Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Multicentre prospective crossover study of the prostatic urethral lift for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia
Multicentre prospective crossover study of the
‘prostatic urethral lift' for the treatment of lower
urinary tract symptoms secondary to benign
prostatic hyperplasia
Anthony L. Cantwell, William K. Bogache*, Steven F. Richardson†, Ronald F. Tutrone‡,
Jack Barkin§, James E. Fagelson¶, Peter T. Chin†† and Henry H. Woo‡‡
Atlantic Urological Associates, Daytona Beach, FL, *Carolina Urological Research Center, Myrtle Beach, SC, †WesternUrological Clinic, Salt Lake City, UT, ‡Chesapeake Urology, Baltimore, MD, USA, §University of Toronto, Toronto, ON,Canada, ¶Urology Associates of Denver, Denver, CO, USA, ††Figtree Private Hospital, Figtree, and ‡‡Sydney AdventistHospital Clinical School, University of Sydney, Sydney, NSW, Australia
• To assess the clinical effect of the ‘prostatic urethral lift'
• Symptom, flow, HRQL and sexual function assessments
(PUL) on lower urinary tract symptoms (LUTS) associated
showed response improvements from baseline results,
with benign prostatic hyperplasia (BPH) through a
similar to results from other published studies, and most
crossover design study.
parameters were markedly improved after PUL vs the shamprocedure in the same patients.
Patients and Methods
• Symptom, flow, and HRQL improvements were durable
over the 12 months of the study.
• Men aged ≥50 years with an International Prostate
• Adverse events associated with the procedure were typically
Symptom Score of ≥13, a maximum urinary flow rate (Qmax)
transient and mild to moderate; one patient (2%) required
of ≤12 mL/s, and a prostate of 30–80 mL were enrolled into
re-intervention with transurethral resection of the prostate
a crossover study after completing a prospective,
in the first year.
randomised, controlled, ‘blinded' pivotal study in which they
• There were no occurrences of de novo, sustained ejaculatory
were control subjects receiving a sham procedure.
or erectile dysfunction.
• Patients were followed for 1 year after crossover PUL at 19
centres in the USA, Canada and Australia. The sham
procedure involved rigid cystoscopy with simulated active
• The PUL can be performed under local anaesthesia, causes
treatment sounds.
minimal associated perioperative complications, allows
• PUL involved placing permanent UroLift® (NeoTract, Inc.,
patients to quickly return to normal activity, provides rapid
Pleasanton, CA, USA) implants into the lateral lobes of the
and durable improvement in symptoms, and preserves
prostate to enlarge the urethral lumen.
sexual function.
• Urinary symptom relief, health-related quality of life
(HRQL) impact, urinary flow parameters, sexual function,
and adverse events were assessed and compared between
prostate, benign prostatic hyperplasia, minimally invasive
the sham and PUL using paired statistical analysis.
surgical procedure, crossover, sham, sexual function
perioperative risk [1–4]. Small UroLift® implants (NeoTract,Inc., Pleasanton, CA, USA) are delivered transurethrally to
BPH is common in men beyond middle age and often causes
separate the lateral lobes of the prostate and relieve
bothersome LUTS that can detrimentally affect a man's
obstruction. Previously published studies have reported
health-related quality of life (HRQL). The ‘prostatic urethral
symptom reduction considerably greater than drugs, faster
lift' (PUL) is a mechanical approach to addressing LUTS that
acting and more durable than thermal therapies, and without
has the potential to offer rapid and significant mitigation of
the more serious complications associated with TURP or laser
symptoms, preservation of sexual function and minimal
[1–4]. We report on a group of patients who underwent a
2013 The AuthorsBJU International 2013 BJU International doi:10.1111/bju.12540
BJU Int 2014;
113: 615–622
Published by John Wiley & Sons Ltd. www.bjui.org
Cantwell
et al.
sham procedure followed by PUL 3–6 months later. These
for devices and support personnel opened packaging
patients allow for analysis of the individual effect of active vs
materials. Then, at appropriate times during the procedure, the
sham procedure, a rare opportunity in medical device clinical
operator simulated the UroLift delivery device sounds by
activating a standard disposable biopsy device that was notinserted into the patient.
