Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Microsoft word - whole thesis
The Effects of
Salvia hispanica L. (Salba) on Postprandial
Glycemia and Subjective Appetite
A thesis submitted in conformity with the requirements
for the degree of Master's of Science
Nutritional Sciences
University of Toronto
Copyright by Amy Sanda Lee 2009
The Effects of
Salvia hispanica L. (Salba) on Postprandial Glycemia
and Subjective Appetite
Master's of Science
Nutritional Sciences
University of Toronto
Dietary interventions have been attempted to lower the risk of obesity, diabetes and CVD by the
reduction of postprandial hyperglycemia and prevention of excess caloric intake. Evidence suggests
an independent predictive role of postprandial glycemia for CVD. Furthermore, due to the possible
role of obesity in the development of CVD and T2D, research has focused on appetite suppression to
reduce excessive food intake. Here we investigate the ability of the novel oil-rich grain
Salvia
hispanica L. (Salba) to lower postprandial glycemia and reduce appetite when added to a
carbohydrate meal. In our first study, we investigated the effects of Salba in escalating doses on both
parameters in healthy individuals. In our second study we compared the effectiveness of ground and
whole forms of Salba on the same parameters. Results confirmed our hypotheses, as Salba given in
either form positively affected postprandial glycemia and mildly suppressed appetite.
I thank Dr. Vladimir Vuksan for taking a chance with a student with absolutely no nutrition
background or research experience whatsoever, and Elena, Pearl, Leanne, Andre, Agnes and Alex for
their neverending support and expertise. I would also still be analyzing my data if it weren't for the
help of the wonderful volunteers at the Risk Factor Modification Centre: Zazeba, Hans, Cathy,
Carmen and Nirangive. I thank my advisory committee members Dr. Valerie Tarasuk and Dr.
Thomas Wolever for their guidance and for always keeping my best interests in mind. Lastly, I thank
my parents, Win and Stephen Lee, for being such willing participants and for their emotional and
financial support, and Dennis, for always being there.
Table of Contents
Acknowledgments . iii
Table of Contents . iv
List of Tables . vii
List of Figures . viii
List of Appendices . ix
1 Introduction . 1
2 Review of Literature . 2
2.1 Introduction to
Salvia hispanica L. (Salba) . 2
2.1.1 Background and Classification . 2
2.1.2 Composition of Salba (Please refer to Tables 2.1 and 2.2) . 3
2.1.3 Salba: Past Clinical Research . 10
2.2 Postprandial Glycemia . 12
2.2.1 The definition of Postprandial Glycemia . 12
2.2.2 The Clinical significance of Postprandial Glycemia . 13
2.2.3 The Effects of Postprandial Hyperglycemia on Health . 14
2.2.4 Dietary control of Postprandial Glycemia . 15
2.2.5 The Components of Salba affecting Postprandial Glycemia . 16
2.3 The Short-term Regulation of Appetite . 26
2.3.1 The Components of Salba potentially affecting Appetite . 28
3 Rationale and Objectives . 33
3.1 Summary and Rationale . 33
3.2 Hypothesis and Objectives . 34
3.2.1 Specific Objectives . 34
3.2.2 Hypotheses . 34
4 Study 1: The Effects of Escalating Doses of Salvia hispanica L. (Salba) on Postprandial
Glycemia and Subjective Appetite in Healthy Individuals . 36
4.1 Abstract . 36
4.2 Introduction . 36
4.3 Methods . 37
4.3.1 Participants . 37
4.3.2 Treatments . 38
4.3.3 Experimental Design . 38
4.3.4 Blood Glucose Analysis . 39
4.3.5 Measurement of Appetite . 39
4.3.6 Study Variables . 39
4.3.7 Statistical Analysis . 40
4.4 Results . 40
4.4.1 Postprandial Blood Glucose Responses . 40
4.4.2 Appetite Scores . 42
4.5 Discussion . 44
5 Study 2: The Effects of Escalating Doses of Whole vs. Ground Salvia hispanica L. (Salba) on
Postprandial Glycemia and Subjective Appetite in Healthy Individuals . 46
5.1 Abstract . 46
5.2 Introduction . 46
5.3.1 Participants . 47
5.3.2 Treatments . 47
5.3.3 Experimental Design . 48
5.3.4 Blood Glucose Analysis . 49
5.3.5 Measurement of Appetite . 49
5.3.6 Study Variables . 49
5.3.7 Statistical Analysis . 50
5.4 Results . 50
5.4.1 Postprandial Blood Glucose Responses . 50
5.4.2 Appetite Scores . 52
5.5 Discussion . 53
6 General Discussion and Conclusion . 57
6.1 Summary . 57
6.2 The Effects of Salba on Postprandial Glycemia . 58
6.3 The Effects of Salba on Subjective Appetite . 59
6.4 Relevance of Findings . 60
6.5 Limitations . 61
6.6 Future Research . 63
6.7 Conclusion . 65
7 References . 66
Table 2.1
Macronutrient Composition of Salba and Common Grains (100g)
Table 2.2
Micronutrient Composition of Salba and Common Grains (100g)
Table 2.3
Total Percentage of Dietary Fiber Content of Certain Common Grains
Table 2.4
Amino Acid Composition of Salba
Table 2.5
Antioxidant Composition of Salba
Table 4.1
Nutritional Information for Experimental Breads
Table 5.1
Nutritional Facts of Salba-enriched Breads
Table 5.2
Nutritional Facts of Control Breads
Table 5.3
Glucose iAUC (min.mmol/L) values (± sem) for all Experimental Breads
Table 5.4
Appetite iAUC's of Experimental Breads
Percent Reductions of Appetite iAUC's of Salba Breads with Respect to
Table 5.5
Calorie-, protein- and fat-matched Control Breads
Graph 2.1 Viscosity of equal amounts of ground Salba and flax
Graph 2.2 Viscosity of different amounts of ground Salba and flax
Graph 4.1 The Effects of Salba on Blood Glucose iAUC (n=12)
Graph 4.2 The Effects of Salba on Incremental Blood Glucose (n=12)
Graph 4.3 The Effects of Salba on Incremental Subjective Appetite (n=12)
Graph 4.4 The Effects of Salba on Satiety Score iAUC's (n=12)
Graph 5.1 The Effects of Salba on the Blood Glucose iAUC of all Salba Breads (n=20)
Graph 5.2 The Effects of the High Dose Breads on Blood Glucose iAUC (n=20)
List of Appendices
Information Form
III - Data Collection Forms
IV - Nutritional Analysis Laboratory Report from Guelph University
V -
Nutritional Analysis Laboratory Report from Dr. David Jenkins
VI - Nutritional Analysis Laboratory Report from Dr. Stephen Cunnane
Today there is much public interest in improving overall health through diet, with increased interest in
whole grains and seeds such as wheat, oats and flax.
