assuming a vigorous provider initiative. In sub-Saharan Africa, if a new IUD were introduced
and marketed as an improved method with clinical advantages it might migrate to the private
sector first, leaving the TCU 380A in the public sector. This is of course hypothetical. Past
experience with the introduction of new methods, such as NORPLANT, cannot be used to
inform this equation because the LnG IUD, although new, is still an IUD and has the attendant
associations and programmatic constraints. In any case, three strategy options follow.
Strategy Option No. 1. An increase in IUD use is most likely in countries where the method is
already established, where its own prevalence is neither too low nor too high, and where total
prevalence is neither too low nor too high. The reasoning is that prospects are unfavorable if the
IUD has not taken hold after all these years, or where total prevalence has not started to rise
(D.R. Congo, etc.). Equally, if IUD or total prevalence is already very high, additional rises are
improbable.
Therefore, the matrix in Table 7 (page 34) isolates countries where both IUD and total
prevalence are intermediate. They fall in the middle cells, with the 21 countries shown in bold.
These deserve special consideration as the most promising sites.
However, the "middle-middle" strategy is only a starting point, and it cannot be the only guide.
No country in sub-Saharan Africa makes the cut, and the only very large country included is
Indonesia, so even if IUD use increases by a half point of prevalence a year in the 21 countries,
there would be only a modest change for the developing world as a whole. Some of the other
giants fall into the very low cells for either IUD prevalence or total prevalence or both. It is
tempting to add at least a few large countries from the less favorable cells, and that is proposed
in the following strategy.
Strategy Option No. 2. USAID is supporting an IUD revitalization effort (Acquire Project) that
identifies eight sub-Saharan countries for special attention. These all fall into the last column and
bottom two rows of Table 7 below (underlined). IUD use has not yet taken hold in these
countries (although it was previously used more in Kenya than it is now). The large countries of
Nigeria and Ethiopia are included, and three in East Africa (Uganda, Kenya, and Tanzania),
where conditions are somewhat more favorable. Ghana is also included, along with Mali and
Guinea. These choices necessarily reflected a blend of Mission interest, central priorities, and
IUDs in Developing Countries-Perkin, Pollack and Ross
available funding (MAQ country partnerships for Ethiopia, Mali, and Nigeria; Global Country Partnerships for Kenya and Uganda, and other sources for Ghana, Guinea, and Tanzania.) A mix of considerations is inevitable through any of the large donors, whereas foundations would be thought of as having somewhat greater freedom of action. The question persists as to what to do, in an ideal world, about sub-Saharan Africa. Sheer need does not discriminate since it is universal. Probability of success is a more realistic criterion, and size also matters—so which countries in the region seem to be the best prospects? Coming down by size, Nigeria and D.R. Congo are large but extremely difficult. Ethiopia may be a more attractive site as it is more homogeneous and manageable, with large donor activities in train, and with a recent rise in prevalence. South Africa deserves consideration; there the injectable is at a world record level and may have topped out since no method can work for everyone. Next in size, after those just named, are the trio of Kenya, Uganda, and Tanzania, followed by Ghana and Mozambique, and then numerous smaller countries. Malawi may deserve particular consideration since its modern-method prevalence is nearly 30%, sterilization prevalence is 6%, and there is an active, committed NGO. Since sterilization has been marketed successfully to 6% in that setting, surely there is potential for the IUD. In any case, the countries just named include five of the eight in the USAID list, so there is considerable overlap. Outside that region, if low IUD-prevalence countries of some size were to be considered, the list would include the Philippines, Bangladesh, Pakistan, Malaysia, Algeria, and Morocco. Finally, some countries with high total prevalence but low IUD prevalence should be considered. If total prevalence is VERY high, as in Colombia (77%), little change is possible. However, Mexico, for example, has 68% total prevalence, of which 9% is for traditional methods, and IUD prevalence is only 14%. Equally, Ecuador has 66% total prevalence, of which a full 16% is for traditional methods, and IUD prevalence is only 10%. Every country is a special case, and any general strategy must be tailored to country specifics. For example, a country undergoing decentralization may not be promising until local officials have chosen their priorities and settled new budgeting/planning procedures. The matrix below is simply a starting point for discussion and for strategy development, classifying countries that have demonstrated initial movement for both IUD use and overall contraceptive use, and identifying others that have yet to do so.
IUDs in Developing Countries-Perkin, Pollack and Ross
Table 7. Countries Classified by Total Prevalence and IUD Prevalence
Read: the high-prevalence countries in the top row vary greatly in the percent of couples using the IUD.
TOTAL PREVALENCE
Prevalence 66%-86%
IranNicaraguaHong Kong
Prevalence 46%-65%
Dominican Republic
BelizeSouth Africa DominicaIndiaEl Salvador Bangladesh Zimbabwe
Prevalence 25%-45%
Saudi Arabia
Trinidad and Tobago Central Af-Guatemala
Sao Tome & Principe Congo D.R.
Botswana
Prevalence 4%-24%
(Source: Macro DHS StatCompiler)
IUDs in Developing Countries-Perkin, Pollack and Ross
Strategy option No 3: the private sector. Here we explore the potential of the private medical
sector for raising IUD use. Although we found in interviews that staff from DKT, MSI and PSI
are all very enthusiastic about contraceptive social marketing broadly, and even about social
marketing of the IUD, there is actually very little being done. This is probably because it is not
an obvious fit. Distribution via social marketing franchising, a new model, provides IUD
insertion training in the private sector. Although the idea is developed and described, programs
are small, and measures of success are short-term and somewhat anecdotal; PSI has an active
program in Pakistan (green Star) where despite a network of 10,000 providers, roughly 4,000
were women, and they distributed 74,000 IUDs through the network in 2005. This was
considered an exceptional accomplishment although actual insertions are unknown. Their other
significant program is Nigeria, where they sold 37,000 IUDs last year through social marketing,
not franchising. These approaches should not be discouraged, and should be further explored,
but their potential is not generalizable at this time. Below we try to narrow the playing field to
identify opportunities to further explore social marketing, which in and of itself seems like a
good model for certain countries.
First, what is the record so far? Figure 7 (page 36) shows the private medical sector to account
for about 30% of IUD use (un-weighted mean of countries). The bars to the right are the most
relevant; they exclude sub-Saharan Africa, where IUD use is trivial and the private sector also
still small, and the Central Asia Republics, which are atypical. The private share is still low in
South and Southeast Asia, at less than 20%, but it is a high 40% in Latin America. Concerning
individual large countries, the private share for IUD use is 44% in India. IUD prevalence
country-wide is 1.6% but further examination of 1999 StatCompiler data indicates prevalence of
6-7% in several states with diverse geographic distribution (Delhi, Arunchal Pradesh, Assam,
Tripurna, Sikkim). Although this is low, the fact that a good percentage of share is private may
indicate a private market in some states. Because of its size, the comments regarding country
selection for expansion do not pertain. In Indonesia the same figures are 57% and 6%. In
Bangladesh and Pakistan IUD prevalence is low, at 1% and 4% respectively. In Mexico, the
picture may be brighter: 14% of married women use the IUD, but we lack information on the
private share. In Brazil IUD use is trivial.
There are distinctly separate groups of countries when looking at private involvement and IUD
prevalence, as shown in Figure 8 (page 36). The cluster in the upper left corner of the chart
represents Vietnam and four Central Asia Republics, where IUD use is popular and is obtained
entirely through government facilities (China is similar). High in the middle is Egypt, with a
very active private sector: 35% of women use the IUD and, among them, 39% use the private
medical sector. To the right is Jordan: 23% of women use the IUD and 78% is in the private
sector.
IUDs in Developing Countries-Perkin, Pollack and Ross
Figure 7. IUD Sources of Supply by Sector
IUD: Source of Supply by Sector
(67 Countries with DHS Surveys)
(Source: Macro DHS)
Figure 8. Private involvement and IUD prevalence
Does Private Involvement Raise IUD Prevalence?
(excludes sub-Sahara Africa)
IUD Prevale
(Source: Macro DHS)
IUDs in Developing Countries-Perkin, Pollack and Ross
Figure 8 illustrates why it cannot be concluded that the larger the private share the higher is total
IUD use. All the countries at the bottom of the chart, below 5% IUD prevalence, represent
places where the private share varies greatly but where total IUD use is nearly trivial. Just above
the 5% line are 13 countries, again with considerable variation in the share of the private sector.
But the real question is whether the private sector can be made more active and thereby raise
total IUD use, in particular countries. This would also give women another channel through
which to obtain the service and perhaps improve continuation, even if total adoptions stayed the
same.
So what is the potential in particular countries for enlarging the private sector? We again
recommend attention to selected countries as spelled out in Table 7 above. Prospects are better
where contraceptive use in general is established and where the IUD has already won a fair
share. Within such a country solid work is needed to detect legal and regulatory brakes on
private medical initiatives. The supply system must be examined, as must the ease of advertising
services. Given a careful assessment, advocacy efforts can then be targeted effectively. Where
one or more NGOs or USAID Cooperative Agencies (CAs) offices are established they can do
much to bring change about (see Table 13 and final recommendations for case studies on page
60). Table 8 below exemplifies diverse funding sources for private sector IUD supply.
Table 8. (DKT) 2004 Social Marketing Program Sales from all sources for IUDs
IUD Funding
Sub-Saharan Africa
DFID, USAID, private
2004 Total
IUDs in Developing Countries-Perkin, Pollack and Ross
II.3 Programmatic requirements for effective IUD use
As described earlier, IUD delivery and uptake are heavily influenced by both "setting"
(socioeconomic and infrastructure conditions that influence access to services) and "program"
(shared commitment to the IUD at policy, provider and clinic levels, including resource
allocation for training, information and education, and marketing to the consumer).
The issue of access is primary. Clearly, the IUD will not be greatly used if there is no access to
it. National estimates for access exist in the "program effort scores" gathered in several cycles,
most recently in 2004 (Ross, Stover, and Adelaja, 2006). These are based upon replies by expert
observers in each country to a questionnaire that asked, among other things, approximately what
proportion of women had "easy and ready" access to each contraceptive method. Those replies
can be compared to the percentage of women in the same countries currently using the method.