Crossover studies have been shown to effectively comparerelative therapeutic effects of pharmaceutical treatments withplacebo or other treatments, but this design has rarely been
Study Procedure the PUL
used to study medical devices [5–8]. The primary challenges
The PUL involves the delivery of permanent
in situ tailored
with conducting a medical device crossover study design are:
transprostatic UroLift® implant (NeoTract, Inc., Pleasanton,
(i) while sham control groups can crossover to active
CA, USA) to reshape the prostatic fossa, allowing for a
treatment, it is not possible to cross active arm subjects back
continuous channel through the anterior aspect of the prostate
to control; and (ii) while ‘blinding' can be maintained for
(Fig. 1) [1–4]. Under cystoscopic visualisation through a 20 F
sham, it is typically not feasible to maintain a ‘blind' when
sheath, the system compresses the obstructing tissue and
these subjects crossover to active treatment. Device trials
delivers through a hollow 19-G needle a monofilament that
consequently use the ‘one-way' instead of the ‘two-way'
traverses the prostate lobe with a metallic tab seated on the
crossover design. We sought to compare the effects of PUL
capsular surface. The monofilament is tensioned and sized
in
when delivered 3–6 months after a sham procedure using this
situ to fit the compressed prostate lobe. A urethral end piece is
self-controlled paired data set.
then affixed to the monofilament, which is trimmed to thenewly fixed length. Typically four implants are delivered tocreate a continuous anterior channel.
Patients and MethodsA crossover study of the PUL procedure after sham control
was conducted at 19 centres in the USA, Canada, andAustralia in men with moderate to severe LUTS secondary to
The IPSS, HRQL (as assessed by the eighth question of the
BPH. While enrolled in a randomised double-blind study
IPSS), and BPH Impact Index (BPHII) were assessed at
published by Roehrborn et al. [3], patients underwent a sham
baseline and 2 weeks, 1 and 3 months after both the sham and
procedure that involved rigid cystoscopy and mimicking
PUL procedures and additionally at 6 and 12 months after the
surgical sounds. After the primary endpoint comparison at 3
PUL. The five-item version of the International Index of
months, these sham controls were unblinded and, if eligible,
Erectile Function (IIEF-5, equivalent to the Sexual Health
offered enrolment into the crossover study, where they were
Inventory for Men [SHIM]) and the Male Sexual Health
treated with PUL and followed to 12 months.
Questionnaire for Ejaculatory Function (MSHQ-EjD) andBother (MSHQ-Bother) were assessed at baseline and 1 and
Eligible patients for the crossover study were aged ≥50 years,
3 months after both the sham and PUL procedures and
provided informed consent, had no prior surgical BPH
additionally at 6 and 12 months after the PUL in patients who
treatment, and were either washed out or naïve to α-blockers
were sexually active. Qmax and PVR were assessed at 3 and 12
and 5α-reductase inhibitors. Each patient had an IPSS score of
months. Safety was assessed at each follow-up visit through
≥13, a maximum urinary flow rate (Qmax) of ≤12 mL/s with a
adverse event reporting. An independent Clinical Events
voided volume of 125 mL, and a prostate of volume of
Committee (CEC) adjudicated all reported events, and an
30–80 mL without an obstructing median lobe. Patients were
independent reviewer over-read each flow waveform using the
excluded for retention, post-void residual urine volume (PVR)
two-second rule.
of >250 mL, active infection, PSA level of >10 ng/mL unlessnegative biopsy, cystolithiasis within 3 months, and bacterial
Statistical Methods
prostatitis within 1 year. The study protocol was approved bythe USA Food and Drug Administration, Health Canada, and
Descriptive statistics were used to describe the baseline and
the Therapeutic Goods Administration of Australia, as well as
follow-up values of all study parameters (IPSS, HRQL, BPHII,
the Institutional Review Boards at each of the 19 enrolling
Qmax, PVR, SHIM, and MSHQ-EjD). Where stated, values are
sites (Clinicaltrials.gov: NCT01294150).