Salvia hispanica L. (Salba) is an ancient oil-rich
whole grain that has a favourable nutritional composition compared to commonly consumed grains and
seeds. Traditionally considered a food and remedy by ancient Aztec and Mayan civilizations, it is no
longer consumed except for in limited areas of Mexico [1]. Awareness of the benefits of functional
foods has spurred the search for less frequently consumed foods to complement the North American
diet [2, 3]. Salba may prove to be an excellent functional food given its unique and superior
Several preliminary studies indicate potentially beneficial physiological effects of Salba on risk factors
for Type 2 diabetes and CVD. In a 6-month crossover study by Vuksan
et al., Type 2 diabetic subjects
consuming 37g of Salba per day experienced lower blood pressure, low-grade body inflammation and
coagulation factors compared to wheat bran control [4]. Vertommen
et al. reported a significant
reduction in waist circumference in healthy individuals after a month of Salba supplementation. This
decrease occurred without a change in body weight, suggesting the specific loss of fat mass. Similarly,
dyslipidemic rats fed Salba for 3 months experienced lower visceral adiposity than rats on a control
maize diet [5]. We hypothesized that the mechanisms of action responsible for the effects seen in these
three studies were mainly the reduction of both postprandial glycemia and appetite.
We investigated the effects of Salba on postprandial glycemia and subjective appetite, both of which
have been suggested to play a role in of the development of Type 2 diabetes and CVD. The research
objectives of this thesis were to assess the ability of Salba to lower postprandial glycemia and appetite
in escalating doses in ground form (Study 1) and whole versus ground form (Study 2) using a
crossover, randomized, double-blind placebo-controlled design. Results from these studies may
provide possible explanations for the long-term effects of Salba seen in previous research in Type 2
diabetes and healthy individuals. If Salba proves to provide health benefits as a functional food, its use
can be further developed to complement the Western diet and provide consumers with a novel grain to
help them adhere to dietary whole grain recommendations.
2 Review of Literature
2.1 Introduction to
Salvia hispanica L. (Salba)
2.1.1 Background and Classification
Salvia hispanica L., commonly known as Chia, is an oil-rich grain that has been consumed for
thousands of years [1]. It was one of the Aztec and Mayan cultures' three main crops, along with
amaranth and corn. Called "running food", it was revered for its medicinal properties and exceptional
nutritional value; so much so, that it played a prominent role in certain religious ceremonies [1].
Recently, renewed interest has emerged for this grain
, as researchers have uncovered its extraordinary
composition and potential beneficial effects on health.
Chia is the mother crop of
Salvia hispanica L., and consists of many varieties with varying nutritional
compositions. Selective breeding of this mother crop yielded a white grain with a more consistent
nutrient composition and greater nutrient density [6]. We studied this variety of
Salvia hispanica L.,
known commercially as "Salba," which is rich in dietary fiber, omega-3 fatty acids, minerals and
vegetable protein, and which has a high antioxidant capacity as well.
Although traditionally considered a grain, it can also be regarded as a seed due to its high oil content.
As well, it does not belong to the
Graminaea family, in which all major cereal grains are
conventionally classified. Excluding its low carbohydrate content, however, Salba contains dietary
fiber, unsaturated fatty acids and other phytochemicals which render it compositionally similar to
typical grains. Thus, Salba may prove to have the health benefits of more commonly consumed whole
grains seen in epidemiological and interventional studies [7-14]. Many of the beneficial effects of
whole grains are attributed to their contents of such nutrients as dietary fiber, minerals, unsaturated
fatty acids, vegetable protein and antioxidants [10, 15].
Exceptionally high in fiber, Salba is also rich in minerals such as iron, calcium, magnesium and
potassium [16, 17] and has an exceptional total antioxidant capacity (TAC) of 84/g, making it
comparable to some berries [18]. With Salba's fat content of 33%, it contains considerably more fat
than the typical whole grain. As such, is also similar to seeds such as flax. Currently however, flax is
considered a whole grain [19]. Salba may also be called a whole grain since it is considered as one by
the medical community [4, 20].
Composition of Salba (Please refer to Tables 2.1 and 2.2)
Table 2.1 Macronutrient Composition of Salba and Common Grains (100g)
Total Carbohydrate
From the USDA Nutrient Data Laboratory [21] and Nutrition Data [22] ; Salba nutrient information determined by laboratory nutrient analysis at the University of Guelph and University of Toronto [17, 23-25]
Table 2.2 Micronutrient Composition of Salba and Common Grains (100g)
Vitamin C (Ascorbic
Acid equivalency)
From the USDA Nutrient Data Laboratory [21]; Salba nutrient information determined by laboratory nutrient analysis at the University of Guelph and University of Toronto [17, 24, 25]
2.1.2.1 Carbohydrates and Dietary Fiber
Salba is composed of 35% total dietary carbohydrate, of which 34% is in the form of dietary fiber [25].
