Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Nkv
International Journal of Antimicrobial Agents 23S1 (2004) S67–S74
Bacterial biofilm formation on urologic devices and
heparin coating as preventive strategy
Peter Tenke , Claus R. Riedl , Gwennan Ll. Jones , Gareth J. Williams ,
David Stickler , Elisabeth Nagy
a
Department of Urology, Jahn Ferenc South-Pest Hospital, H-1204 Budapest, Köves u. 2-4, Hungary
b
Department of Urology, Thermenklinikum Baden, Baden, Austria
c
Cardiff School of Biosciences, Cardiff University, Cardiff, Wales, UK
d
Faculty of Medicine, Institute of Clinical Microbiology, University of Szeged, Szeged, Hungary
In the process of endourological development a variety of foreign bodies have been invented besides urinary catheters, on which biofilm
can be formed. Bacteria in the biofilm are less susceptible to antibiotics. An additional problem of medical biomaterials in the urinary tractenvironment is the development of encrustation and consecutive obstruction. The most promising prevention strategy for bacterial biofilmsis the production of materials with anti-adhesive surfaces such as heparin. Although heparin-coated ureteral stents are expensive, they justifytheir cost. Our studies show that such devices are protected against incrustation and biofilm formation for a longer period of time: 6–12months, both in vitro and in vivo.
2004 Elsevier B.V. and the International Society of Chemotherapy. All rights reserved.
Keywords: Biofilm formation; Antimicrobial susceptibility; Incrustation; Obstruction; Heparin coating
manent urinary catheters and stents are the most commonbiomaterial implants comparable to contact and intraocular
It is known that bacterial biofilms can colonise the sur-
lenses or hip and knee implants
faces of both tissues and implanted medical devices. Theprocess of biofilm formation and the impact on the develop-ment and clinical course of infectious diseases, however, are
2. Mechanism of biofilm formation
still poorly understood. Effective preventive and therapeuticstrategies still need to be developed for device-associated
The formation of biofilm generally consists of several
infections. By definition, a biofilm is an accumulation of
main steps: the first step is the deposition of the microorgan-
microorganisms and their extracellular products forming a
isms, next follows their attachment by microbial adhesion
structured community on a surface.
and anchorage to the surface by exopolymer production. Af-
It is evident that with the steadily increasing number of
ter this process their growth, multiplication and dissemina-
biomaterial devices used in urology for urinary drainage
tion can be observed (
(catheters, ureteral and prostatic stents) as well as implants
The initial event in this process is bacterial adhesion and
for replacement of lost body functions (sphincters and other
the deposition of a host urinary component on the surface
continence devices, penile prosthesis), biofilm formation
of the biomaterial leading to the formation of a condition-
and device infection is an issue of growing importance. In
ing film. This film consists of proteins, electrolytes and
addition, new tissue surfaces are created by using bowel
some unidentified molecules The types of compo-
segments for partial or complete replacement of the lower
nents that form the conditioning film depend on the surface
urinary tract. According to a North American survey, per-
characteristics (chemistry, charge and hydrophobicity).
Many of the protein molecules in the conditioning film
∗ Corresponding author. Tel.: +36-12847610; fax: +36-12856380.
play an active role in the bacterial adhesion process. The
E-mail address: [email protected] (P. Tenke).
conditioning film does not cover the entire implant surface
0924-8579/$ – see front matter 2004 Elsevier B.V. and the International Society of Chemotherapy. All rights reserved.
doi:10.1016/j.ijantimicag.2003.12.007


P. Tenke et al. / International Journal of Antimicrobial Agents 23S1 (2004) S67–S74
3. Antimicrobial susceptibility of biofilms
Bacteria within the biofilms differ both in behaviour
and in phenotypic form from the planktonic, free-floatingbacteria. Conventional clinical microbiology can detectonly the planktonic, free-floating bacteria, which are ab-solutely different from bacteria enclosed in the biofilm
The failure of antimicrobial agents to treat biofilms has
been attributed to a variety of mechanisms In general, organisms encapsulated in the biofilm grow moreslowly than the planktonic organisms, probably because theencapsulated bacteria have a decreased nutrient and oxy-gen supply leading to a decreased metabolic rate and, asa consequence, to a decreased antimicrobial susceptibility.