Allowing for some measurement error in the access measure (with over-estimates being more
common than under-estimates), see Figure 9 below.
High levels of IUD use occur only where IUD access is quite common. At the upper right (box)
we find eleven countries, also noted earlier, as making up three of the principal country clusters.
Each cluster has its own causal dynamics, but high access is common to all three groups:
• China and Vietnam
• The five Central Asian Republics • Egypt, Jordan, Turkey, and Lebanon
At the other extreme on the bottom of the chart is the entire body of sub-Saharan Africa countries, where use is negligible and access ratings tend to be low. In practice, easy and ready access to the IUD rests on a composite of programmatic factors: the presence, in fairly close proximity, of a facility where both skilled personnel and supplies are present, where training for the method is current, and where the staff are motivated to suggest its use. Those in turn rest upon certain policy and programmatic features, and a failure in any one of these factors can render the IUD, or any other method, nearly inaccessible. In many African countries, the simple ratio of providers to women is so low as to settle any question of broad, easy IUD access. In addition, the distraction of competing burdens can affect provider motivation. An IUD insertion often requires a follow-up visit, with some cases requiring provider action to address bleeding, do a removal, or counsel the client. Under time and caseload pressures the temptation may well exist to suggest the simpler injectable or pill.
IUDs in Developing Countries-Perkin, Pollack and Ross
Figure 9. Dependence of IUD Prevalence upon IUD Access
Data from 76 countries illustrate that IUD prevalence is
high only
where IUD access is high
nce (% Usin
Percent of Women with Access to the IUD
(Source: Ross, Stover, and Adelaja, 2006)
In summary, the five most significant influences on IUD uptake are:
1. Low access (discussed above).
2. Absence or presence of competing methods: as in the antipathy to sterilization in the
Middle East which has left a kind of vacuum that the IUD has moved into, or the lack of both sterilization and re-supply methods in the former USSR, which has left an opening for the IUD to become the principal method. Likewise, easier access to competing methods such as pills or injectables. In both cases, there is not a provider shortage.
3. Myths: The bad reputation that has grown up around the IUD among the elites in some
countries, together with unfavorable rumors of all sorts that hurt its image among potential users (vs. the satisfaction registered by many actual users). The myths regarding a foreign body placed inside of the body.
4. The inherent drawbacks of the device: the need for a clinic visit and a pelvic exam;
changes in menstrual patterns; lack of complete invisibility (partner can detect the strings); early side effects; some failures/perforations; foreign body in the uterus.
5. Medical barriers: provider biases, fears and lack of motivation, e.g. in HIV areas
reluctance to have bodily contact or touch bodily fluids; the need to deal with side effects and decision-making around removal and failures.
IUDs in Developing Countries-Perkin, Pollack and Ross
II.4 Ranking factors influencing programmatic initiatives supporting
access and uptake
Teasing apart the exact ranking of influences upon programmatic stability and growth is nearly
impossible given the multiple factors that interconnect settings and programs. Adding method-
specific concerns to the equation increases the level of guesswork. However, we can discuss
certain essentials as follows.
The currently available IUD is inexpensive. Insertion, although easy, still requires at least a mid-
level provider. Failures are rare and not likely to present a significant barrier, so little change can
be expected in either price, ease of insertion, or failures. However, discontinuations are common,
and are related primarily to side effects of bleeding and pain. Decreasing these would
substantially improve the overall reputation of the IUD and extend use time considerably while
also attracting more new users by increasing the pool of satisfied clients. The difficulty of course
is that a reduction in discontinuations rests upon either a modified device or large scale
improvements in setting-related limitations such as training, supervision, direct to consumer IEC
and marketing.
Kenya's experience affords some insights into important influences, drawn from work there with
the USAID-funded revitalization project. The proportion of modern method users relying on the
IUD dropped from 20.7% to 7.6% between 1989 to 2003, while use of the injectable jumped
from 18.4% to 45.4%. Use of female sterilization decreased significantly over the same time
period. During these sharp changes in method mix, overall use has stagnated. Does this represent
the increasingly stressed health care delivery "setting", compromised by increasing levels of
HIV/AIDS and the need to focus on treatment? Or, the migration of trained health care providers
that has left huge gaps for both physicians and nurses? Or, more likely, both? A service delivery
assessment indicated that along with these macro level influences, the losses in trained providers
has probably led to a secondary decrease in provider knowledge and in accurate information for
consumers. Given that the method technology has not changed for the worse over this period,
one would have to assume that the problems are related primarily to the changing environment,
at least in Kenya.
Returning to the initial question as to whether it is possible to rank the factors that impact IUD
use, the answer is unfortunately negative, apart from noting that certain influences are necessary
but by no means sufficient, such as access. The profile of determinants is so different from one
country to another as to preclude a relative ranking of programmatic influences that is
generalizable to all settings.
II.5 Successful and unsuccessful programs: lessons learned
If "success" means that the IUD is widely used, the lessons depend entirely upon which of the
six clusters of countries we focus upon. Success in China and Vietnam rested upon a powerful
push from the top. Success in the Middle East cluster rested not upon any top-down push but
upon other factors, including the private medical sector's own availability, growth, and initiative.
Success in the former USSR Republics reflected a vacuum of alternative contraceptives and a
IUDs in Developing Countries-Perkin, Pollack and Ross
widespread clinical infrastructure. In the mixed pattern throughout Asia and Latin America the lessons are very much country-specific. Within any cluster, IUD adoption is elevated if the essentials for service delivery are present, and depressed if they are not. This involves the usual list of physical access, training, supervision, public education, and so on. Proper counseling can be quite important; some studies show that continuation on a method is greater if a woman is informed about alternative methods and given the one of her choice. The "commonality" question is an important one. In one sense, there is no commonality in what makes for IUD success due to the fundamental differences between the country clusters. In another sense, however, there are commonalities in the necessary conditions of clinical capacity, supplies, provider training, and links between women and facilities. The woman and the trained clinician have to meet in a facility with equipment and supplies. Some countries have lacked one or more of these conditions: poor transport for women, poor training, few clinicians, few facilities, little equipment, or poor supply lines. Where any single one of these is lacking, IUDs will not be inserted and IUD prevalence will be low. Even if all necessary conditions are present they are not sufficient, as testified by certain Latin American and Asian countries that could have qualified but nevertheless have had very low IUD use. Brazil is the standout example. As to the "why" question, we can only speculate that a blend of national policies (formal or informal), views of the medical establishment, the religious environment, and ad hoc historical factors, account for the dominance of one method against another. In Brazil, the IUD never took off but sterilization did, on a sub rosa basis in the context of caesarian deliveries. Unique factors are present in each country. To actually change the IUD's position in a place like Brazil, a significant policy and consumer directed marketing campaign would have to be implemented over an extended period of time. It remains that, within any of the main clusters of countries, IUD use will tend to be greater where the essential conditions mentioned above are more favorable. Those in turn rest partly upon policy positions and the vigor with which they are implemented. International leverage can focus on the policy and medical establishments, in addition to direct programmatic assistance. For foundations, these efforts should be selective where moderate investments can have an impact.
II.6 Knowledge and acceptance of the IUD
It is important to consider what we know about acceptance of the IUD among donors, policy
makers, program managers and providers, as well as what we know about subgroups of clients
that may inform knowledge and acceptability. Lacking survey data on most of these groups, we
can comment only generally. The principal donors are fully cognizant of the IUD due to their
long history with it and in some cases, their expenditures for supplying it. However, their level of
commitment varies, as does their appreciation of a better method mix for continuation rates and
pregnancy prevention. The stand-out is USAID, with its prominent revitalization push, which
has galvanized efforts by certain CAs and by some of the Missions. This project's objective is to
change the international context by alerting other donors and policy makers to the advantages of
IUDs in Developing Countries-Perkin, Pollack and Ross
the IUD; this high level of advocacy should not be underestimated in terms of global impact. It
suggests that similar efforts by a few other major donors could be very useful and could
significantly reinforce the USAID efforts (see recommendations in Section IV). Most other
bilateral donors are not as specific in their foreign assistance as to focus on one method for one
program for service delivery. Many participate in basket funding (see recommendations).
Policy makers are another matter; they are very diverse within the countries, as well as in the
regional bureaus and missions of the UNFPA, USAID, and elsewhere. We have little empirical
information, but it is clear that some policy makers have very little interest in the IUD or for that
matter in contraceptive provision. Many of them have strong leverage or influence with
ministries regarding service delivery initiatives, and they are the target group of any
revitalization project.
Program managers and providers have been very little studied regarding their commitment to
the IUD or to other methods, so, as with the above groups, they are difficult to quantify.
However, it is clear from field impressions that both managers and providers in some countries
can be sharply prejudiced against the IUD. Even where there is no overt prejudice against the
method, its general lack of use in a country can induce timidity by providers, who fear mistakes
and, under time pressures, may avoid pelvic examinations and prefer the easy alternative of the
injectable at-hand. In other countries, the IUD may be the method of choice by providers, as in
Vietnam, China, and the Central Asian Republics. Like anywhere else in the world, whatever
providers are trained in and have considerable experience with, they migrate toward.
Clients also stand along as a group. For them, a wealth of information is available on both
knowledge and acceptability (use). In sub-Saharan Africa, the IUD is little used when all couples
are taken together, but the interesting question is whether use might be higher in the sensitive
sub-groups. In general, IUD use increases with age and parity, and it usually varies by residence
and education. Table 9 provides the results, based on 145 surveys in Macro's StatCompiler.
The picture for sub-Saharan Africa remains the same no matter what sub-group is examined:
IUD use is uniformly very low. That is an unfavorable outcome, because if use was decidedly
greater at the upper ages or parities, this would signal a basic acceptability that could gradually
penetrate the other groups. Use is not as low in urban areas and among the best educated groups,
but age and parity seem not to matter. They are somewhat confounded with rural residence, and
the results might be slightly different if we examined age and parity within rural and urban areas
separately, however the StatCompiler does not allow this. So while cross-tabulations might show
slightly higher use in more specialized sub-groups, the overall levels are so low that the
conclusion would likely be the same. That is, for program planning, the populations are
uniformly starting at near-negligible levels. Table 9 shows that no subgroup in sub-Saharan
Africa has significant IUD use.