Thus, merely 1% of Salba is available carbohydrate. Dietary fiber can be defined as the "edible parts of
plants or analogous carbohydrates that are resistant to digestion and absorption in the human small
intestine with complete or partial fermentation in the large intestine. Dietary fiber includes
polysaccharides, oligosaccharides, lignin, and associated plant substances." [26] For example, the
structural supports of plants' stems and leaves are considered dietary fiber. All types of fiber are non-
starch polysaccharides except for lignin, which is an alcohol derivative [27].
A common method of classifying fibers is by their solubility in water and ability to gelatinize [28, 29].
Soluble fibers are hygroscopic and able to form a gel, and include psyllium husks, guar, beta-glucan
from oats and barley and naturally-occurring pectins in fruit [27]. The soluble fibres are typically
fermented to a greater extent than insoluble fibres, although all non-starch polysaccharides are partially
fermented to some degree in the large intestine [30].
According to the American Association of Cereal Chemists, dietary fibers promote beneficial
physiological effects including laxation, and blood cholesterol and glucose attenuation [26]. Research
supports this claim: it has been found that fiber improves glycemic control [31, 32], lowers blood lipid
levels [33] and may even reduce the risk of colon cancer [34].
Table 2.3 Total Percentage of Dietary Fiber Content of Certain Common Grains
From the USDA Nutrient Data Laboratory [21]; Salba nutrient information determined by laboratory nutrient analysis at the University of Guelph and University of Toronto [17, 23-25]
2.1.2.2 Dietary Fat Content
Salba is composed of approximately 33% fat. Of that, 68% is in the form of omega-3 polyunsaturated
fatty acid, 19% omega-6 polyunsaturated fatty acid, 6% monounsaturated fatty acid and 16.4%
saturated fatty acid. The omega-3 polyunsaturated fat is in the form of alpha-linolenic acid [24].
Polyunsaturated omega-3 fatty acids include the 18-carbon alpha-linolenic acid (ALA), 20-carbon
eicosapentaneoic acid (EPA) and 22-carbon docosahexaneoic acid (DHA). Animal products such as
fish are excellent sources of EPA and DHA, while ALA can be found in plant foods such as vegetables
and seeds. They are all the precursors to eicosanoids including prostaglandins, thromboxanes, and
leukotrienes, which have extensive hormonal functions in the body. Yet they are all essential fatty
acids, meaning that the body is unable to produce them. However, the human body has a limited ability
to form EPA and DHA from ALA [35-37]. These reactions occur competitively with omega-6 fatty
acids, and as such, the formation of long-chain omega-3 fatty acids is most effective when their number
is not significantly less than that of the omega-6 fatty acids [38, 39].
Thus the ratio of dietary omega-3 to omega-6 fatty acids is especially important given the competition
between omega-3 and omega-6 analogues for the same conversion pathway. The conversion of ALA to
EPA is estimated to be anywhere from 0.2 to 21%, and further synthesis of EPA to DHA is especially
limited with approximately 0-9% of ALA being metabolized to DHA [40-42]. The efficiency of this
pathway varies between species and even between sexes, with women demonstrating a greater capacity
to convert EPA and DHA from ALA [37, 41, 42]. Unfortunately, it is estimated that the typical North
American consumes omega-6 and omega-3 fatty acids in ratios between 14:1 and 20:1, while scientists
recommend a ratio of 4:1[36]. Thus, regular ingestion of Salba may help counteract the imbalance of
omega-6 to omega-3 fatty acids in the typical North American diet given its ratio of 1:3. Preliminary
data suggest that Salba increases blood levels of EPA. After participants consumed approximately 37g
a day for 12 weeks, their ALA and EPA blood levels nearly doubled, indicating the effective
conversion of ALA to EPA [4].
Experimental and epidemiological studies have demonstrated that ingestion of fish oil, which is rich in
both EPA and DHA, reduces risk factors associated with cardiovascular disease such as hypertension
and insulin resistance [37, 40, 43]. Fewer studies have investigated the effects of ALA on such
parameters, but preliminary results suggest that this form of polyunsaturated fat may also play a
beneficial role. For instance, a 2-year study assessing the intake of ALA-enriched margarine versus
LA-rich margarine reported significantly lower c-RP levels in the former diet condition [43]. In
addition, perilla oil (rich in ALA) effectively suppressed fatty acid synthase activity and decreased
hepatic and plasma triacylglyerol levels compared to maize oil (rich in linoleic acid) in rats [44].
Further studies in rats suggest that regular ingestion of ALA from Salba
can significantly decrease diet-
induced dyslipidemia compared to ingestion of linoleic acid (LA) in the form of maize oil [5]. It is
important to note, however, that conversion of ALA to EPA is more efficient in rats than in humans.
Nonetheless, these preliminary results are promising and warrant further study into the effects of
dietary ALA on possible risk factors for CVD.
2.1.2.3 Dietary Protein Content
Salba contains 22% vegetable protein [17]. The quality of a protein, usually expressed as a Protein
Efficiency Ratio (PER), is dependant on the percentage of protein that is likely to be used by the body
[45]. The PER is measured by feeding rats a diet containing 9-10% of the protein for 4 weeks and
calculating the weight gain per unit of protein consumed. The value is then adjusted proportionately to
the PER that would be obtained if the PER of a casein diet were 2.5. Casein, derived from skim milk, is
a high quality protein and thus a standard of comparison of protein quality [46].
The adjusted PER of Salba is 91% (using casein as the standard). The PER of Salba is higher than that
of soy protein, a highly regarded source of protein often used as a meat substitute by vegetarians [16].
Additionally, the protein in Salba has no limiting factors for the adult diet (based on the limiting amino
acid, lysine) meaning that it contains all essential amino acids. Thus, it is a complete and balanced
source of protein [1]. As well, Salba
contains no gluten, a protein found in wheat [1]. Therefore, it can
safely be safely consumed by individuals with celiac disease.