This may lead to a less susceptible genotype selecting a re-sistant population. Furthermore, antimicrobial binding pro-teins are poorly expressed in these slow-growing biofilmbacteria.
The biofilm matrix itself often delays or impedes the dif-
fusion of antibiotic molecules into the deeper layer of thefilm (extrinsic resistance).
Bacteria within the biofilm are phenotypically different
Fig. 1. Formation of biofilm.
from their planktonic counterparts. Antimicrobial agentswould therefore frequently fail to eradicate them. Bacteria
completely, but rather forms a "mesh-like" covering
within a biofilm activate many genes, which change their
Several factors are thought to influence bacterial adhe-
surfaces and other molecular targets, reducing the suscep-
sion to foreign body surfaces, such as biomaterial surface
tibility to antimicrobial agents (intrinsic resistance). It is
characteristics, bacterial surface features and the behaviour
suggested that these phenotypic changes are more impor-
of microorganisms and the presenting clinical condition
tant for antimicrobial resistance than the external resistance
mechanisms such as biofilm matrix or glycocalyx.
The biofilm is usually built up of three layers (
Bacteria within a biofilm can analyse the external en-
The linking or conditioning film is attached to the surface
vironment, develop interbacterial communication and may
of a tissue or biomaterial, the biofilm base consisting of
transfer genetic information and plasmids within biofilms.
microorganisms and the surface film acts as an outer layer
As a consequence, bacteria in biofilms may survive the use
where planktonic organisms can be released free-floating
of antibacterial agents at concentrations 1000–1500 times
and spread to the surrounding compartments The
higher than needed to eradicate planktonic bacteria of the
development of the biofilm is shown in
same species.
Fig. 2. Composition of the biofilm.

P. Tenke et al. / International Journal of Antimicrobial Agents 23S1 (2004) S67–S74
Fig. 3. Scanning electron microscope of a developing biofilm. Formation of the biofilm on the (a) outer surface of the polyurethane stent and (b) innersurface of the polyurethane stent.
4. Management of biofilm infection
ure of eradicating chronic bacterial infections with biofilms.
Antimicrobial treatment may be effective in "young"
Urine cultures of planktonic bacteria and the definition of
biofilms that developed within 24 h or less
their antimicrobial susceptibility may contribute to the fail-
Wollin et al. demonstrated that ciprofloxacin and ofloxacin
P. Tenke et al. / International Journal of Antimicrobial Agents 23S1 (2004) S67–S74
care. The crystalline deposits can be hard and abrasive andcan traumatise the bladder mucosa and urethra. Obstructionof urinary flow through the catheter may cause either in-continence due to leakage of urine around the catheter orpainful distention of the bladder due to urinary retention.
Bacteriuria is always found in these patients, therefore re-tention and vesico-ureteral reflux may facilitate ascendinginfection of the urinary tract, culminating in episodes ofpyelonephritis, septicaemia and shock Thus, unde-tected catheter blockage may lead to life-threatening compli-
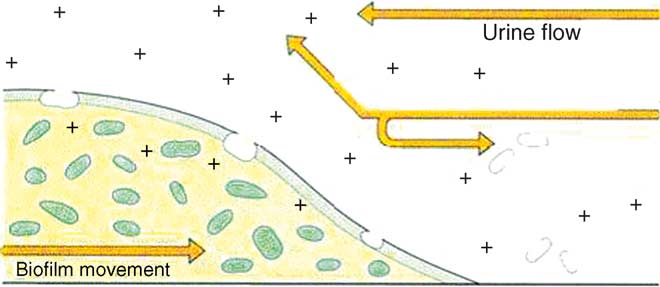
Fig. 4. Effect of antibiotics (+) on biofilm progression (adapted from
cations Several studies reported that up to 50%of patients undergoing long-term catheterisation will requireunscheduled catheter replacement because the flow of urine
rapidly reached a high urinary concentration and were able
has been blocked by crystalline deposits
to penetrate into conditioning bacterial biofilms and onto the
Current approaches in preventing catheter blockage by
stent surface. Significantly greater amounts of both antibi-
encrustation (i.e. replacement of the catheter, changing the
otics were absorbed onto the biofilm than to the stent surface
type or size of catheter, increasing fluid intake, administra-
It was also demonstrated that ciprofloxacin concen-
tion of cranberry juice or acidifying drugs and washing the
trations on biofilms surrounding urinary stents were signif-
bladder/catheter with acidic, antiseptic or saline solutions)
icantly higher than ofloxacin concentrations
are frequently ineffective Recurrent catheter blockage
Other studies showed that ciprofloxacin and ofloxacin might
gives patients the reputation as "blockers" ffec-
prevent microbial adhesion and biofilm formation for a
tive procedures to prevent encrustation are definitely needed.