Ever use of the IUD gives a picture for sub-Saharan Africa that closely parallels the dismal
picture for current use, so it is not that the IUD was once popular and has fallen into disfavor or
has lost out to competing methods. At least the age pattern makes sense: ever use rises a little
and peaks at 3.9% to 4.0% for ages 35-44 (see Table 9; full country data are in APPENDIX 6).
Knowledge: In sub-Saharan Africa, the IUD is less known than any other method except
vasectomy. The percent knowing about the IUD, at 42%, is well below the percent knowing of
IUDs in Developing Countries-Perkin, Pollack and Ross
female sterilization (53%). Of course knowledge of all methods is low in sub-Saharan Africa, but
the differential is greatest for the IUD: there is a difference of 21 points between its average and
the all-country average (42% vs. 63%). (Full country data for knowledge appear in APPENDIX
7).
In Tables 9, 10, and 11 below, the contrast is extreme between sub-Saharan Africa and the other
regions—so extreme as to return our attention to the puzzle of causality. It is true that selected
countries in Asia and Latin America also ignore the IUD, but every country in sub-Saharan
Africa does so.
Table 9. Percent Using the IUD, by Age, No. of Children, Residence, and Education
South and
Africa/West
Southeast
countries
No. of living children
Highest educ. Level
* All countries receive equal weights.
(Source: Macro DHS StatCompiler)
IUDs in Developing Countries-Perkin, Pollack and Ross
Table 10. Ever Use of the IUD, by Age and Region
Africa/West
Southeast
countries
*All countries receive equal weights
(Source: Macro DHS StatCompiler)
Table 11. Knowledge of IUD and Other Methods, by Region
Africa/West
Southeast
Asia Rep.
countries
*All countries receive equal weights
(Source: Macro DHS StatCompiler)
II.7 Status of other IUD revitalization program, research, and
advocacy efforts
The work so far is an excellent start but it is only that. Most developing countries are not yet engaged, and the UNFPA, IPPF, EU, and other major players do not seem yet to be on board. Far more is needed if the IUD is to enjoy a major rejuvenation. Most of the funding for current revitalization efforts has come from USAID, either centrally or from the Missions. Funds have gone to various organizations. The most active CAs in this work have been EngenderHealth, FHI, and the Population Council (separate summaries below). In some countries they have worked together, and they are closely involved in the MAQ (USAID's
IUDs in Developing Countries-Perkin, Pollack and Ross
Maximizing Access and Quality Initiative) working group. Much of the recent work concerning revitalization considerations has come from these organizations6. EngenderHealth (EH) is using various USAID funding streams for activities in several countries, as well as doing extensive work centrally to marshal data and analyze biomedical aspects. Countries in which EH is involved either directly or through technical assistance to other CAs include the following (some of this involves MAQ funds and some Global Partnership funds): Kenya, Uganda, Nigeria, Mali, and Ethiopia. Using field support funds, Tanzania and Guinea are also included. FHI is active in Kenya and Uganda, with lesser activities in Ethiopia and Guinea. EH and FHI are co-chairing an IUD Working Group through MAQ (which met March 27, 2006 in Washington); it developed the IUD ToolKit, with an associated CD Rom containing the content of the website for wide distribution7. The operational details of projects in the countries vary a good deal, concerning guidelines, training, field experiments, etc. They also vary in geographic scope. Some, such as guidelines and policy work, are national; others work in a few provinces or local areas. The Population Council is involved in several IUD activities. Three fall under the USAID FRONTIERS Project, one falls under the International Contraceptive Access (ICA) Foundation, and another falls under the Center for Biomedical Research's NICHD Contraceptive Development Center grant. All FRONTIERS efforts are focused on the TCu 380A, while the ICA is focused on the LNG IUD. CBR's research is focused on new technology. ♦ The FRONTIERS work is intended to strengthen health system capacity for IUD services. It
is collaborating with FHI, EH, and University of Southampton, UK in several sites in Latin America, Africa, and Asia. Studies are on-going or recently completed in Guatemala, Honduras, Ghana, Kenya, and India. Additional assessments were completed by FHI in El Salvador, and by the University of Southampton in Nepal and China.
♦ The second activity is to follow-up earlier, completed efforts to extend coverage of IUD
services through on-site training of nurses in rural areas in Guatemala and Honduras. This was scaled-up by the University Research Corporation (URC) in Guatemala and by EngenderHealth in Honduras through USAID bilateral programs. The intent is to document sustainability of the service models over several years and to examine counseling, insertion and removal, and infection prevention.
6 There are four presentations (PPTs) from EngenderHealth through its ACQUIRE Project, all by Roy Jacobstein.:
• "IUD ‘Revitalization': Scientific, Programmatic, and Contextual Considerations," presented at the 2005
meeting of the American Public Health Association.
The next three presentations were given at the IUD Standardization Workshop, Webuye, Kenya, Nov. 2005
• "The Latest Scientific Knowledge and Guidance about IUDs: PID, Infertility, HIV/AIDS (and other
provider concerns)."
• "The ACQUIRE Project and the IU(C)D ‘Revitalization' Initiative."
• "Change Comes with Strings Attached: Thinking about Change in the Context of IUD ‘Revitalization.'"
7 The IUD Toolkit is available online at www.iudtoolkit.org.
IUDs in Developing Countries-Perkin, Pollack and Ross
♦ The third activity is to cooperate with the MAQ activity (above). Several staff have
collaborated in developing and/or reviewing elements of the web-based toolkit. Country case studies on Guatemala, Kenya and Pakistan are instructive of ways in which foundations might use diverse approaches to increase access to the IUD.
♦ The ICA Foundation is a partnership between the Population Council and Schering OY
(Finland) and Schering AG (Germany). It provides LNG IUD donations of up to one percent of the unit sales of Mirena in developed countries (excluding US sales) to public sector counterparts in developing countries. In addition, counterparts have the option of purchasing additional LNG IUDs from the Foundation (matched to 3% of the unit sales, again excluding US sales) at the current public sector price of US$40. The ICA Foundation is currently cooperating with partners in Brazil, Ecuador, Indonesia, Kenya, Nigeria and South Africa and donating LNG IUS to national counterparts.
II.8 Case-in-point: The USAID Kenya Mission
The USAID Kenya Mission is involved with IUD revitalization and has begun to report findings
from some projects. FHI repeated a situation analysis in their work area and got results that
agreed with an earlier situational analysis to explain low IUD use. The key obstacles were:
• Provider bias
• Pressures on time use • Staff worries about infection
• Lack of IUD training for a long time
• Staff then lack confidence due to inexperience, so the injectable is easier • The hierarchical character of the medical community, which, in turn, means an agency
cannot simply conduct nurse training on the IUD and must generate buy-in at each level of the professional community.
As a next step, some field actions may be taken in the context of the above to pursue renewed clinical expertise, retraining, advocacy etc. The Kenya Mission reports that the MOH is not actively opposed to the IUD – it is just distracted by HIV/AIDS and Safe Motherhood; also the ‘plain vanilla' FP program has receded. IUD retraining requires both interested women acceptors and IUD practitioners who are still active. Both are scarce. There is a history of stock outs that adds to the problem of consistent provider commitment. (The national program also suffered leadership changes, funding cuts, and supply problems). Nationally, the IEC arm of the program weakened greatly about 8 years ago, decreasing any public stimulus for the IUD. Parallel: when Pepsi pulled out of Kenya Coca Cola thought it could relax on advertising, but then its sales fell. For FP incessant IEC, stimuli are needed as new couples come of age, etc. An interviewee with the Family Planning Association of Kenya (FPAK) explained that IUD use declined partly because all funding fell some years ago including funds for training, so meaningful training ceased. Community mobilization also fell – most CBD agents were stopped
IUDs in Developing Countries-Perkin, Pollack and Ross
for lack of funds. FPAK used to provide all methods free but lost funding so it now charges about one-third of the real cost. Also, it is partly true that women do not like the IUD since they dislike the idea of a foreign object in the body, and some users had failures that caused distrust of the method. To re-energize the IUD, several steps are essential:
• Training. Need better counseling by the providers in the clinics.
• Mobilize at the community level – especially by using CBD workers. They are trusted by
the women (who dislike coming directly to the clinics).
• Fix commodity security; there were serious deficiencies in IUD supplies in 2004-05.
• Strengthen IEC in general with particular attention to unmet need and to the large
numbers of women who want longer-term protection of at least 3-5 years.
"Do all that and the women WILL respond." As to clinical capacity in rural areas, it was augmented earlier by the CBD workers, who were each attached to a local clinic, which was often just held in a tiny town or market crossroads.
In summary, many countries face classic constraints on adoption of any method: poor conditions for IEC, supply, training, counseling, and the local infrastructures required to contact women personally. These are probably accentuated in the case of the IUD, where clinical capacity – both specialized and ongoing insertion training and specialized equipment --play a large role. Moreover the profit motive in the private sector of maternity homes, etc. probably tilts toward the injectable in some countries since it offers repeat charges and is quick and easy.
IUDs in Developing Countries-Perkin, Pollack and Ross
SECTION III SUPPLY ISSUES
Overview
Considerable programmatic constraints influence both supply of, and demand for IUDs in
developing countries. Although it is impossible to predict the opportunity that would arise if a
new IUD were introduced that had specific health advantages, these cannot be adequately
examined without some promise of reasonable public sector pricing for the new device as a start.
The following section examines potential solutions to supply-related barriers to expanded
hormonal IUD access.
III.1 Cost/Benefit
With an eight to 10 year potential life span, a LnG IUD priced at US $5.00 would be competitive
relative to other contraceptives. This pricing would make the LnG cost competitive with the TCu
380A in the market today because of the duration of use. According to a recent report from the
Alan Guttmacher Institute (Vlassof 2004), the average costs of insertion, follow up, removal and
overhead for an IUD is US $22.38. This calculation does not include the cost of the device. If we
project that a LnG IUD will be available to the public sector for US$5.00 or less, the total comes
to around US$27.00 or $9 per year with an average use of three years. The TCu 380A total costs
would be slightly lower at US$23 for three years, or US$7.50/year.