Table 2.4 Amino Acid Composition of Salba
Nutritional laboratory analysis performed at the University of Guelph [23]
2.1.2.4 Antioxidant Capacity
An antioxidant is a substance that, when present at low concentrations compared to those of an
oxidizable substrate, significantly prevents or delays oxidation of the substrate triggered by a pro-
oxidant [47]. A pro-oxidant, or reactive oxygen species, is a toxic substance that can cause oxidative
damage to lipids, proteins and nucleic acids, resulting in various pathologic events or diseases. The
antioxidant capacity of a compound is defined as the ability of the compound to reduce pro-oxidants
Antioxidant capacity is often expressed as Total Antioxidant Capacity, or TAC, per gram. The TAC is
the sum of the oxidant radical antioxidant capacity (ORAC), as determined with an ORAC assay, of
both the lipophilic and hydrophilic components of the compound [18]. Salba has a TAC value of 84/g,
while that of lowbush blueberries, generally considered an excellent source of antioxidants, is 96/g.
Berries such as the raspberry and strawberry have TAC's of 49/g and 36/g, respectively [48].
Water and methanol extracts of the Chia meal remaining after pressing to remove oil demonstrate high
antioxidant activity [1]. It is because of this antioxidant activity that the omega-3 fatty acids in Salba
are very stable and also why Aztecs were able to store the grain for long periods of time with low risk
of rancidity. Scientific interest on the health benefits of antioxidant consumption has focused mainly on
the effects of beta-carotene and vitamins E and C on CVD [49]. Numerous epidemiological studies
have demonstrated an association between the consumption of antioxidant-rich foods such as fruits and
vegetables and nutrients such as vitamin E and flavonoids and lowered risk of mortality from
cardiovascular events [49-56]. One 2-year interventional trial called the Cambridge Heart Association
Study reported that consumption of vitamin E reduced the risk of CVD-related events such as non-fatal
myocardial infarction [57]. Most randomized trials, however, show no protective effects of antioxidant
supplementation on cardiovascular events [49, 58-60]. Therefore, although some health benefits have
been documented, more research is needed.
Table 2.5 Antioxidant Composition of Salba
Concentration (mol/kg
I – Nonhydrolyzed
Chlorogenic acid
II – Hydrolyzed
From Salba Group http://www.salba.info/antioxidants.html [61]
2.1.3 Salba: Past Clinical Research
The field of nutrition has recently begun to focus on the benefits of foods beyond fulfilling basic
nutrient requirements; such foods are called "functional foods" [3]. Due to a rich nutrient composition
and promising preliminary clinical data, Salba
is a grain that could be considered a novel functional
food. Past studies done with Type 2 diabetics, healthy individuals and rats have all demonstrated a
possible role for Salba in improving risk factors for Type 2 diabetes and CVD such as glucose
metabolism and adiposity. However, very little research has been conducted with this grain.
In a 6-month randomized, crossover study, Salba demonstrated the ability to decrease risk factors for
CVD in Type 2 diabetes [4]. Diabetic individuals undergoing conventional therapy and adhering to a
CDA-recommended diet consumed 37g of either Salba or wheat bran (control) per day for 3 months.
The Salba group experienced a significant reduction in systolic blood pressure of 6.3 ±4.2 mmHg
compared to baseline [4]. Although it did not reach significance, diastolic blood pressure dropped as
well, by an average of 1 ±1.3 mmHg compared to baseline. Likewise, a significant decrease in HbA1c
was seen in the Salba group compared to baseline, but not to control. With regards to coagulation
factors, fibrinogen and VonWillebrand factor levels in the Salba group both decreased significantly
compared to baseline only. Low-grade body inflammation, as measured by c-RP, was significantly
lower in the Salba group compared to the control group at the end of the 12 weeks.
Vertommen
et al. conducted a 1-month fluidity study in which 12 health individuals consumed 50g of
Salba per day [20]. Diastolic blood pressure decreased significantly from 66.1 ± 8.4 to 61.5 ± 7.0
mmHg. Fasting serum triglycerides tended to decrease from 89 ± 52 to 69 ± 22 mg/dL (p = 0.07).
Finally, a decrease in waist circumference was reported without a concurrent decrease in body weight,
which could be due to the specific loss of fat mass. No side effects were observed in either study and
all safety parameters remain unchanged. Both authors speculated that Salba's beneficial health effects
were due to the rich nutrient content naturally occurring in this ancient grain.
Most recently, Chicco
et al. studied the effects of a diet including Salba in rats on parameters such as
insulin sensitivity and visceral adiposity. The animals were placed on a 5-month sucrose-rich diet to
induce dyslipidemia and insulin resistance, followed by a diet enriched with either Salba or maize [5].
The objective was to compare the effects of the ALA from Salba versus linoleic acid (LA) from maize.
Results demonstrated a significant decrease in visceral adiposity (epididymal and retroperitoneal fat)
relative to body weight for the rats on the Salba diet. Thus these results also suggest a role for Salba in
lowering adiposity. With regards to insulin sensitivity, it was found that insulin resistance was
normalized without changes in insulinaemia in the rats receiving the Salba diet.
The results of these three studies suggest a possible role for Salba in the prevention of risk factors for
Type 2 diabetes and CVD, as it has been demonstrated that Salba reduces blood pressure, coagulation
factors and adiposity. Given the promising results of these studies, Salba demonstrates potential as an
excellent functional food, and as such, further research into this grain may prove to be beneficial for
2.2 Postprandial Glycemia
2.2.1 The definition of Postprandial Glycemia
Postprandial glycemia refers to the elevations of blood glucose concentration that normally occur in
response to a meal [62]. In healthy individuals, the ingestion of a typical carbohydrate-rich meal causes
transient increases in plasma glucose that peak approximately 30-60 mins after the meal and return to
the fasting, preprandial level in approximately 2-3 hours, depending on the type and amount of
carbohydrate. Levels of postprandial glycemia in healthy, nondiabetic individuals rarely exceed 7.8-8.8
mmol/L; however, they can peak at 10 mmol/L after some large meals [62, 63]. The dynamics of the
postprandial glucose response are tightly regulated as part of the body's glucose homeostasis such that
postprandial variations in glycemia reflect the interplay between intestinal absorption, endogenous
release and tissue uptake of glucose from circulation.