short period of time Goto demonstrated that
Since the significance of biofilm formation has been
other drugs such as trimethoprim-sulphamethoxazole and
appreciated as the main problem of all implants and bio-
tobramycin were less potent against biofilms compared to
material devices, modification of the biomaterial surface
the fluoroquinolones According to Kumon, a com-
was regarded the most promising prevention strategy for
bination therapy with fluoroquinolones and macrolides or
bacterial biofilms. A variety of techniques have been de-
fosfomycin seems to be most effective against biofilm infec-
signed for this purpose, including the controlled release
tions Most researchers believe that antibiotics
of antimicrobial agents or antiseptics (such as minocy-
can only slow down the progress of biofilm formation by
cline, rifampicin, gentamicin, nitrofurantoin) incorporated
eliminating unprotected planktonic bacteria and stopping or
in the device material, surface coatings with silver and
reducing the metabolic activity of bacteria on the biofilm
other metals, surface modifications to change or increase
surface However, during an acute
hydrophobicity or to create functional groups with intrinsic
febrile phase of a biofilm infection, antimicrobial therapy is
antimicrobial activity, and anti-adhesive surfaces such as
reasonable and essential because the planktonic and not the
heparin and phosphorylcolin
biofilm bacteria are responsible for febrile reactions
Heparin with its antithrombogenicity and its strong elec-
tronegativity that repells cellular organisms is an excellentcandidate for an anti-adhesive stent coating. In 1987, Rug-
5. Biofilms in catheter-associated urinary tract
gieri et al. showed a 90% reduction of bacterial adhesion
on urinary catheter surfaces by heparin coating brandt et al. demonstrated the reduction of stent encrustation
An additional problem of medical biomaterials in the
by heparin coating in an experimental setting
urinary tract environment is the development of encrusta-tion and consecutive obstruction. When the drained urinarytract becomes infected by urease-producing bacteria such as
6. An in vitro examination of the ability of
Proteus mirabilis, the bacterial urease generates ammonia
heparin-coated catheters to resist encrustation by
from urea and elevates the pH of the urine. In this alka-
crystalline P. mirabilis biofilm
line environment, crystals of magnesium ammonium phos-phate (struvite) and calcium phosphate (hydroxyapatite) are
The ability of three catheter types to resist encrusta-
formed and trapped in the organic matrix surrounding the
tion and blockage by crystal-generating urine cultures of
cells. Progression of these encrustations eventually blocks
P. mirabilis isolated from patients encrusted catheters was
the catheter lumen Clinical experience and
examined in a laboratory model of the catheterised blad-
laboratory studies have shown that all types of catheters cur-
der Catheters (14 French) were inserted aseptically
rently available are vulnerable to blockage by crystalline
through a section of silicone tubing. They were attached to
P. mirabilis biofilms The complications result-
a glass outlet at the base of the vessel into a 200 ml glass
ing from catheter encrustation seriously compromise patient
chamber maintained at 37 ◦C. The catheter balloon was

P. Tenke et al. / International Journal of Antimicrobial Agents 23S1 (2004) S67–S74
inflated with water securing the catheter in position and
der at 0.5 ml per minute. The models were operated until the
sealing the outlet from the vessel "bladder". The catheter
catheters were blocked with encrustation. Low vacuum scan-
was then attached to a drainage tube and reservoir bag.
ning electron microscopy (REM) was performed to visually
Sterile pooled human urine was supplied to the bladder via
assess the extent of encrustation at catheter cross-sections 1,
a peristaltic pump. Thus, a residual volume of about 30 ml
4, 10 and 30 cm from the tip.
was collected in the vessel below the level of the catheter
Whereas the hydrogel-coated latex catheter was blocked
eyehole. As urine was supplied to the model the overflow
after an average of 28.1 h in four experiments, time un-
drained through the catheter into the collecting-bag.