reported as the mean (standard deviation). The changebetween baseline and 3 months for the sham procedure vs thePUL was compared using a paired Student's
t-test, in which
Control (Sham) Procedure
each patient served as their own control. Additionally, a
The sham control procedure was conducted in a manner that
general estimating equation model (GEE) was fitted to each
simulated PUL. A visual obstruction was erected in the room
study output parameter. The change from baseline was the
so that the recumbent patient could not see the operator or
dependent variable; baseline score and visit were the
endoscopy image. During rigid cystoscopy, the operator called
independent variables. In this model, an exchangeable
2013 The Authors
616 BJU International 2013 BJU International
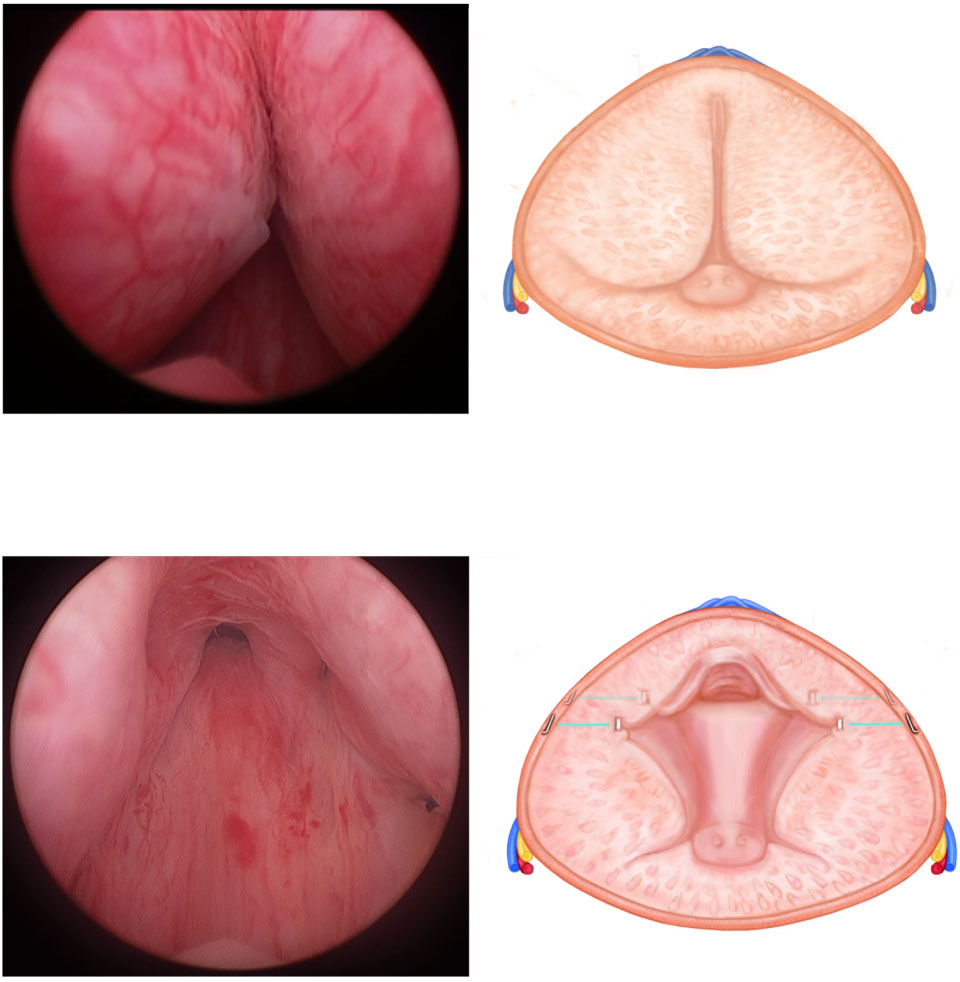
PUL for the treatment of LUTS
Fig. 1 The Prostatic Urethral Lift procedure.