Oral contraceptives are less per year of protection. If we look at the cost of oral contraceptives
for three years, 39 cycles x $0.25 is equal to US$9.75. With an estimated cost for 12 re-supply
visits at $1 per visit, the total method cost for oral contraceptives would be just under $22 for 3
years or $7 per year of protection. This is probably an over-estimate though because of the
marketing direct to the consumer, saving much of the cost of re-supply visits. DMPA is more
expensive, with product costs of 12 x$0.90 or US$10.80 for 3 years. Visits to receive DMPA,
would also be more costly since in most cases a clinic based health professional would
administer the injection. An estimate of $2 per visit for DMPA would bring the total cost for 3
years to $35 or $12 per year.
In terms of costs, using the above estimates the break point for the LnG IUD is around three
years. If used longer, the cost of IUD per couple years of protection would continue to decrease
relative to other methods requiring re-supply.
III.2 Product patents, intellectual property and licensing
Many of the issues relating to patents, intellectual property, and licensing would be avoided in a public-private partnership. The company would give preferential pricing to the public sector in return for subsidization of Phase 3 clinical trials or other development costs, all negotiated up front. The public sector would not need to have a non-exclusive license since devices would be purchased directly from the manufacturer.
IUDs in Developing Countries-Perkin, Pollack and Ross
III.3 Public-Private Partnership
The time would seem ideal for the development of a LnG IUD Public/ Private Partnership. At
least three new LnG IUDs are in the development pipeline. Contrel (Ghent Belgium) has
developed the Femilis TM and completed a small scale clinical evaluation with good results
(Wildemeersch 2005) Another Belgium company is developing a generic Mirena device. The
third LnG IUD is in early stage development by a US company.
Contrel and the US company have shown interest in discussing a public-private partnership.
They are especially interested in providing a preferential price to the public sector in return for
the public sector covering the significant costs of the Phase 3 trials.
It appears that the Mirena LnG has been registered in 107 countries across Asia, North and
South America, and Europe. In Africa it has only been registered in South Africa and Egypt. The
Concept Foundation, established some 15 years ago in Bangkok to act as a non-profit
registration/distribution arm for the public sector, is willing and prepared to provide assistance
with registration of a generic or second generation device (subject to the availability of funds).
Where Mirena is not registered, or in the case of a new device, the per country cost is estimated
at US$30,000. This includes filing and registration but does not include the cost of labor or of
obtaining the data in the file, which would vary from case to case.
The provision of funds to cover the costs of clinical trial liability insurance would be a further
attraction. These costs could be significant if a US arm is included in the Phase 3 trial, which
would be necessary for FDA approval. USAID purchasing requires approval by a stringent drug
regulatory authority including either the FDA or the European Medicines Agency (EMEA).
Program Related Investments (PRIs) are essentially low interest loans with funds disbursed
directly from a foundation to the company involved. A PRI would be attractive to at least one of
the prospective partners. Funds would be used to develop the manufacturing infrastructure. The
loan would be repaid from profits on devices the company sells in the private sector. There is
some risk that the company will not sell enough devices in the private sector to generate
significant revenue and could default on the loan.
Advanced Purchase Contracts (APCs) are agreements that guarantee the purchase of a minimum
agreed upon number of devices (drugs or vaccines in other cases). This is usually based on
demand forecasts. The APC reduces the risk to the manufacturer as they invest to expand
production capacity. In this case, donors could agree to an APC of, for example, 200,000 devices
per year for five years at the public sector price. This is of course more complicated than it
sounds, and is not well tested yet, but should be further explored.
III.4 Supply Options
Generic Mirena
The Belgium company noted above, which has reverse engineered the Mirena device, has
qualified a new polyethylene for the T and has proceeded through Phase 2 clinical testing. Since
IUDs in Developing Countries-Perkin, Pollack and Ross
it is a generic device, that is, identical to Mirena in dimensions and drug release, they believe that a demonstration of bioequivalency is all that is required for registration, obviating the need for time consuming and costly Phase 3 trials. Filing and registration for the generic device is expected by the end of 2007, according to the company. Public sector agencies could facilitate registration and the safe introduction of the device. In addition to cost, there are several concerns that make the widespread distribution of a generic Mirena less attractive than it might first appear. As described earlier in the paper, Mirena has been reported to be "difficult to insert". The inserter that comes with the device is more complicated relative to other simple tube inserters. The arms of the Mirena are loaded into the insertion tube and require that the tube be inserted part way into the uterine cavity where the arms are deployed before proceeding to push the inserter tube the rest of the way to the fundus. One recent report described higher than expected uterine perforations (Van Houdenhoven 2006). The retrospective study from Holland reports an estimated incidence of uterine perforation of at least 2.6 per 1000 insertions, likely based on study design to be under-reported. Training providers in insertion would be an important component of any introduction effort as the insertion is different than insertion of the more familiar TCu 380A, which uses a pull technique. In summary:
• The generic Mirena device might be ready for market in roughly 2 years.
• The biggest advantage is that Mirena's performance is well documented. If
bioequivalence could be demonstrated, expedited approval by an appropriate drug regulatory authority might be obtainable.
• The estimated cost to the donors would be the cost of registration in developing
countries, however the public sector pricing is not confirmed.
• The disadvantage is that the insertion is difficult and the introduction would have to place
training emphasis on insertion technique. Poor placement may lead to higher expulsion rates than with the TCu 380A, but more data are needed. Available studies comparing the TCu 380A to the Mirena LnG to date suggest that the TCu 380A has a better continuation rate.
Femilis Device The Femilis device (Wildemeersch 2005) is described as easier to insert while maintaining high efficacy and safety. The device employs "coaxial fiber technology" for steroid reservoir and release. Piet Wildemeersch believes the Femilis device will provide protection for ten years. This is not yet based on actual use experience. Advantages include moving quickly into Phase 3 trials, working with an existing, albeit new, device. The Femilis is slimmer than Mirena and can be inserted through a simple tube in which the arms of the T remain outside the tube and self deploy when the device enters the uterine cavity. Disadvantages include a complex and expensive set of options to guarantee a low public sector price.
IUDs in Developing Countries-Perkin, Pollack and Ross
• The Femilis IUD is more advanced in the development process than the US Lng IUD
under development but requires further trials. It might be ready for broader introduction in 6-8 years.
• The advantage is product design with ease of insertion. • Assuming similar progestin release to Mirena, there are no device-specific disadvantages,
however, long-term performance has not been documented.
US Company LnG IUD A US company is developing a new, "second generation" levonorgestrel IUD. This will be a slim device that can be inserted through a simple plastic tube. The flexible arms of the device remain outside the inserter and self deploy once the device enters the uterine cavity. A copper version of this device has been on the European market (UK and Netherlands) for several years with a good record. There are a number of advantages to choosing this option. The device represents an easier to insert, second generation IUD, using a frame that is registered and sold in Europe as a Copper T version. No perforations have been reported with this device, although the manufacturers have no reason to research this as an outcome. The major disadvantage is the timeline. It will take 3-4 years for the US device to complete the preclinical work required for advanced clinical study. This means that the device is unlikely to be widely available for at least a decade. The company has projected that the formulation, IND filing, toxicology, and pre-Phase 3 clinical studies would cost $2-3 million over 3-4 years. In summary:
• It could take up to a decade before there is widespread introduction of the device.
• The cost to donors would be estimated US$8-10 million for Phase 3 trials and the cost of
the US trial arm, which might be covered by USAID or NIH.
• The advantage is that the device design has been tested and does not appear to have
insertion or perforation problems (to be verified during trials).
• There are not device-related disadvantages, but the time line makes this a long-term
investment and strategy.
Purchase Mirena from Schering There have been a number of contacts with Schering to negotiate a substantially reduced price for Mirena in the public sector. Schering expressed the need for training and counseling in association with Mirena. They are anxious to preserve the reputation of the product. The Population Council and Schering have established the International Contraceptive Access (ICA) Foundation that can provide Mirena at a public sector price of $40, compared to the US price of $400. During 2006 the Foundation expects to donate 25,000 devices. There has been no indication that Schering is prepared to consider a significant downward revision in the public sector price.
IUDs in Developing Countries-Perkin, Pollack and Ross
• There does not appear to be a public sector purchase option.
III.5 Timeline
WHO has prepared a budget and timeline for the Phase 3 multicentered trial of a new LnG IUD.
The timeline extends over a nine-year period with a total cost (excluding a US arm) of $7.7
million. WHO is proposing a side by side trial with Mirena. Ten centers would participate with a
total of 400 subjects per site. One year is estimated for preparation, two years for recruitment,
five years for follow up, and a final year of analysis. It is expected that a preliminary analysis of
the data could be carried out during year five, when sufficient data on three years of use have
accumulated. This would permit registration to proceed with initial approval for three years of
use. This time of continued use would be extended as subsequent data on longer use become
available (see Table 12).
IUDs in Developing Countries-Perkin, Pollack and Ross
Table 12. WHO Cost Estimate and a timeline for Phase III Clinical Trial of a New LnG/Mirena IUD
Preparation 2 year recruitment
Project manager / Epidemiologist
Data collection/ management contract cost
Data monitoring cost
Statistical support cost: planning and preparationP5
Statistical support cost: analysis
Contract for product development
TSA site cost with institutions for study
Coordination and GCP training meetings
Site visits (number)
Management and administrative support
Grand total
experimental group
subjects per site
TSA cost per subject per year
TSA cost per year
Excludes product development cost
For comparison, Implanon vs Jadelle study cost per subject per year averaged $109
(Source: WHO, personal communication with Dr. Paul Van Look, February 27, 2006.)
IUDs in Developing Countries-Perkin, Pollack and Ross
SECTION IV CONCLUSIONS AND RECOMMENDATIONS
IV.1 Conclusions
Our review confirmed that the currently available IUDs perform well as highly effective,
reversible, long-acting contraceptive methods. Like all methods, the IUD has both strengths and
weaknesses. It requires a clinical intervention and pelvic exam for placement and cannot be
tolerated by some women because of side effects. For the majority of others, it provides years of
uncomplicated protection.