til blockage was significantly longer for the heparin-coated
The three catheters tested were a latex catheter with hy-
catheter (58.2 h) and silicone catheter (54.8 h) in the setting
drophilic coating, a silicone catheter and a heparin-coated
described. However, all three types of catheters were vul-
silicone catheter. After inoculation of the sterilised urine
nerable to Proteus blockage.
with the P. mirabilis strain, the organisms were allowed to es-
Encrustation (REM) was observed only on hydrogel-
tablish themselves in the model for 1 h. The peristaltic pump
coated and silicone catheters especially around the eyeholes
was then switched on and fresh urine supplied to the blad-
and balloon but no encrustation was found on heparin-coated
Fig. 5. (a) A heparin-coated ureteral stent which remains unaffected by biofilm formation and incrustation. (b) An uncoated ureteral stent with a biofilmformed on its inner surface.

P. Tenke et al. / International Journal of Antimicrobial Agents 23S1 (2004) S67–S74
Fig. 6. Incrustation, biofilm formation and colour change of heparin-coated ureteral stents (Heparius) and uncoated polyurethane stents (PUR) within a6-week observation period. Y-axis is—0: no change, 1: moderate change and 2: significant change.
catheters; the blockage was caused by plugs of clear gel-like
tomy tubes remained unaffected for the whole 6–8 weeks
indwelling periods, whereas uncoated tubes got obstructedwithin 2–3 weeks.
This pilot study showed that no biofilms were de-
7. Clinical evaluation of encrustation and biofilm
tectable on heparin-coated stents whereas significant
formation on heparin-coated ureteral stents and
biofilms were demonstrated in 33% of uncoated stents.
Mild incrustation was observed in 10% of heparin stentscompared to significant incrustation in 50% of uncoated
In a pilot study the encrustation of heparin-coated
stents, and incrustations/biofilms were demonstrable on
ureteral stents was compared to uncoated polyurethane
uncoated stents as early as 2 weeks after implantation
stents. Twenty heparin-coated and 20 uncoated stents were
inserted into obstructed ureters in a prospective randomisedstudy under sterile conditions and left indwelling for peri-ods between 2 and 6 weeks. The stents were then removed
8. Extended indwelling times for heparin-coated
under sterile conditions, sealed in sterile covers and sent
for electron-microscopic evaluation. Nephrostomy tubeswere used in two patients with permanent bilateral external
In 10 patients with permanent ureteral stent drainage,
urinary drainage suffering from frequent encrustation ob-
heparin-coated stents were left indwelling for 6–8 months
struction of their silicone catheters that resulted in repeated
(Group I). In all patients bacteriuria was demonstrable at the
emergency visits. In these patients a heparin-coated and an
time of heparin-coated stent insertion; from previous stents.
uncoated nephrostomy tube were used simultaneously for
In three patients with uretero-enteral anastomosis stricture
either side so that direct comparison of encrustation status
in an ileal conduit, heparin-coated stents were left for 1 year
was possible.
Electron microscopy showed a significant difference be-
No obstruction/blockage of the stents was observed dur-
tween heparin-coated and uncoated ureteral stents.
ing this time in group I. On REM none or only minimal en-
the two types of stents that react to biofilm
crustations were found after this prolonged indwelling time.
formation in different ways. Two weeks after the insertion,
In the difficult bacteria-exposed Group II situation, the
two types of deposits could be detected on the surfaces of
silicone stents were found to be obstructed after 7 weeks
the uncoated stents—amorph anorganic deposits consisting
while the hydrogel-coated stents in 5 months, whereas all of
of mineralised crystals and another of bacterial biofilms.
the heparin-coated stents were unaffected after 12 months
Heparin-coated stents were unaffected by encrustations. Af-
of indwelling time.
ter 6 weeks of indwelling time, all uncoated stents showed
Heparin-coated ureteral stents are more expensive than
varying degrees and forms of deposits. Within the limited
standard stents. However, with longer indwelling times and
observation period of this pilot study none of the uncoated
reduction of the number of stent exchange procedures, the
stents became totally obstructed. The heparinized nephros-
total costs for heparin stents should be reduced compared to

P. Tenke et al. / International Journal of Antimicrobial Agents 23S1 (2004) S67–S74
Fig. 7. Costs per year for heparin-coated ureteral stents (Heparius) and uncoated polyurethane stents (PUR).