Glucose is absorbed from the small intestine through the secondary active sodium-glucose
cotransporter SGLT-1 and sodium-independent facilitative transporter GLUT-5 situated in the brush-
border membrane [64]. SGLT-1 activity is mainly regulated by diet [65]. Studies show however, that
angiotensin II may play a role in regulating sodium-glucose cotransport. In animal models, angiotensin
II was shown to inhibit SGLT-1 activity by suppressing its translation and to increase SGLT-2
expression and function in tubular cells [66]. From the enterocytes, glucose reaches the liver through
the portal vein. Here, high postprandial glucose concentrations activate glucokinase, in turn promoting
hepatic glucose uptake. At the hepatic level, glucose exceeding immediate needs is stored as glycogen
or converted to fat. The rest of the glucose is transported into the circulation and reaches pancreatic
beta cells where it stimulates insulin release and secretion, as glucose is the most powerful stimulator
of insulin production and release [67].
Insulin is produced in the beta cells of the Langerhans pancreatic islets. Insulin release is triggered by
high postprandial glucose concentrations following a carbohydrate meal, which activates the beta-cell
glucokinase with a low affinity for glucose. Consequently, increased amounts of glucose enter the citric
acid cycle producing large amounts of ATP, and subsequently insulin exocytosis is stimulated [68].
The endogenous hepatic and renal glucose release and tissue uptake of glucose from circulation are
antagonistically regulated by insulin and glucagon, the latter hormone of which is secreted by
pancreatic alpha cells [68]. The absorbed glucose stimulates insulin secretion and suppresses glucagon
secretion, which together cause an 80% reduction in endogenous glucose production by suppressing
hepatic glycogenolysis and gluconeogenesis [68]. The rate-limiting step for cellular glucose uptake is
glucose transport, enhanced by both hyperglycemia and insulin.
Up to 75% of the glucose available postprandially is taken up by an insulin-independent mechanism.
Another 25% is taken up through insulin-dependent GLUT-4 transporters in peripheral tissues, mainly
in skeletal muscle (80%) and to a lesser extent in heart and adipose tissue [68]. Moreover, in these
tissues, insulin directs intracellular glucose metabolism by activating key enzymes such as glycogen
synthase and pyruvate dehydrogenase, while suppressing lipolysis, inhibiting hepatic gluconeogenesis,
increasing glucose uptake in the muscle and promoting glucose oxidation [69]. Incretin hormones such
as glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP), which are released
distally in the ileum upon carbohydrate intake, amplify insulin secretion beyond the levels induced by
glycemic increases [70].
2.2.2 The Clinical significance of Postprandial Glycemia
Postprandial glycemia reflects the body's ability to regulate glucose levels. Based on this principle, an
individual's glycemic response to a set amount of carbohydrate can be a diagnostic tool for insulin
resistance and the onset of diabetes. There is much interest in reducing postprandial glycemia in people
at risk for diabetes since doing so appears to decrease diabetes risk and associated cardiovascular
complications [71, 72]. Most importantly, growing epidemiological evidence suggests that postprandial
glycemic rises are an independent and modifiable predictor of cardiovascular disease. Studies show
that, even after adjusting for other cardiovascular risk factors, the relationship between 2-hour
postprandial glycemia and cardiovascular risk remains direct and continuous, extending below and
beyond the cut-off points for impaired glucose tolerance [737475] ] ]. Furthermore, postprandial
glycemic spikes have detrimental effects on blood coagulation, body inflammation and endothelial cell
function and, together with 2-hour glycemia, are considered equally predictive of atherosclerosis [33-
Epidemiological data suggest that the glucose response to an oral glucose tolerance test (oGTT) is a
better predictor of heart disease risk than fasting glucose level. For instance, the Diabetes Intervention
Study (DIS), a prospective trial of newly detected occurrences of Type 2 diabetes, reviewed health
information for approximately 1100 adults over 11 years. The main outcome measures were
myocardial infarction and death. The participants who died had significantly higher postprandial
glycemia at baseline than those who did not. Interestingly, fasting blood glucose at baseline was not
associated with death. The researchers concluded that strict control of postprandial hyperglycemia is
necessary to reduce the risk of MI and death in Type 2 diabetic individuals [76].
The above study, however, proves only that high postprandial glycemia predicts, not causes,
cardiovascular complications and death. The STOP-NIDDM trial was an intervention trial in which the
effects of altering postprandial glycemia were investigated. Started in 1995, it tested the effectiveness
of the α-glucosidase inhibitor acarbose on the development of Type 2 diabetes. Acarbose specifically
lowers postprandial glycemia by inhibiting the action of α-glucosidase, an enzyme required for the
breakdown of carbohydrates to monosaccharides. Its effect is to slow down carbohydrate absorption,
and therefore glucose appearance, into the circulation. Individuals receiving acarbose had a 25% less
chance of developing diabetes than a placebo group [77]. They were also less likely to experience
cardiovascular events and hypertension [78].