The currently available TCu 380A is easy to insert and does not have a significant complication
or expulsion rate; concerns about its relationship to pelvic infections have been resolved for the
most part and WHO does not advise against use in HIV positive women. Although TCu 380A
IUD users experience a temporary increase in menstrual bleeding, research shows that it is not
significant over time. Removal of the device is technically easy and return to fertility is
immediate.
The greatest barriers to higher use are due less to shortcomings of the device itself than to access
and the necessity of clinic based care by trained and willing providers, who are all too rare in the
least developed countries. Myths about IUDs migrating, or dislike of a foreign body, may be
frequent barriers, but they are probably not as important as access issues. Long-term
continuation of use is likely affected by counseling at insertion and provider comfort with
management of side effects. Based on recent operations research and past experience (Frontiers
in Ghana, Guatemala, Honduras; Amkeni/ EH and FHI in Kenya, Guinea), infrastructure
capacity for ongoing training and counseling is critical for both uptake and continuation.
Additional, fundamental barriers are a lack of advocacy and political will to invest on a large
scale in sustainable institutionalization of the IUD to maintain or increase use. It must be an
integral part of available health services, and this makes Africa an unusually challenging
environment for IUD revitalization. In places where even the essential vaccinations fail to reach
large populations in need, a clinic-dependent contraceptive method is hard to sustain, and
especially so in competition with easy-adoption methods such as the pill and injectables. For
poor, young couples and women, the IUD does not compete well.
Nevertheless, the current TCu 380A is highly effective and relatively inexpensive at a public
sector price and an investment worth considering by foundations. Probably because of this
cost/benefit relationship, family planning advocates continue to seek means to invest in or
explore expanded use where fertility rates are high. Despite this, the number of donors investing
in "family planning" programs or a particular family planning method is currently small. Most
bilateral and multilateral donors are investing in broader, integrated reproductive or even
"health" programs, leaving un-earmarked funds to be deployed at the country or local level.
IUDs in Developing Countries-Perkin, Pollack and Ross
More and more, earmarked funds go toward prevention of infectious diseases. USAID appears to be the significant interested government donor yet many expect US family planning monies to decrease over the next few years. The future influence and role of the Foundations should be considered in this context. In the past, "foreign assistance" provided support for service delivery that might not be available in the future. Foundation replacement of this is out of the question, and so leveraging whatever there is, and catalyzing change at the national and global level seems more appropriate. Cost aside, public health arguments for the introduction of a hormonal IUD are not yet firm. On a population basis, reductions in menstrual bleeding and in anemia rest on the LnG IUD's impact per woman, on the distribution and level of anemia in the population, and on adoption of the LnG IUD by large numbers of anemic women. The marginal advantage of the LnG IUD on a population basis is unknown and not calculable with current information. However, with additional research it may prove to be substantial for some high-anemia populations, albeit less likely for SSA populations where anemia is multifactoral. Two other public health considerations should be weighed: its use for older women at risk of serious bleeding from other intrauterine problems, and the human rights argument that individuals who can benefit from it should have access to it. In these instances, women in Africa are no different than anywhere else at baseline, and probably in greater need because of the lack of access to safe surgical procedures to treat excessive uterine bleeding and intrauterine pathology. It is entirely possible that eventual availability of an inexpensive hormonal IUD marketed for its health benefits could gain acceptance first in an older population and ultimately in a younger population some time in the future when the environment can be more accepting of non-barrier methods. Short-term prospects, over the next 5 years, for SSA are quite mixed regarding prospects for any IUD. Although revitalization efforts under USAID support appear to have increased uptake in sites with retraining, improved supply chains, and expanded education for clients, the sustainability of these programs remains unknown and their national adoption is yet to emerge. Intervention activities must be incessant due to discontinuations and to the movement upward of women by age and parity that makes for constant shifts in the relevant audience. A short-term increase of uptake in response to an intervention will not repeat if the intervention does not repeat. National commitment to a large IUD push will compete with provider shortages in health care and with the resource drains resulting from traumatic impact of the AIDS epidemic. A deeper commitment to contraception, including the IUD, can ease the burden of many health problems in every country. Foundations can highlight the leverage that contraception offers for lessening those burdens. They can advance advocacy at the highest levels and partner with other donors in a common strategy, thus leveraging the investment of USAID and the private sectors commitment, such as MSI. The longer term outlook hinges partly upon a calculus that compares a generic Mirena device available sooner (assuming that a lower price could be negotiated), to a potentially improved device that will require several years to develop. The question may be posed as to whether donors prefer a long-term vision for a new IUD that may take a decade to develop and bring to market, or accept the limitations of the generic Mirena as the best opportunity to add a new IUD to the method mix. Both of these can be posed against a concentration just upon the TCu 380A now widely available.
IUDs in Developing Countries-Perkin, Pollack and Ross
IV.2 Recommendations
The following recommendations do not take into account any funding level pre-determined by
the participating Foundations, or particular country/regional interests other than those
specifically expressed by the Hewlett Foundation.
1. Investing in a new hormonal IUD—A Long-term Goal
Our strong recommendation to the Foundations is to invest in bringing a hormonal IUD to an expanded market by taking advantage of an immediate opportunity to secure public sector pricing for the future through a partnership with a US company. This may be a time-limited opportunity and thus should be included in a timeline for long-term planning. Although funding for the WHO phase 3 clinical trial is requested, this funding of approximately 8 million USD will not be required for 2-3 years. Minimal interim funding to support the due diligence and the business plan and independent progress reports will be the short-term cost. Our reasons for this include the following:
a. We believe that the hormonal IUD represents a remarkable innovation in IUD
design and utility.
b. The current Mirena will likely not have a public sector price (at least as of today)
but is now proving its potential in private markets.
c. A new device would offer an opportunity to re-introduce the IUD as a long-term,
highly effective method. In countries where contraceptive prevalence is in the middle range but IUD use is low, there is a significant opportunity for a new IUD.
d. Investing in a new hormonal IUD while using a design that has already been
tested as a copper releasing IUD for excellent performance and relative ease of insertion will minimize investment failure risk
e. Improvements in the IUD will expand the universe of providers and reduce risk of
f. The public health advantage of a hormonal IUD—specifically its impact on
anemia—has not been tested in populations where bone marrow suppression is the cause of anemia. For many reasons it is unlikely that IUD uptake will significantly improve in the most compromised setting of sub-Saharan Africa, as explained below. It is true, however, that there should be a positive impact on anemia in a more general population of women. Therefore, we believe that there is a public health argument for investing in a hormonal IUD for use in low-resource settings other than sub-Saharan Africa and, that in the course of development over the next decade, opportunities in SSA could likely arise.
g. The hormonal IUD has a quality of life impact given its secondary effects of
reduction of menstrual bleeding and decrease of abnormal bleeding from intrauterine abnormalities. These health outcomes affect a percentage of women of all socioeconomic classes. This translates into fewer surgical interventions for bleeding and thus increased safety in places where surgery is impossible and/or unsafe, especially for the poor. It is impossible to say whether the health benefit
IUDs in Developing Countries-Perkin, Pollack and Ross
of the hormonal IUD would be enough to catalyze new interest even in SSA, but we agreed that the potential might exist in several countries within the next 5-10 years.
h. We believe the US company to be a reliable and financially secure partner that
understands the public sector and is willing to move forward on a Public-Private Sector Partnership.
2. Investing in the expanded use of the TCu 380A: Short-term goals
Uptake of the TCu 380A in the rudimentary setting of sub-Saharan Africa cannot be expected to improve appreciably in the foreseeable future. In light of that, and the resounding and universal pressures to give attention to barrier methods for HIV protection (such as the female condom), we have reluctantly concluded against recommending an ambitious new IUD revitalization program in sub-Saharan Africa. That is not to say that current activities are misplaced. Foundation and USAID support for NGOs that are focusing on the IUD should be continued and encouraged for an extended period. Foundation support is especially important given the environment in Washington that predicts a continued decrease in general family planning support. Country-specific revitalization projects will only continue for another year or two with any certainty, so assuring funds to continue some of the activities and support for evaluation of the outcomes is well advised and could be done through continued general support for the involved NGOs. The results thus far show some short-term promise in attracting clients, however, data on sustainability is scant. One recent report from FHI's work in Kenya suggests that the investment cost in training providers and generating demand in that environment is high. This is consistent with our findings. However, continued involvement will provide the interested foundations with regular feedback and will identify any important opportunities that emerge; continued involvement will also provide lessons about IUD provision in difficult settings that can be transferred elsewhere.
3. Investing in the private sector
DKT, MSI and PSI all have moderate programs attempting to expand the private market for IUD use either through social marketing (product sales) or social market franchising (more than just about the product, this includes training and IEC that penetrates the private sector). Although these programs may never win a large market share they represent an opportunity to reach the "working poor". If these markets grow, it will represent, on a relative scale, significant expansion in some places. It is a model that needs to be further referenced in the literature and then might be more easily replicated (not only for IUD delivery). In the private sector, support for MSI to expand IUD services and service social marketing and to test post-abortion IUD provision in the context of the private sector is a good option. In fact, Dana Hovig (at MSI) refers to PSI's Green Star Pakistan service social market model when discussing MSI's strategy to expand IUD services. Literature on the success of sustainable private sector contraceptive commodity supply and uptake after donor subsidies are withdrawn is specific only to pills and condoms. The application of the
IUDs in Developing Countries-Perkin, Pollack and Ross
model to IUD supply and demand is relatively new but could be important (www.psp-one.com).
4. Foundation comparative advantage
Foundations' comparative advantage will be to invest in activities that USAID is not supporting or likely to support for one reason or another, whether in collaboration with others (USAID cannot support MSI), in choice of countries (some NGOs would not sign the GAG rule which ultimately limits FP funds), or in kinds of activities (such as post-abortion IUD insertion).