(a & b) Before treatment, the enlarged lateral
lobes obstruct the urethra. (c & d) After
transurethral delivery through a 19 gauge
needle, the UroLift® implants reshape the
prostate to allow for a patent channel through
the anterior aspect of the prostatic fossa.
correlation structure and identity link were used and P values
Table 1 Baseline characteristics for patients who elected crossover PUL
for each follow-up interval compared with baseline were
procedure 3–6 months subsequent to receiving a rigid cystoscopy shamprocedure.
calculated using SAS (SAS Institute, Inc. Cary, NC) and R (TheR Foundation, Vienna, Austria); a P < 0.05 was considered to
Cross-over PUL (n = 53)
indicate statistical significance.
Mean (SD, range)
64 (8.0, 50–79)
Prostate volume, mL*
40.3 (9.9, 30–68)
23.3 (5.5, 13–34)
HRQL (IPSS question 8)
6.3 (3.0, 1–12)
8.8 (4.2, 2.0–30.0)
Between February and December 2011, 66 men underwent a
67.8 (66.44, 0–262)
sham procedure as part of a ‘blinded' randomised study [3].
2.26 (1.85, 0–8)
12.8 (8.3, 1–25)
After unblinding at 3 months, 53 subjects (80%) elected to
9.5 (10.0, 3–14)
enrol in this crossover study and undergo PUL (Table 1). Overthe 12-month follow-up, no PUL patient required α-blocker
*Baseline data was used for those patients who did not have data collected immediatelybefore crossover.
therapy and one (2%) progressed to a standard TURPintervention, which was completed without complication.
The mean (SD) crossover PUL procedure time was 53 (15)
patient enrolled in North America underwent general
min for delivering a mean (range) of 4.4 (2–8) implants in
anaesthesia; 44/46 (96%) of procedures were conducted under
prostates ranging in volume from 30 to 70 mL. While
local anaesthesia using cold lidocaine with sedative and the
Australian standard of care required general anaesthesia, no
remaining two (4%) used prostatic block. Of the 53 patients
2013 The Authors
BJU International 2013 BJU International 617
Cantwell et al.
Table 2 Baseline, follow-up, and change in each outcome measure (IPSS, HRQL, BPHII, MSHQ-EjD, MSHQ-Bother, and IIEF-5) after control sham therapy
followed by crossover PUL in the same patient cohort. Each parameter is presented as the mean (SD). The 3-month change in each parameter in the
control vs crossover period was compared using a paired Student's t-test.
Control sham therapy period
Crossover PUL period
(paired sample size, n)
HRQL, IPSS question 8 (52)
IIEF-5 (SHIM) (36)
*Baseline value was defined as the value before the initial sham procedure for the Control group and the value before PUL for the Cross-over group. Note that the baseline value for theCrossover group was 3–6 months after the sham procedure.
undergoing crossover PUL, 41 underwent void trial after the
Fig. 2 Comparison of the IPSS from baseline to 3-month follow-up for
procedure. No postoperative catheterisation was required for
patients who underwent sham procedure and later crossover PUL
27 (66%) of these tested patients, and the mean catheter
procedure. Also plotted are the ‘blinded' and randomised results from
duration for all patients was 33 h. The PUL patients reported a
Roehrborn et al. [3] on PUL only patients. Crossover PUL IPSS improvement
mean (SD) complete return to preoperative activity by 6.5 (6.8)
is significantly greater than that of sham and closely mimics prior
published results. Values shown are the mean absolute IPSS, error bars
represent the 95% CI.
The therapeutic effect of the PUL was significantly greater
than that seen for the sham procedure in this crossover study.
The mean IPSS improvement after crossover PUL (11.1
points) was 122% greater than after sham (5.0 points) at 3
months (P < 0.001; Table 2). The IPSS reduction seen in
crossover PUL patients closely mimics that of previously
published randomised results (Fig. 2) [3]. Improvements in
HRQL(IPSS question 8) and BPHII, were also significantly
greater for crossover PUL patients vs sham (P < 0.001 and P =0.024, respectively). Qmax showed stepwise improvement,
increasing from 7.9 (2.4) mL/s at baseline to 10.3 (4.6) mL/s 3months after sham and further increasing to 12.0 (6.1) mL/s
and 12.5 (5.3) mL/s at 3 and 12 months after crossover PUL,
respectively (Fig. 3). The PUL showed clinically and
statistically significant improvement in IPSS, HRQL, BPHIIand Qmax throughout the course of the 12-month study(Tables 3,4). Sexual function was maintained with no
pelvic pain/discomfort (21%) (Table 5). No patient required a
significant degradation in SHIM or MSHQ-EjD at any time
blood transfusion and haematuria typically resolved within 3
point after the PUL, and the general trend was improvement
days. The patients who reported pelvic pain or discomfort at
in all measures after the PUL (Table 3). Ejaculatory function
the 1 month visit rated their pain on a visual analogue scale.