2.2.3 The Effects of Postprandial Hyperglycemia on Health
According to the World Health Organization, a blood glucose concentration above 11.0 mmol/L in the
2 hours after a 75g oral glucose tolerance test (oGTT) is considered hyperglycemia and thus represents
their main diagnostic criteria for diabetes [79]. According to their recommendations, this post-
challenge blood glucose level distinguishes individuals "with significantly increased premature
mortality and increased risk of microvascular and cardiovascular complications." Blood glucose levels
between 7.8 to 11.0 mmol/L are considered indicative of impaired glucose tolerance, and therefore
increased risk of developing Type 2 diabetes and cardiovascular complications. Normoglycemia is
defined as a post-challenge blood glucose concentration of 7.8 mmol/L and below. However, the WHO
stresses that there is no threshold for predicting the risk of Type 2 diabetes and CVD such that post-
oGTT blood glucose concentrations of approximately 5.50 mmol/L and above are linearly correlated
with developing these diseases.
Postprandial hyperglycemia is detrimental to health because it causes oxidative stress [80, 81].
Oxidative stress can be defined as an imbalance between the production of oxidation products and the
ability of antioxidant mechanisms to neutralize them, resulting in an excess of oxidation products [80].
Common oxidants such as superoxide (O -
2 ), hydrogen peroxide (H2O2), peroxynitrite (ONOO-) and
hydroxyl radical (OH-) can cause vascular damage. Furthermore, hyperglycemia is particularly
detrimental to the vascular system because the endothelial cells of the vascular endothelium are unable
to regulate glucose uptake, allowing excess glucose to enter the cytoplasm [81].
Excess glucose can lead to the production of the above oxidation products, or reactive oxygen species,
by a number of enzymes and pathways. One mechanism to explain the overproduction of reactive
oxygen species (ROS) has been demonstrated by Brownlee
et al. [72]. According to their research,
high glucose concentrations within these cells results in the overproduction of superoxide by the
mitochondrial electron-transport chain. Glucose is metabolized first through glycolysis and then the
tricarboxylic acid cycle, producing electron donors. These are needed to create a proton gradient across
the inner mitochondrial membrane via the electron transport chain. Excess glucose causes the
overproduction of electron donors, which in turn increases the proton gradient across the mitochondrial
membrane. Finally, a prolonged period of O -
2 production occurs when a gradient threshold level is
reached. The result is the initiation of four pathways that lead to vascular damage: increased glucose
entering the polyol pathway, increased production of advanced glycation end-products (AGEs),
increased protein kinase C activity and increased flux through the hexosamine pathway [71, 81].
Indeed, it has been shown that diabetics have higher levels of ROS. Even typical levels of glycemia
attained through an oGTT cause a decrease in plasma antioxidant capacity, indicating oxidative stress.
In one study done by Ceriello
et al., diabetic subjects were given two meals with different amounts of
carbohydrate. Postprandial glycemia and susceptibility of subjects' LDL cholesterol to oxidation were
measured after each meal. Oxidative modifications to LDL render it more atherogenic. It was reported
that the susceptibility of LDL to oxidation was significantly higher after the meal that elicited the
greatest hyperglycemia, confirming the hypothesis that hyperglycemia causes oxidative stress [82].
2.2.4 Dietary control of Postprandial Glycemia
Dietary approaches aimed at adequate glycemic control principally target the reduction of the overall
glycemic response (blood glucose spikes and AUC) in conjunction with a reduction in insulin
secretion. Related dietary approaches include changes of the type and amount of carbohydrate with
preference for carbohydrates with slow or low availability. These carbohydrates maintain adequate
postprandial glycemic control in terms of both 2-hour glycemia and postprandial spikes, without
excessive rises in serum triglycerides and insulin and without reducing carbohydrate intake below the
recommended daily amount [83].
One measure of the nutritional value of a carbohydrate food can be expressed by its Glycemic Index
value. The Glycemic Index (GI) is a classification system for carbohydrate-rich foods based on their
ability to raise blood glucose [84]. The GI value for a food is calculated by dividing a subject's glucose
AUC for a 50-g carbohydrate test meal by that of the same subject's AUC for 50g glucose or white
bread, and multiplying the number by 100 [85]. Expressing the glycemic response of a food as a
proportion of the glycemic response of a standard carbohydrate meal greatly reduces between-subject
variation and creates a system that can be applicable to almost anyone. Experiments to determine the
GI value of a food usually require a minimum of 10 subjects [86]. Many such experiments have been
conducted, and as a result, the GI values of many carbohydrate-rich foods are known.
Carbohydrate foods that are absorbed quickly, such as white bread and cornflakes, cause a rapid spike
in blood glucose and thus have high GI values according to the equation above. On the other hand,
foods that are absorbed slowly and which promote slower, steadier increases in blood glucose have low
GI values. Beans and pasta are examples of low-GI foods. The presence of dietary fiber and physical
characteristics such as botanical structure help determine the GI of a carbohydrate. It should be noted,
however, that GI cannot be predicted from the fiber content of a food.
This concept has proven instrumental in evaluating the preventative role of low-glycemic index foods
in cardiovascular health [87] and in the development of diabetes [88] in both the healthy population
and in individuals at risk. Both prospective observational and clinical trials have been undertaken to
compare the effects of high-glycemic and low-glycemic diets on risk factors for diabetes and
cardiovascular disease. Low-glycemic diets tend to be associated with lower blood lipids and glycated
haemoglobin. Brand-Miller performed a meta-analysis of 14 trials and reported that low GI-diets
reduced HbA1c in nine of the studies and reduced glycated proteins (HbA1c and fructosamine) by
7.4% of the starting values [89]. Further, prospective studies done by Stevens
et al [90], Hodge
et al.
[91] and Schulze
et al. [92] all showed that the risk of developing diabetes was significantly greater on
a high-GI diet. This effect is not always consistent, however [93, 94]. Taken together, the results of
clinical trials studying the effects of diet GI on glycemic control suggest a small but significant
improvement on low-GI diets.
2.2.5 The Components of Salba affecting Postprandial Glycemia
There are several components of this grain that may lower postprandial glycemia; including its fiber,
polyunsaturated fat and protein contents. Each of these nutrients has been repeatedly and separately
shown to reduce glycemic responses. Together, they may act additively to provide Salba with an ability
to lower postprandial glycemia.