We favor action programs or case studies in countries where contraceptive prevalence
is moderate, between 25-65%, and IUD use is lower than expected. The case studies
should address both private and public sector expansion, to help determine more
thoroughly what the country specific barriers are. These in-depth studies could be
brief, but were still beyond the scope of this project as they require country visits.
Examples of these are available on the Frontiers project website for Guatemala,
Ghana and Kenya8. According to John Townsend of the Population Council, there
are also proposals for India, Honduras, and Nepal.
Working with NGOs already active in family planning in the country to execute a
strategy based on findings could prepare for the introduction of the new IUD in years
to come. Thus, matching countries to WHO's phase 3 trial sites would be best.
We checked on trial sites with Paul Van Look at WHO and recommend consideration
of Mexico, the Philippines, and Indonesia. Brazil is currently included on their list
among trial sites but the literature does not describe the barriers to IUD use there
other than to suggest that sterilization takes up the long-term method portion of the
users. We cannot be sure how introduction of the new IUD might be received.
IV.3 Specific Actions
Based on the above recommendations, the Foundations should consider the following
actions/funding opportunities:
1. Identify a consultant or team to develop a business plan that creates a partnership
between donors and the US company to support the research and development of a new hormonal IUD assuring low public sector pricing for the future.
2. Continue to support grantees who are already engaged in IUD revitalization projects.
Also, plan for ways to assure continued support for projects now using USAID funds having a one-two year horizon, since those funds may well cease and the lessons of those projects may be lost due to premature termination. Continuity of funding can be critical to an accurate evaluation of success. A full evaluation component, carried through to capture the full experience of the projects, will inform future investments, both in the sub-Sahara Africa trials and in several countries elsewhere.
8 www.popcouncil.com/pdfs/frontiers/FR_full reports/Guatemala_IUD.pdf
IUDs in Developing Countries-Perkin, Pollack and Ross
3. Consider a specific investment in social marketing/franchising projects with the intention
of further describing private sector capacity and replicable models.
4. Identify a grantee (a) to design a collaborative project that takes into account currently
completed or proposed case studies of IUD availability, acceptability and use, and (b) to identify 2-4 additional countries to prepare for the new hormonal IUD introduction (Table 13). Ideally the grantee would match several countries with between 25-65% IUD contraceptive prevalence and IUD prevalence above 5%, where health systems can accommodate increased IUD provision in both public and private sectors, acceptability for ICA donated Mirena LnG9, and potential WHO study sites. The case studies, and ultimately the projects, should be designed with intent to publish findings on several topics for use in high level advocacy once the new IUD trials are completed. These countries would be primed for introduction of the new IUD.
Table 13. Sample matrix of country case studies
Hewlett Case
PopCo (Frontiers)
WHO Study
Case Studies
Potential
active
IV.4 Research Priorities
The team did not design a research agenda. The recommended projects described above all
involve research at either the clinical or operations level and seemed to be specific enough based
on our findings and review of other ongoing activities through USAID with the following
exceptions:
Although literature exists that describes the clinical safety and efficacy of post-abortion IUD,
there is very little literature on actual programmatic implementation. These programs would
9 The ICA is prepared to make a long-term stable donation of LnG IUDs to one or two countries based on requests from the Hewlett Foundation. (John Townsend, email communication to Sara Seims May 23, 2006)
IUDs in Developing Countries-Perkin, Pollack and Ross
only be possible in well established "institutionalized" public sector services or in well organized private sector services where abortion is legal. Operations research demonstrating service delivery implementation and demand for the method would be a contribution to the literature and inform some settings where advocacy requires an evidence base. If the Foundations decide to support the development of a new hormonal IUD, it will be critical to learn more about the significance of amenorrhea in different populations and how to counsel and communicate about the advantage of the new IUD to both providers and clients. This will also be important for marketing. Very little is known about attitudes and how effective counseling could impact both choice and continuation.
IUDs in Developing Countries-Perkin, Pollack and Ross
SECTION V
REFERENCES
Andersson K, Odlind V, Rybo G. Levonorgestrel- releasing and copper-releasing (Nova-T)
IUDs during five years of use: a randomized comparative trial. Contraception 1994;49:56-72.
Andersson K, Ryde-Blomqvist E., Lindell K, et al. Perforations with intrauterine devices. Contraception 1998;57:251-255.
Andrade AT, Pizzarro Orchard E. Quantitative studies on menstrual blood loss in IUD users. Contraception 1987; 36: 129-44.
Bahamondes L, Diaz J, Marchi NM, et al. Performance of copper intrauterine devices when
inserted after an expulsion. Human Reprod 1995; 10:2917-2918.
Baveja R, Bichille LK, Coyaji KJ, et al. Randomized clinical trial with intrauterine devices
(levonorgestrel intrauterine device [LNG], CuT 380Ag, CuT 220C and CuT 200B). A 36-month
study. Indian Council of Medical Research Task Force on IUD. Contraception 1989;39:37–52
Chi I. What we have learned from recent IUD studies: a researcher's perspective. Contraception
2003; 68: 3-10.
Contraceptiononline. www.contraceptiononline.org. Accessed March 20, 2006.
Davie JE, Walling MR, Mansour DJ, et al. Impact of patient counseling on acceptance of the
levonorgestrel implant contraceptive in the United Kingdom. Clin Ther 1996;18:150–159
Farley TM, Rosenberg MJ, Rowe PJ et al. Intrauterine devices and pelvic inflammatory disease:
an international perspective. Lancet 1992; 339: 785-788
Fedele L, Bianchi S, Raffaelli R, et al. Treatment of adenomyosis-associated menorrhagia with a levonorgestrel-releasing intruterine device. Fertile Seril. 1997: 68; (3):426-9.
Feldblum PJ, Caraway J, Bahamondes L. et al. Randomized assignment to copper IUD or depot-medroxyprogesterone acetate: feasibility of enrollment, continuation and disease ascertainment. Contraception 2005; 72:187-191.
FHI. Hormonal Contraception and HIV: Science and Policy. Final Statement. Africa Regional Meeting. Nairobi September 19-21, 2005.
Finger WR. Injectables use increasing rapidly. 1995 Network. 15(4):20.
Franks AL, Beral V, Cates W Jr, Hogue CJ. Contraception and ectopic pregnancy risk. Am J Obstet Gynecol 1990;163:1120-1123.
Grimes DA. Intrauterine devices. In: Hatcher RA, Trussell J, Stewart F, et al.(eds) Contraceptive technology. Ardent Media, Inc. New York. 2004(A).
Grimes DA. Cochrane Library 2004. http://www.cochrane.org/reviews/en/ab003036.html
IUDs in Developing Countries-Perkin, Pollack and Ross
(text/html) Sat, 28 Jan 2006 02:49:55
Harrison-Woolrych M., Ashton J., Coulter D. Uterine perforation on intrauterine device perforation: is the incidence higher than previously reported?. Contraception 2003;67:53-56.
Hatcher RA, Trussell J, Stewart F, et al (eds). Contraceptive Technology, 18th Revised Edition, Ardent Media, Inc., New York, 2004; (9):223. Hidalgo M, Bahamondes L, Perrotti M, et al. Bleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena) up to two years. Contraception 2002;65:129–132.
Hormonal Contraception and HIV: Science and Policy; Africa Regional Meeting Nairobi 19-21 September 2005. World Health Organization, 2006. Available at: http://www.who.int/reproductive-health/stis/hc_hiv/nairobi_statement.pdf
Hubacher D, Grimes DA. Noncontraceptive health benefits of intrauterine devices: a systematic review. Obstet Gynecol Surv 2002;57:120-128.
Hurskainen R, Paavonen J. Levonorgestral-releasing intrauterine system int the treatment of heavy menstrual bleeding. Curr Opin Obstet Gynecol. 2004;16(6):487-90.
Jensen JT. Contraceptive and therapeutic effects of the levonorgestrel intrauterine system: an overview. Obstet Gynecol Surv 2005; 60 (9):604-612
Jonsson B, Landgren BM, Eneroth P. Effects of various IUDs on the composition of cervical mucus. Contraception 1991; 43:447-458
MacPhail, PA. Forging Effective Strategies to Combat Iron Deficiency Anemia. Abstract presented at the International ILSI Conference, Atlanta Georgia. May 2001
Mandelin E, Koistenin H, koisteninR, et al. Levonorgestrel-releasing intrauterine device-wearing women express contraceptive glycodin A in Endometrium during midcycle: another contraceptive mechanism? Hum Reprod 1997;12:2671-2675.
Mohllajee AP, Curtis KM, Peterson HB. Does insertion and use of an intrauterine device increase the risk of pelvic inflammatory disease among women with sexually transmitted infection? A systematic review. Contraception. 2006 73; 145-153.
Mosher WD, Martinez GM, Chandra A, et al. Use of contraception and use of family planning services in the United States: 1982-2002. Adv Data. 2004 Dec 10; (350):1-36 (p. 18).
OMNI website. http://www.jsi.com/intl/omni/safr.htm Pakarinen P, Toivonen J, Luukkainen T. Randomized comparison of levonorgestrel- and copper-releasing intrauterine systems immediately after abortion, with 5 years' follow-up. Contraception
IUDs in Developing Countries-Perkin, Pollack and Ross
2003;68:31–34.
Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the U.S. Collaborative Review of Sterilization. Am J Obstet Gynecol 1996;174:1161-1168;
Rivera R, Yacobson I, Grimes D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am J Obstet Gynecol 1999;181:1263-1269.