other stents. Heparin-coated stents cost four times the price
of uncoated stents; the ratio is 2.3 for nephrostomy tubes and6.5 for urethral catheters. If a stent exchange procedure is
[1] Reid G, Habash M. Oral fluoroquinolone therapy results in drug
200 (a very conservative estimate), the costs
adsorption on ureteral stents and prevention of biofilm formation.
per year for various stents and variable indwelling times are
Int J Antimicrob Agents 2001;17:317–20.
[2] Biering-Sorensen F. Urinary tract infection in individuals with spinal
cord lesion. Curr Opin Urol 2002;12:45–9.
Similar calculations have been made for nephrostomy
[3] Choong S, Whitfield H. Biofilms and their role in infections in
tubes and urethral catheters to show the advantage of
urology. Brit J Urol 2000;86:935–41.
heparin-coated devices. Each institution has to check its
[4] Costerton JW. Introduction to biofilm. Int J Antimicrob Agents
own regime with regard to cost effectivity, but the excellent
[5] Habash M, Ried G. Microbial biofilms: their development and sig-
qualities of the heparin-coated urologic drainage devices
nificance for medical device-related infections. J Clin Pharmacol
add a new possibility to the urologic armamentarium. Be-
sides the possible cost reduction for permanent urinary
[6] Kunin CM, Chin QF, Chambers S. Formation of encrustations on
drainage, significant reduction of patients morbidity due
indwelling urinary catheters in the elderly: a comparison of different
to less obstruction, less emergency visits and less invasive
types of catheter materials in "blockers" and "non-blockers". J Urol1987;138:899–902.
procedures are compelling arguments.
[7] Liedl B. Catheter-associated urinary tract infections. Curr Opin Urol
[8] Reid G. Biofilms in infectious diseases and on medical devices. Int
J Antimicrob Agents 1999;11:223–6.
[9] Keane PF, Bonner MC. Characterization of biofilm and encrustation
Further research in the field of biofilm physiology,
on ureteric stents in vivo. Brit J Urol 1994;73:687–91.
development and function is mandatory. Mechanisms
[10] Goto T, Nakame Y, Nishida M, et al. In vitro bacterici-
for prevention and control of biofilm formation and
dal activities of beta-lactamases, amikacin and fluoroquinolonesagainst Pseudomonas aeruginosa biofilm in artificial urine. Urology
catheter encrustation have to be found. Heparin coating
seems one possible solution but further development of
[11] Donlan RM. Biofilm formation. A clinically relevant microbiological
catheter materials resisting bacterial colonisation is to be
process. Healthcare Epidemiol 2001;33:1387–92.
[12] Kunin CM. Urinary tract infections: detection, prevention and man-
The future goal is to define easier methods for diagnosing
agement. 5th ed. Williams and Wilkins, 1997. p. 226–78.
[13] Kumon H. Management of biofilm infections in the urinary tract.
and quantifying biofilm infection and to develop antimicro-
World J Surg 2000;24:1193–6.
bial agents, which are effective against bacteria enclosed in
[14] Nickel JC, Downey J. Movement of Pseudomonas aeruginosa along
the biofilm. It is also important to identify molecular targets
catheter surfaces. Urology 1992;39:93–8.
of biofilm bacteria as well as the urinary components that
[15] Wollin TA, Tieszer C, Riddell JV. Bacterial biofilm formation, en-
are involved in biofilm formation. An ideal surface device
crustation and antibiotic adsorption to ureteral stents indwelling inhumans. J Endourol 1998;12:101–11.
to resist protein has to be developed. Bacterial adhesion and
[16] Desgrandshamps F, Moulinier F. An in vitro comparison of
the interaction between the biomaterial surface and urine
urease-induced encrustation of JJ stents in human urine. Brit J Urol
also need to be defined.