showed a statistically significant difference between the sham
The mean pain scores after the PUL showed no significant
procedure, which decreased ejaculatory function, and the PUL
difference those after the sham procedure (2.71 and 2.67 out
treatment, which increased ejaculatory function, at 3 months
of 10, respectively; P = 0.9). There was no incidence of de
novo, sustained erectile dysfunction or retrograde ejaculation.
One patient progressed to TURP 12 months after treatment
due to persistent nocturia.
The adverse events reported for PUL were typically mild to
Related adverse events were also examined using the Clavien-
moderate and resolved within 2 weeks; the most commonly
Dindo classification. Most were mild, typically Class I or II,
occurring events were dysuria (36%), haematuria (26%), and
while none were Class IV or V. There were two Class III
2013 The Authors
618 BJU International 2013 BJU International
PUL for the treatment of LUTS
Table 3 IPSS, HRQL, BPHII, SHIM, MSHQ-EjD, and change from baseline (after sham procedure and before PUL) after PUL. P values were obtained from a
general estimating equation.
12 Months
n (paired)
Mean % change (95% CI)
−18 (−27, −10)
−46 (−53, −39)
−48 (−56, −40)
−43 (−52, −34)
−37 (−46, −27)
HRQL (IPSS question 8)
n (paired)
Mean % change (95% CI)
−20 (−32, −9)
−43 (−53, −33)
−49 (−59, −39)
−44 (−53, −35)
−41 (−53, −29)
n (paired)
Mean % change (95% CI)
−41 (−59, −23)
−52 (−64, −41)
−53 (−64, −42)
−44 (−58, −30)
n (paired)
n (paired)
n (paired)
events, each of those was a patient who presented in hospital
when he underwent TURP; the remaining implants were left
for urinary retention; one was discharged the same day with a
in situ as they were asymptomatic; the patients will be
catheter and the other was readmitted for 2 days.
In all, 48 patients, with a total of 215 implants, underwentcystoscopy at 12 months. An independent reviewer found no
evidence of encrustation on the implants delivered within the
The results of this crossover study show that, with each patient
prostate, no increase over baseline in oedema or inflammation,
serving as his own control, the PUL procedure is associated
no de novo strictures, and no evidence of abnormal pathology
with a clinically and statistically significant treatment effect
in the prostatic urethra. Surface encrustation was observed on
beyond sham therapy. The crossover PUL LUTS improvement
10 implants (4.7%) that were inadvertently delivered such that
is consistent with that observed when comparing separate
part of the implant was exposed to urine within the bladder.
randomised groups. The mean (SD) 3-month IPSS
Two of these 10 implants were removed using cystoscopic
improvement after crossover PUL was virtually identical to
grasping forceps and two were removed from a single patient
that seen with a separate group of patients in a ‘blinded'
2013 The Authors
BJU International 2013 BJU International 619
Cantwell et al.
Fig. 3 Qmax shows stepwise improvement starting at baseline, improving 3
baseline of enrolment was 10.6 points, again consistent with
months after sham, and further improving 3 months after crossover PUL.
the 10.8 and 12.3 point improvements at 12 months reported
The improvement after crossover is stable through to the 12-month
in prior studies [3,4]. A possible explanation for this
cumulative effect is that dilatation during the sham procedure
does not fully dissipate by 3 months but appears to no longercontribute to overall effect by 12 months.