Ross, J., Stover J, and. Adelaja D, Profiles for Family Planning and Reproductive Health Programs: 116 Countries. Second Edition. Futures Group, 2004. Ross, J., Stover J, and . Adelaja D. Family Planning Programs in 2004: Efforts, Justifications, Influences, and Special Populations of Interest. Submitted for publication. 2006. Ross J, Stover J, Adelaja D. Profiles for family planning and reproductive health programs. 2005; Futures Group, Glastonbury Connecticut.A3-A8. Ross, J., Winfrey W. Unmet need in the developing world and the former USSR: An updated estimate. International Family Planning Perspectives. 2002; Vol. 28, no. 3. Sept. 2002. Saleem S, Hills FA, Salem HT, et al. Mechanism of action of the intrauterine device: evidence for a specific biochemical deficiency in the endometrium, Hum Rerop 11: 1996; pp 1220-1222 Shelton JD.Risk of clinical pelvic inflammatory disease attributable to an intrauterine device. The Lancet; 2001;357,9254;443. Sinei SK, Schulz KF, Lamptey PR et al. preventing IUCD –related pelvic infection: the efficacy of prophylactic doxycycline at insertion. Br. J. Obstet Gynaecol 1990; 97:412-9. Sivin I. 2006 Pre-publication communication. Sivin I Dose- and age-dependent ectopic pregnancy risks with intrauterine contraception. Obstet Gynecol 1991; 78:291-298 Sivin I, Schmidt F. Effectiveness of IUDs: a review. Contraception 1987;36:55-84. Sivin I, Stern J. Health during prolonged use of levonorgestrel 20 micrograms/d and the copper TCu 380Ag intrauterine contraceptive devices: a multicenter study. International Committee for Contraception Research (ICCR). Fertil Steril 1994;61:70–77. Stewart A, Cummins C, Gold L, et al. The effectiveness of the levonorgestrel-releasing intrauterine system in menorrhagia: a systematic review. BJOG 2003; 108(1):74-86. Suvisaari J, Lahteenmaki P. Detailed analysis of menstrual bleeding patterns after postmenstrual and postabortal insertion of a copper IUD or a levonorgestrel-releasing intrauterine system. Contraception .1996;54:201-208. UNDPA/UNFPA/WHO/WB/RHR. Long-term reversible Contraception. Contraception 1997; 56:341-352.
IUDs in Developing Countries-Perkin, Pollack and Ross
UNICEF, MI. Vitamin and Mineral Deficiency. A Global Progress Report. 2004 The Micronutrient Initiative. Ottawa, Canada. UN Population Division, "World Contraceptive Use 2003," Wallchart. New York: United Nations, 2004. UN Population Division, World Population Prospects: The 2004 Revision, Vol. I. New York: United Nations 2005. Van Kets H, Van der Pas H, Thiery M, et al. The GyneFix implant systems for interval, postabortal and postpartum contraception: a significant advance in long-term reversible contraception. International Study Group on Intrauterine Drug Delivery. Eur J Contracept Reprod Health Care. 1997 Mar;2(1):1-13. Van Houdenhoven K., van Kaam K.J.A.F., van Groottheest A.C., et al. Uterine perforation in women using a levonorgestrel-releasing intrauterine system. Contraception 2006. 73;257-260.
Vlassof M, Singh S, Darroch JE et al. Occasional Report number 11. Alan Guttmacher Institute, New York. 2004 Walsh TL, Bernstein GS, grimes DA. Effect of prophylactic antibiotics on morbidity associated with IUD insertion: results of a pilot randomized controlled trial. Contraception 1994; 50:319-27).
WHO. Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C.Contraception. 1997 Dec;56(6):341-52. World Health organization Scientific Group, Mechanism of Action, safety, and efficacy of intrauterine devices, WHO, Geneva (1987) Technical report Series Wilcox AJ, Weinberg CR, Armstrong EG, Canfield RE. Urinary human chorionic gonadotropin among intrauterine device users: detection with a highly specific and sensitive assay. Fertil Steril 1987;47:265-269.
Wildemeersch D, Batar I, Affandi B et. al. The frameless intrauterine system for long-term reversible contraception: A review of 15 years of clinical experience. J Obstet Gynaecol. Res. 2003; 29(3): 164-173.
Wildemeersch D, Janssens D, vrijens M et al, Ease of insertion, contraceptive efficacy and safety of new T-shaped levonorgestral-releasing intrauterine systems. Contraception 2005; 71: 465-469.
Wildemersch D, Schact E, Wildemeersch P et al. Development of a miniature, low dose, frameless intrauterine levonorgestral-releasing system for contraception and treatment: a review of initial clinical experience. Reprod Biomed Online. 2002; 4(1):71-82.
Xiao B, Wu SC, Chong J, et al. Therapeutic effects of the levonorgestrel-releasing intrauterine system in the treatment of idiopathic menorrhagia. Fertil Steril 2003;79:963–969
Zhang J, Feldblum PJ, Chi IC, Farr MG. Risk factors for copper T IUD expulsion: an epidemiologic analysis. Contraception 1992; 46:427-433.
IUDs in Developing Countries-Perkin, Pollack and Ross
SECTION VI APPENDICES
VI.1 APPENDIX 1: NUMBERS OF IUD USERS AND OTHER
(Source: UN 2004; UN 2005)
Table 1. Numbers of IUD Users, IUD Prevalence, and IUD Share of All Use
Most IUD Users are in a few countries -- both globally and within each region.
Size counts: even the low-prevalence countries of India and Indonesia have large numbers.
Other countries, with high IUD prevalence, are small.
Countries at ceilings for either total prevalence or IUD prevalence are poor bets for IUD increases.
Those in a middle range may be of greatest interest.
Developing World Total
Rest of Developing World
TOP 40 COUNTRIES for
Percent (ex-
Cumulative
TOP 40 COUNTRIES for
IUD Share
No. of Users
IUD Prev.
of all Use
55.3 HALF
90.2 9/10
IUDs in Developing Countries-Perkin, Pollack and Ross
Table 2. IUD prevalence, share of all use, and numbers of users, for all countries by region
This is a basic reference table: within each region countries are listed alphabetically.
IUD Share
IUD Share
No. of IUD
No. of IUD
Prevalence (% of users)
Users IUD
ASIA, EAST
ANGLOPHONE SS AFRICA
ASIA, SOUTH CENTRAL
FRANCOPHONE SS AFRICA
Central African Rep.
CENTRAL ASIA REPUBLICS
MIDDLE EAST/ N. AFRICA
IUDs in Developing Countries-Perkin, Pollack and Ross
Table 2, continued
IUD Share
IUD Share
No. of IUD
No. of IUD
Prevalence (% of users)
Users IUD
Antigua and Barbuda
Dominican Republic
Saint Kitts and Nevis
Saint Vincent and the G
Trinidad and Tobago
IUDs in Developing Countries-Perkin, Pollack and Ross
VI.2 APPENDIX 2: DISCONTINUATION RATES
(Source: Macro DHS StatCompiler)
APPENDIX 2: One Year Contraceptive discontinuation rates for 19 countries
(Reasons for discontinuation are treated as competing risks, so reasons add to totals.)Dashes indicate absence of data. Means in the top row for all reasons are similar when done only for countries with data on all 3 contraceptive methods.
IUD discontinuation rates have been falling in 8 countries (in bold). Reason for discontinuation
All reasons
Method failure
To become pregnant
Side effects, health
All other reasons
Injections
Pill IUD ns
Pill IUD ns
Pill IUD ns
Sub-Saharan AfricaZimbabwe 1994
N. Africa/ W.Asia/EuropeArmenia 2000
Morocco 2003-2004 (1)
Central AsiaTurkmenistan 2000
South & Southeast AsiaBangladesh 1993/94 (2)
Bangladesh 1999/2000 (3)
Bangladesh 2004 (4)
Indonesia 2002/2003
IUDs in Developing Countries-Perkin, Pollack and Ross
Philippines 1993
Philippines 1993
Philippines 1998
Philippines 2003
Latin America & CaribbeanBolivia 1994
Dominican Rep1991
Dominican Rep1996
Dominican Rep1999
Dominican Rep 2002
Guatemala 1998/99
Nicaragua 1997/98
First-year contraceptive discontinuation rates due to method failure, desire for pregnancy, health reasons, or other reasons, according to specific method.
Note: Based on 5 years of calendar data. The rates apply to the 3 - 63 month period prior to the survey; exposure during the month of interview and the two months prior are excluded to avoid the biases that may be introduced by unrecognised pregnancies. These cumulative discontinuation rates represent the proportion of users discontinuing a method within 12 months after the start of use. The rates are calculated by dividing the number of women discontinuing a method by the number exposed at that duration. The single-month rates are then cumulated to produce a one-year rate. In calculating the rate, the various reasons for discontinuation are treated as competing risks. Methods with less than 125 months of exposure are not shown.
VI.3 APPENDIX 3. Numbers with Unmet Need by Region, Spacing/Limiting, and Marital Status, 2000 (in thous
(Source: Ross and Winfrey, 2002.) (see "Demand" section in text)
Sub-Saharan Africa
Asia except China Middle East/N. Africa
NUMBERS WITH UNMET NEED
DEVELOPING COUNTRIES
Currently Married
Percent Distribution
PERCENTAGES WITH UNMET NEED
DEVELOPING COUNTRIES
Currently Married
OTHER AREAS (a)
Note (a): Percents for last 4 areas pertain to women of all marital statuses
VI.4 APPENDIX 4. Projections of IUD Use.
(Source: Macro DHS StatCompiler and UN 2005).
See Text section on Projections.
APPENDIX TABLE FOR PROJECTIONS
Projections of IUD prevalence, users, and adopters at a half percent rise per year in IUD prevalence*
Adoption
Prevalence
Rate (% of
Prevalence
after 5 yrs at
Adoptions
1/2% rise/yr
MWRA in 2005
Users in 2005
during 2006
LESS DEVELOPED
REGIONS
15.5 18.00 941,040,280 145,843,807 56,625,597 6.0
98,386,639
39,189,647
14,820,792
Central African Republic
Sao Tome & Principe
5,415,300
38,960,900
ASIA Total
706,783,937
130,074,064
49,840,287
Eastern Asia
305,269,089
108,967,781
40,318,875
S. Central Asia
291,899,852
5,723,599
3,497,101
PROJECTION TABLE CONTINUED
SE Asia
98,398,283
10,214,179
4,128,239
Central Asia Rep.