2.2.5.1 The Effects of Carbohydrates and Dietary Fiber on Postprandial
Although 35% of Salba is carbohydrate, 34% of it in the form of fiber [25]. Thus, it contains only 1%
available carbohydrate. As mentioned previously, 2.3% of the fiber is soluble and 32% is insoluble.
Both types of fiber have beneficial effects on health and have been shown to lower postprandial
glycemia, although the effects of soluble fiber on blood glucose have been more pronounced [95, 96].
The mechanisms put forth to explain fiber's ability to lower glycemia include slowing the rate of
digestion of starchy polysaccharides in the stomach,
slowing the rate of passage of the contents of the
stomach into the duodenum, lowering the rate of hydrolysis of polysaccharides in the upper small
intestine, lowering the rate of diffusion of carbohydrates in the small intestine and reduction of the rate
of absorption of monosaccharides through the microvilli of the epithelial cells in the jejunum and upper
ileum [86, 97, 98].
The Effects of Different Types of Fiber on Postprandial Hyperglycemia
Soluble fibers are hygroscopic, meaning they have the ability to absorb liquids, and when in contact
with liquids, expand to form a gel-like substance. Examples include gums and mucilages from oatmeal,
barley and legumes, and pectin found in fruits such as apples and strawberries [29]. Because it absorbs
liquids and expands in the stomach, soluble fiber slows the rate of gastric emptying. As a result, food is
absorbed over a longer period of time, slowing the rate of glucose absorption and release into the
bloodstream and thus reducing postprandial glycemia [29].
Extensive research supports the premise that soluble fiber significantly decreases postprandial glycemia
[98, 99]. Furthermore, clinical studies have demonstrated enhanced glucose tolerance and increased
insulin sensitivity in individuals consuming soluble dietary fibre supplements [95]. Soluble fibre has
also been shown to blunt the increase in and insulin following a glucose load [100]. The majority of
studies reviewed by Pilch found that soluble fibre intake results in an enhanced glucose tolerance and
increased insulin sensitivity [29]. In one parallel study, Anderson
et al. provided diabetic subjects
supplements containing either psyllium or cellulose twice daily for 8 weeks [83]. Participants who
ingested the psyllium, which contains mainly soluble fiber, experienced a 19.2% and 11.0% reduction
in average lunch postprandial glycemia and fasting glucose serum total, respectively. Soluble fiber has
also been linked to reduction of blood lipids [101]. In the same study, serum total cholesterol and LDL
cholesterol were reduced in the psyllium group by 8.9% and 13.0%, respectively.
Salba is composed of 32% insoluble fiber. Insoluble fibers are those that bind to liquids, and include
celluloses and lignins. This type of fiber has been associated with reducing GI transit time, increasing
fecal bulk, improving laxation and maintaining healthy intestinal and colonic pH levels [98].
It has been suggested that insoluble fiber has minimal effects on postprandial glycemia, gastric
emptying and nutrient absorption [98]. For instance, Samra and Anderson demonstrated that varying
the amount of insoluble fiber in a preload has no effect on postprandial glycemia [102]. Yet, Schenk
et
al. showed that insoluble fiber in cereal increases the rate of glucose uptake without affecting glucose
absorption, resulting in lower postprandial glycemia [103]. Greater glucose uptake was achieved with a
greater secretion of insulin in response to the insoluble fiber. Weickert
et al. reported that consumption
of 31g insoluble fiber for 3 days significantly improved whole-body insulin sensitivity compared to
white bread control [104]. In another study, increased intake of insoluble fiber in the form of wheat
fibre- and oat fibre-enriched bread over 24 hours reduced the glycemic response to a subsequent white
bread meal by 31% and 32%, respectively [105]. Therefore, there is evidence to suggest that insoluble
fiber affects glycemic responses.
The Effects of Fiber on Postprandial Glycemia: Mechanisms of Action
Soluble Fiber: Mechanisms of Action
Viscosity is one of the most important rheological properties of soluble fiber, as it largely determines
the health benefits of the fiber [106]. Viscosity is defined as a liquid's resistance to flow; it can also be
described as a liquid's "thickness". Viscosity is positively correlated with a fiber's ability to improve
glycemic control and lower blood lipids [107].
When viscous fiber interacts with the liquid contents of the stomach and intestines, it forms a
gelatinous matrix and expands. This viscous mixture prolongs absorption of carbohydrates by slowing
gastric emptying, and therefore slowing the release of glucose into the bloodstream [108]. The
gelatinous fiber mixture may also trap ingested food, resulting in fewer nutrients being absorbed in a
given amount of time and thus less glucose released into circulation. Soluble fibre reportedly traps
carbohydrates to slow their digestion and absorption, serving to prevent wide swings in blood sugar
and insulin levels throughout the day [28]. Other mechanisms proposed to explain this phenomenon are
resistance to the mixing action in the intestine, inhibition of enzyme activity and increased mucin
production [98, 106, 109]. Lastly, the production of short-chain fatty acids may explain soluble fiber's
ability to lower postprandial glycemia [98]. Butyrate, propionate and acetate are the short-chain fatty
acids are by-products of soluble fibre fermentation in the colon, and there is evidence that diets
enriched with short chain fatty acids reduce fasting glucose levels and postprandial glycemia [110,
111]. Some animal studies have found that the presence of acetate may result in a reduction in blood
glucose possibly by inhibiting endogenous glucose production [112]. Additionally, it has been
suggested that the production of acetate can lead to enhanced extrahepatic insulin secretion in the
presence of raised blood glucose [113].