By contrast, urinary flow rate change was more durable after
sham rigid cystoscopy. At 3 months after sham cystoscopy,
there was a 2.4 mL/s increase in Qmax from baseline. Aftercrossover PUL, Qmax further improved 2.5 mL/s at 3 months
and was maintained to 12 months. The cumulative 12 months
Qmax improvement of 4.6 mL/s is similar to the 4.0 mL/s
improvement reported in both randomised and open labelstudies [3,4]. The continued improvement in flow after the
sham procedure may be a result of a lingering dilatory effect
from rigid cystoscopy.
For a minimally invasive approach, patient satisfaction is oftendetermined by return to normal activity and perioperativecomplications [9]. Morbidity associated with the PUL
Table 4 Qmax and PVR change from baseline (after sham procedure and
before PUL) after PUL. P values were obtained from a general estimating
procedure was low as was the need for postoperative
catheterisation. Adverse events were as expected after a rigidcystoscopic intervention, with most events transient and either
12 Months
mild or moderate. Pelvic pain was tracked carefully, and visual
analogue scores were not different between the PUL and sham
n (paired)
procedures. On average, PUL patients returned to normal
preoperative activity in less than a week, which is considerably
more rapid than the 4–6 weeks typical of other BPH therapies
[10]. In PUL procedures conducted in the USA and Canada,
Mean % change (95% CI)
all were conducted with local anaesthesia (96%) or prostate
n (paired)
After the crossover PUL procedure, no patient had new onset,
sustained ejaculatory or erectile dysfunction. Further, sexual
function measures in the ‘erectile function', ‘ejaculatory
Mean % change (95% CI)
9.26 (67.35, −48.84)
4.67 (55.70, −46.36)
function', and ‘ejaculatory bother' domains improved after
PUL at every time point, although most changes were notstatistically significant. This preservation in overall sexualfunction after a BPH procedure stands in contrast to the
randomised study, at 11.1 (7.2) vs 11.1 (7.7), respectively [3].
41–65% rates of ejaculatory dysfunction and 7–10% rates of
In both comparisons, the improvement after the PUL was
erectile dysfunction reported for TURP or laser procedures
significantly greater than the effect of sham rigid cystoscopy.
[11–13]. Iatrogenic sexual dysfunction can significantly affect
This high level of repeatability serves as a validation of the
HRQL [14]. One study has shown that 19% of men would
consistent therapeutic effect of the PUL. Both ‘blinded' and
even forego treatment for cancer if it compromised their
crossover (open-label) PUL patients had rapid, durable relief
sexual function [15]. While erectile function is more
with minimal morbidity and virtually no sexual compromise.
commonly analysed, ejaculatory function has also been foundto be of high importance to many patients [16]. The increase
There was a change in IPSS score at 2 weeks for both sham
in ejaculatory function after PUL compared with the
and crossover PUL. For the sham procedure, this could be due
functional compromise after the sham procedure, suggests that
to the psychological effect of undergoing a treatment and the
PUL may be uniquely suited to treat LUTS while preserving
temporary urethral dilatation associated with rigid cystoscopy.
sexual function and is consistent with the prior randomised
From 2 weeks to 3 months, the sham effect begins to diminish,
while the PUL effect continues to improve. In the longer term,the 12-month IPSS improvement from the time of crossover
The primary strength of the present study lies in the statistical
was 8.7 points, but the cumulative improvement from true
power associated with the paired measures analysis that was
2013 The Authors
620 BJU International 2013 BJU International
PUL for the treatment of LUTS
Table 5 Overview of adjudicated adverse events of interest.
0–3 months
Control (n = 53)
Cross-over (n = 53)
Cross-over (n = 53)
pelvic pain/discomfort
urgency incontinence
urinary tract infection
erectile dysfunction*
retrograde ejaculation*
*Sexual dysfunction adjudicated as new onset and sustained. Related, device or procedure related; SAE, serious adverseevent; AE, adverse event.