11,139,713
5,161,575
1,893,219
N. Africa/Middle East
56,680,305
8,910,177
3,455,424
Libyan Arab Jamahiriha
United Arab Emiratess
3,237,300
Latin American & Caribbean
75,952,099
5,742,755
2,424,181
4,860,620
Antigua and Barbudau
Dominican Republic
Saint Kitts and Nevisu
Saint Vincent & Grenadinesu
Trinidad and Tobagou
23,921,700
2,752,683
1,099,564
47,169,779
2,035,060
* Regional figures are aggregates of country numbers so regional results weight countries by their numbers
VI.5 APPENDIX 5: Preferred method of contraception for
future use
(Source: Macro DHS StatCompiler)
(columns do not add to 100 due to omitted methods)
Injection
Sub-Saharan Africa
Benin 2001
Botswana 1988
Burkina Faso 2003
Burundi 1987
Cameroon 2004
CAR 1994/95
Chad 1996/97
Comoros 1996
Cote d'Ivoire 1998/99
Eritrea 2002
Ethiopia 2000
Gabon 2000
Ghana 2003
Guinea 1999
Kenya 2003
Liberia 1986
Madagascar 2003/2004
Malawi 2000
Mali 2001
Mozambique 2003
Namibia 2000
Niger 1998
Nigeria 2003
Rwanda 2000
Senegal 1997
South Africa 1998
Sudan 1990
Tanzania 1996
Togo 1998
Uganda 2000/01
Zambia 2001/02 (2)
Zimbabwe 1999
North Africa/West
Asia/Europe
Armenia 2000
Egypt 2000
Jordan 2002
Morocco 2003-2004
Tunisia 1988
Turkey 1998
Yemen 1997
Central Asia
Kazakhstan 1999
Kyrgyz Republic 1997
Turkmenistan 2000
Uzbekistan 1996
South & Southeast Asia
Bangladesh 2004 (7)
Cambodia 2000
India 1998/99
Indonesia 2002/2003
Nepal 2001
Pakistan 1990/91
Philippines 2003
Sri Lanka 1987
Thailand 1987
Vietnam 2002
Latin America &
Caribbean
Bolivia 2003
Brazil 1996
Colombia 2000
Dominican Republic 2002
Ecuador 1987
El Salvador 1985
Guatemala 1998/99
Haiti 2000
Mexico 1987
Nicaragua 2001
Paraguay 1990
Peru 2000
Trinidad & Tobago 1987
VI.6 APPENDIX 6. Percent of Married Women Ever Using the IUD, by Age and Region
(Source: Macro DHS StatCompiler)
15-19 20-24 25-29 30-34 35-39 40-44 45-49 Total
0.1 1.0 1.8 3.1 3.9 4.0 3.7
6.7 19.7 31.5 38.3 40.4 36.1 30.1
12.5 38.2 60.4 75.5 76.4 73.6 65.1
3.1 7.6 12.0 14.4 15.3 15.4 13.4
5.8 12.5 17.7 19.4 19.2 17.3 14.6
3.0 8.3 12.9 15.7 16.5 15.6 13.5
*Countries receive equal weights.
15-19 20-24 25-29 30-34 35-39 40-44 45-49 Total
0.0 0.5 1.1 1.9 2.2 4.1 2.3
0.0 15.6 17.9 23.9 20.1 13.4 8.1
0.2 0.1 0.9 1.3 1.9 2.4 2.6
0.0 0.4 0.7 0.9 0.5 0.6 0.5
0.2 0.1 0.5 1.1 2.0 3.1 4.3
0.0 0.1 0.1 0.2 0.2 0.6 0.3
0.0 0.0 0.1 0.6 0.2 0.2 0.0
0.0 1.2 1.9 2.0 2.5 1.1 1.2
0.2 0.4 2.0 2.9 0.5 3.7 0.9
0.0 0.1 0.8 1.6 3.4 4.0 3.0
0.0 0.0 0.3 0.8 1.1 0.5 0.4
0.0 0.1 0.4 1.2 1.1 3.8 5.5
0.0 0.4 1.5 3.2 5.1 6.2 5.0
0.0 0.2 0.2 0.8 0.5 0.8 0.2
0.0 2.2 2.3 4.0 4.7 3.3 2.8
0.0 0.3 1.2 1.6 1.7 1.4 2.0
0.0 0.5 1.1 1.3 3.2 3.7 3.5
0.0 0.2 1.0 1.2 1.4 1.3 1.5
0.0 0.2 0.3 0.8 1.0 0.8 0.9
0.4 0.4 1.4 3.6 4.1 2.2 2.7
0.1 0.4 1.3 2.4 5.7 5.0 2.7
0.0 0.4 1.8 5.2 8.6 8.3 7.6
0.1 0.2 0.4 0.7 1.1 0.5 0.9
0.0 0.5 1.9 1.6 3.1 3.8 4.0
0.0 0.0 0.4 0.3 0.8 1.1 1.5
0.5 0.7 2.7 5.3 7.2 6.7 4.6
South Africa 1998
0.3 0.6 1.4 2.5 2.3 2.0 1.5
0.2 0.5 2.8 2.4 1.0 1.2 5.5
0.4 1.2 2.1 2.8 3.7 4.6 6.2
0.3 0.2 1.6 1.5 2.1 1.3 1.4
Zambia 2001/02 (2)
0.3 0.7 1.3 3.1 6.1 4.2 6.5
0.1 1.0 1.8 3.1 3.9 4.0 3.7
2.8 7.5 16.5 26.1 28.1 20.5 16.3
67.0 66.3 61.5 52.9
4.4 22.7 41.5 57.5 63.7 61.9 57.1
3.2 7.3 12.5 17.2 20.3 24.3 20.9
4.8 28.1 39.5 43.9 40.9 35.3 27.5
9.6 23.4 40.9 46.4 52.8 42.3 30.5
1.1 4.5 7.2 9.8 11.0 7.1 5.4
6.7 19.7 31.5 38.3 40.4 36.1 30.1
14.5 42.7 67.6 79.8 76.6 76.2 65.5
Kyrgyz Republic 1997
7.6 32.5 52.3 69.0 74.4 76.7 67.3
12.9 35.0 60.2 72.1 74.3 66.5 59.0
75.5 76.4 73.6 65.1
Bangladesh 2004 (7)
2.8 3.9 4.8 3.8 3.6
0.7 3.9 7.1 8.4 7.5 4.6 3.2
Indonesia 2002/2003
0.1 0.9 1.7 1.8 1.7 0.5 0.4
0.4 1.4 4.1 4.9 4.1 3.6 1.8
3.1 5.7 7.2 9.0 8.5 8.6 7.7
Philippines 2003
7.4 14.1 18.6 16.5 17.3 17.4 12.8
15.4 41.4 63.6 69.5 70.6 70.2 66.4
3.1 7.6 12.0 14.4 15.3 15.4 13.4
Latin America & Caribbean
6.0 16.6 24.0 27.4 25.1 21.0 19.0
1.3 2.0 3.3 5.2 3.8 4.1 4.1
34.8 36.3 41.1 34.7 35.4
Dominican Republic 2002
7.2 16.3 24.8 23.5 19.3 19.9 9.1
6.8 11.2 11.5 10.7 12.0 16.0 9.4
Guatemala 1998/99
0.0 0.1 0.2 0.4 0.4 2.3 3.1
17.5 28.2 31.6 27.0 22.2 22.0 12.6
9.2 17.5 28.1 32.3 33.5 26.3 21.1
2.2 6.7 14.1 15.5 15.5 13.0 11.8
4.8 17.2 26.3 33.9 35.3 31.4 24.3
Trinidad & Tobago 1987
5.8 12.5 17.7 19.4 19.2 17.3 14.6
3.0 8.3 12.9 15.7 16.5 15.6 13.5
VI.7 APPENDIX 7. Percent of Currently married Women
Who Know Each Method
(Source: Macro StatCompiler)
Regional Summary*
Injections
Sub-Saharan Africa
43.6 77.2
North Africa/West Asia
92.3 92.9
Central Asia Rep.
98.1 79.3
South & Southeast Asia
80.1 90.7
80.8 92.7
64.6 84.0
*Countries receive equal weights
Country Detail
Injection
Sub-Saharan Africa
Burkina Faso 2003
Cote d'Ivoire 1998/99
91.8 94.7 74.3 45.4
Madagascar 2003/2004
56.9 61.5 32.5 12.6
Mauritania 2000/01
Mozambique 2003
South Africa 1998
Zambia 2001/02 (2)
72.6 70.1
North Africa/West
Asia/Europe
99.4 68.1 74.9 15.7
Morocco 2003-2004 (3)
75.6 73.4
Central Asia
Kazakhstan 1999
Kyrgyz Republic 1997
Turkmenistan 2000
Uzbekistan 1996
66.4 75.6
South & Southeast Asia
Bangladesh 2004 (7)
Indonesia 2002/2003
Pakistan 1990/91
Philippines 2003
86.3 79.9
Latin America &
Caribbean
Dominican Rep. 2002
El Salvador 1985
Guatemala 1998/99
Trinidad & Tobago 1987
85.7 82.7
OVERALL MEANS
84.0 77.0
74.7 65.7 35.9
Source: http://www.hewlett.co/uploads/files/iud.pdf
ANSWERS FOR SCIENCE. KNOWLEDGE FOR LIFE.™ High Resolution Separation and Quantitation of Charged and Polar Analytes P/ACE™ MDQ PLUS CAPILLARY ELECTROPHORESIS SYSTEM A Robust, Automated Analytical ToolSCIEX Capil ary Electrophoresis (CE) has global y proven itself in many different research projects and industrial applications, including biological science, chemistry, forensic science, food and beverage, petrochemical, biofuels, environmental safety and more…
Platinum Priority – Prostate Cancer Editorial by Derek J. Rosario, Liam Bourke and Nancy L. Keating on pp. 574–576 of this issue Cardiovascular Morbidity Associated with GonadotropinReleasing Hormone Agonists and an Antagonist Peter C. Albertsen , Laurence Klotz Bertrand Tombal , James Grady ,Tine K. Olesen Jan Nilsson a University of Connecticut Health Center, Farmington, CT, USA; b Division of Urology, University of Toronto, ON, Canada; c University Clinics Saint Luc/Catholic University of Louvain, Brussels, Belgium; d Ferring Pharmaceuticals, Copenhagen, Denmark; e Department of Clinical Sciences, Lund University,