P. Tenke et al. / International Journal of Antimicrobial Agents 23S1 (2004) S67–S74
[17] Goto T, Nakame Y, Nishida M. Bacterial biofilms and catheters
[28] Morris NS, Stickler DJ, Winters C. Which indwelling urethral
in experimental urinary tract infection. Int J Antimicrob Agents
catheter resists encrustation by Proteus mirabilis biofilms? Brit J
[18] Kumon H, Hashimoto H. Catheter-associated urinary tract infections:
[29] Kunin CM. Detection, prevention and management of urinary tract
impact of catheter materials on their management. Int J Antimicrob
infections. 4th ed. Philadelphia: Lea and Febiger, 1987. p. 245–9.
[30] Warren JW, Muncie HL, Hebel JR, et al. Long-term urethral catheter-
[19] Morris NS, Stickler DJ, McLean RJ. The development of bacterial
ization increases risk of chronic pyelonephritis and renal inflamma-
biofilms on indwelling catheters. World J Urol 1999;17:345–50.
tion. J Am Geriatr Soc 1994;42:1286–90.
[20] Reid G, Potter P, Dalenay G, et al. Ofloxacin for treatment of urinary
[31] Cools HJM, Van der Meers JWM. Restriction of long-term indwelling
tract infections and biofilms in spinal cord injury. Int J Antimicrob
urethral catheterisation in the elderly. Brit J Urol 1986;58:683–8.
[32] Getliffe KA. The characteristics and management of patients with
[21] Shigeta M, Komatsuzawa H, Sugai M, et al. Effect of the growth
recurrent blockage of long-term catheters. J Adv Nursing 1994;20:
rate of Pseudomonas aeruginosa biofilms on the susceptibility to
antimicrobial agents. Chemotherapy 1997;43:137–41.
[33] Capewell AE, Morris SL. Audit of catheter management provided
[22] Tsukamoto T, Matsukawa M, Sano M, et al. Biofilm in complicated
by district nurses and continence advisors. Brit J Urol 1993;71:259–
urinary tract infection. Int J Antimicrob Agents 1999;11:233–6.
[23] Choong S, Wood S. Catheter associated urinary tract infection and
[34] Stickler DJ, Morris NS, Williams TJ. An assessment of the ability
encrustation. Int J Antimicrob Agents 2001;17:305–10.
of a silver-releasing device to prevent bacterial contamination of
[24] Sofer M, Denstedt JD. Encrustation of biomaterials in the urinary
urethral catheter drainage system. Brit J Urol 1996;78:579–88.
tract. Curr Opin Urol 2000;10:563–9.
[35] Stickler DJ, Zimakoff J. Complications of urinary tract infections
[25] Mobley HLT, Warren JW. Urease-positive bacteria and obstruction
associated with devices for long-term bladder management. J Hosp
of long-term urinary catheters. J Clin Microb 1987;25:2216–7.
[26] Warren JW. Catheter-associated urinary tract infections. Int J An-
[36] Ruggieri MR, Hanno PM, Levin RM. Reduction of bacterial adher-
timicrob Agents 2001;17:299–303.
ence to catheter surface with heparin. J Urol 1987;138:423–6.
[27] Bull E, Chilton CP, Gould CAL, et al. Single-blind, randomised,
[37] Hildebrandt P, Rzany A, Bolz A, et al. Immobilisiertes heparin als
parallel group study of the Bard Biocath catheter and a silicone
inkrusteirungsresistence beschichtung auf urologishen implantaten.
elastomer coated catheter. Brit J Urol 1991;68:394–9.
Biomed Tech 1997;42:123–4.
Source: http://www.uronovis.de/uploads/media/neueste_klinische_veroeffentlichung.pdf
October 2007, Volume 16, No.1 Current Issues in Medical Management Varenicline: The Newest Pharmacotherapy for Smoking CessationAndrew L. Pipe, CM, MD, Robert Reid, PhD, MBA, Bonnie Quinlan, RN, BScN, APN Minto Prevention and Rehabilitation Centre, University of Ottawa Heart Institute, Ottawa, Ontario Smoking cessation is deemed to be the most powerful of all the is essential, in all professional settings, that systematic approaches to
B I O D I V E RS I T Y H U M A N H E A LT H populations ofdisease-causingorganisms in check • assures sufficient food and water supplies warnings oftoxins and otherenvironmentalhealth hazards • provides source materials fordrugs • provides models for medicaldiscoveries Center for Biodiversity and Conservation American Museum of Natural History