permitted because each patient served as his own control. The
results from this self-controlled data set, which included
The authors would like to thank Drs Rodney Anderson, Kyle
open-label PUL therapy, corroborates previously published
Anderson, and Parker Eberwein for serving on the CEC, and
results from a randomised study. In contrast to the
Drs Harchi Gill and James Yu for conducting independent
randomised study, the analysis of the self-controlled data set
review of flow waveforms. In addition, the authors want to
may provide more insight into what the patient response
express appreciation to the staff of NeoTract, Inc., Five
might be outside of a clinical study. In everyday use, the
Corners Pty. Ltd., CMX Research, Inc., QST Consultations,
patient generally has free will to choose the treatment, perhaps
LTD, and Myraqa, Inc. for their assistance in study conduct,
in view of previous other treatment failures. It could be argued
manuscript preparation, and statistical analysis. This study was
that the crossover phase comes closer to assessing the results
funded by NeoTract, Inc.
expected for a commercialised product under a free willchoice. The fact that the results from the randomised andcrossover phases are similar is reassuring.
Conflict of Interest
Conversely, some weaknesses of the study must be recognised;
A.L.C.l., W.K.B., S.F.R., R.F.T., J.B., J.E.F. l., and P.T.C. have been
notably, the duration of follow-up is only to 1 year at this
investigators for the Neotract sponsored study from which this
point. An earlier study showed a similar reduction in IPSS at 1
data has been extracted.
year (10.4 vs 10.6 points observed in the present study) and
H.H.W. and P.T.C. have been consultants to Neotract and hold
2-year durability of LUTS improvement, thereby providing
stock in Neotract.
some evidence of the longevity of this minimally invasivetherapy [2]. Additionally, as the present study includedopen-label PUL therapy, the possibility of a placebo effect
cannot be excluded. However, the consistency between the
Woo HH, Chin PT, McNicholas TA et al. Safety and feasibility of the
3-month results in the present study and in a prior
prostatic urethral lift: a novel, minimally invasive treatment for lower
randomised study indicate a true therapeutic effect.
urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia(BPH). BJU Int 2011; 108: 82–8
In conclusion, the PUL is associated with early symptom relief,
Chin PT, Bolton DM, Jack G et al. Prostatic urethral lift: two-year results
low morbidity and preservation of sexual function. Not
after treatment for lower urinary tract symptoms secondary to benign
surprisingly, PUL reduces symptoms more than rigid
prostatic hyperplasia. Urology 2012; 79: 5–11
Roehrborn CG, Gange SN, Shore ND et al. The prostatic urethral lift for
cystoscopy at 3 months and the results of this open label
the treatment of lower urinary tract symptoms associated with prostate
self-controlled study corroborate earlier findings in a
enlargement due to benign prostatic hyperplasia: the L.I.FT. study. J Urol
randomised study.
2013; 190: 2161–7
2013 The Authors
BJU International 2013 BJU International 621
Cantwell et al.
McNicholas TA, Woo HH, Chin PT et al. Minimally invasive prostatic
2010. Available at: http://www.auanet.org/education/guidelines/benign
urethral lift: surgical technique and multinational experience. Eur Urol
-prostatic-hyperplasia.cfm. Accessed November 2013
2013; 64: 292–9
13 Thangasamy IA, Chalasani V, Bachmann A, Woo HH. Photoselective
Ikemoto I, Kiyota H, Ohishi Y et al. Usefulness of tamsulosin
vaporisation of the prostate using 80-W and 120-W laser versus
hydrochloride and naftopidil in patients with urinary disturbances caused
transurethral resection of the prostate for benign prostatic hyperplasia: a
by benign prostatic hyperplasia: a comparative, randomized, two-drug
systematic review with meta-analysis from 2002 to 2012. Eur Urol 2012;
crossover study. Int J Urol 2003; 10: 587–94
Kirby RS. A randomized, double-blind crossover study of tamsulosin and
14 Haltbakk J, Hanestad BR, Hunskaar S. How important are men's lower
controlled-release doxazosin in patients with benign prostatic hyperplasia.
urinary tract symptoms (LUTS) and their impact on the quality of life
BJU Int 2003; 91: 41–4
(QOL)? Qual Life Res 2005; 14: 1733–41
Miyakita H, Yokoyama E, Onodera Y et al. Short-term effects of
15 Helgason AR, Adolfsson J, Dickman P, Fredrikson M, Arver S, Steineck
crossover treatment with silososin and tamsulosin hydrochloride for
G. Waning sexual function-the most important disease-specific distress
lower urinary tract symptoms associated with benign prostatic
for patients with prostate cancer. Br J Cancer 1996; 73: 1417–21
hyperplasia. Int J Urol 2010; 17: 869–75
16 Arai Y, Aoki Y, Okubo K et al. Impact of interventional therapy for
Nishino Y, Masue T, Miwa K, Takahashi Y, Ishihara S, Deguchi T.
benign prostatic hyperplasia on quality of life and sexual function: a
Comparison of two alpha1-adrenoceptor antagonists naftopidil and
prospective study. J Urol 2000; 164: 1206–11
tamsulosin hydrochloride, in the treatment of lower urinary tract
17 McVary KT, Gange SN, Shore ND et al. Treatment of LUTS secondary to
symptoms with benign prostatic hyperplasia: a randomized crossover
BPH while preserving sexual function: randomized controlled study of
study. BJU Int 2006; 97: 747–51
prostatic urethral lift. J Sex Med 2014; 11: 279–87
Patel VR, Sivaraman A, Coelho RF et al. Pentafecta: a new concept for
reporting outcomes of robot-assisted laparoscopic radical prostatectomy.
Eur Urol 2011; 59: 702–7
Correspondence: Henry Woo, Suite 406, SAN Clinic, 185 Fox
10 AUA Practice Guidelines Committee. Diagnosis and treatment
Valley Road, Wahroonga, NSW 2076, Australia.
recommendations. Chapter 1 in AUA guideline on management of benignprostatic hyperplasia. J Urol 2003; 170: 530–47
11 Roehrborn CG, McConnell JD, Bary MJ et al. American Urological
Association Guideline: Management of Benign Prostatic Hyperplasia
Abbreviations: BPHII, BPH Impact Index; HRQL, health-
(BPH). American Urological Association Education and Research Inc.
related quality of life; IIEF, International Index of Erectile
2003. Available at: http://www.auanet.org/education/guidelines/benign
Function; MSHQ-EjD, Male Sexual Health Questionnaire for
-prostatic-hyperplasia.cfm. Accessed November 2013
Ejaculatory Function; Q
12 McVary KT, Roehrborn CG, Avins AL et al. American Urological
max, maximum urinary flow rate; PUL,
Association Guideline: Management of Benign Prostatic Hyperplasia
prostatic urethral lift; PVR, post-void residual urine volume;
(BPH). American Urological Association Education and Research Inc.
SHIM, Sexual Health Inventory for Men.
2013 The Authors
622 BJU International 2013 BJU International
Source: http://urologypartners.co.uk/repository/BJUI_-_Crossover_Study_-_01-2014.pdf
AMERICAN JOURNAL OF UNDERGRADUATE RESEARCH VOL. 11, NOS. 3 & 4 (2012-13) SAR and Pharmacophore Based Designing of Some Antimalarial and Antiretroviral Agents: An INTERNET Based Drug Design Approach Soumendranath Bhakat* Department of Pharmaceutical Sciences Birla Institute of Technology Mesra, Ranchi-835215 INDIA
LITHIUM, A STRATEGIC ELEMENT FOR ENERGY IN THE WORLD MARKET Robert Bruce Wallace∗ RESERVES AND RESOURCES STRUCTURE OF THE LITHIUM INDUSTRY VIII. MEXICO'S LITHIUM/POTASSIUM SALAR IX. I. INTRODUCTION This paper does not seek to be ground breaking originality, since numerous commentators and researchers, ranging from reporters, geologists, mining engineers, scientists of different fields and organizations, some economists, and official government institutions such as the United States Geologic Survey (USGS), have delved into the intricacies of the sources, production, demand, prices, competitive industrial structure, and even the geopolitics of lithium, its compounds, and its minerals for a good many years. What the paper does seek is to gather together different dispersed sources of information, both technical and economic, and present a coherent, critical general analysis. Furthermore, though there is unfortunately a lack of sufficient hard data regarding a pending development of what appears to be a huge lithium-potassium deposit in Mexico straddling the limits of Zacatecas and San Luis Potosí, I shall include an analysis of what has been divulged publicly via the internet, most of which is hopeful expectation.