Viscosity of Flax versus Salba
Even though Salba contains only 2.3% soluble fiber, this fiber is especially viscous as evidenced by the
comparison of the viscosity of ground Salba and ground flaxseed performed in our lab. Various
amounts of each were added to 200 mL water, and the viscosity of both were measured using a
Synchro-electric viscometer (Brookfield Ltd. Stoughton, MA, USA) at 22°C with a sheer of rpm 12
and with spindle E. Viscosity was measured at regular intervals for 1 to 3 hours. (Graphs 2.1 and 2.2)
We found that Salba was approximately three times more viscous than an equal amount of flaxseed
[114]. Interestingly, flax has on average 10g soluble fiber per 100g, making its soluble fiber content
more than three times greater than that of Salba (2.3g per 100g). Therefore, when accounting for the
difference in content, one gram of Salba has a comparable level of viscosity as approximately 9g
flaxseed, a fact that may contribute to Salba's ability to reduce postprandial glycemia.
Graph 2.1 Viscosity of equal amounts of ground Salba and flax
) 120
ille
u 100
Time (mins)
Graph 2.2 Viscosity of different amounts of ground Salba and flax
Time (mins)
Insoluble Fiber: Mechanisms of Action
Insoluble fiber may not delay gastric emptying as soluble fiber does, as a limited number of studies
have demonstrated its glucose-lowering ability [98]. It may, however, lower postprandial glycemia by
increasing glucose uptake into tissues. In the study done by Schenk
et al. greater glucose uptake was
achieved with a greater secretion of insulin in response to insoluble fiber [103]. Insoluble fiber also
increases transit time in the small intestine, which may decrease the amount of food absorbed, lowering
postprandial glycemia [115].
2.2.5.2 The Effects of Fat on Postprandial Glycemia
Thirty-three percent of Salba is fat, a factor contributing to its glucose-lowering ability. Research has
demonstrated that adding fat to a carbohydrate meal significantly lowers postprandial glycemia [116,
117]. The addition of fat to a carbohydrate meal does not affect postprandial glycemia in a linear
fashion, however. It has been shown that the greatest relative reduction in postprandial glycemia is seen
for small amounts of fat [86, 118]. When the ratio of fat to carbohydrate is approximately 0.05-0.2g fat
to 1g carbohydrate in a meal, fat reduces blood glucose by the greatest proportion. In most meals with
carbohydrate and fat, fat comprises 20-45% of the total energy [86]. In the experiments conducted for
this thesis, the ratios of fat to carbohydrate in the experimental meals of both studies were 0.07, 0.13
and 0.18 for low, intermediate and high doses, respectively. This represents a percentage of calories
from fat of 11%, 18% and 24 %, respectively.
It must be noted that the AUC of a glucose response for a carbohydrate and fat meal may not portray
the full extent that postprandial glycemia is altered. Adding fat to a carbohydrate meal may also change
the shape of the glucose response curve since not only does fat slow the rise in blood glucose, but it
slows the fall as well [86]. Therefore, the area under the curve for glucose could be quite similar for
carbohydrate foods with or without fat, but the shapes of the curves different. For instance,
Cunningham and Read demonstrated that adding fat to a soup meal delayed the occurrence of the peak
glucose level [119]. Thus, it has been shown that the addition of fat to a carbohydrate-rich meal lowers
the peak rise of glucose, suggesting that fat stabilizes glucose levels in the blood.
The Effects of Different Types of Fat on Postprandial Glycemia
Various studies have demonstrated that different types of fat affect postprandial glycemia differently.
For instance, it appears that postprandial glycemia decreases as the degree of unsaturation increases
[120, 121]; however this effect isn't consistent [122, 123]. Joannic
et al tested glucose and insulin
responses to four meals in which the ratio of monounsaturated fatty acids and polyunsaturated fatty
acids, and the type of carbohydrate (potatoes or par-boiled rice) were varied [124]. The two kinds of fat
used were a mixture of 70% high-oleic sunflower oil and 30% rapeseed oil (high MUFA to PUFA
ratio), and a mixture of 60% sunflower oil and 40% soybean oil (low MUFA-PUFA ratio). Blood
samples were taken every 30 mins for 3 hours post-consumption. Results showed that the glucose
AUC's for both types of fat mixtures did not differ significantly. However, at 30 mins, the glucose
response was significantly lower for both PUFA meals compared to the two MUFA meals. Thus, this
study demonstrated that the degree of fat saturation affected the early postprandial glucose response
[124]. Additionally, Gatti
et al. established that the co-ingestion of saturated fat and white bread did
not affect postprandial glycemia, while olive and corn oil reduced it [120].
Conflicting results were found in a study testing the effects of potato with either butter or olive oil.
Potatoes eaten with 100 g butter significantly reduced the blood glucose response area, while potato
with olive oil (40 and 80g) or 50g butter had no affect [122]. Further, MacIntosh
et al. studied the
glucose responses to three types of fat: butter, Sunola oil (MUFA) and sunflower oil (PUFA). No effect
of the degree of saturation on glycemic responses was found. The fact that MacIntosh
et al. studied
only males, administered a different amount of fat and measured blood samples only 2 hours post-
consumption may explain the discrepancy between their findings and those of Joannic
et al.
The effects of the type of fat on postprandial glycemia may be explained in part by the binding affinity
of intestinal fatty acid binding protein (FABP2)[86, 121]. Fat is first hydrolysed by pancreatic lipase to
fatty acids and monoglycerides, which are absorbed into enterocytes in the small intestine. Here they
are reassembled into triglycerides that are incorporated into chylomicrons. Some of the absorbed fatty
acids enter the portal circulation as free fatty acids, however, and go straight to the liver. Different free
fatty acids are absorbed in different amounts, partly due to differences in their affinities for intestinal
FABP2. A high level of free fatty acids in the portal vein is purported to increase hepatic glucose
Human FABP2 has the highest affinity for long-chain fatty acids such as palmitic, stearic, oleic and
linoleic acids [121]. It appears that binding affinity decreases as the chain length decreases [121];
however this effect isn't consistent in rats [125]. Nonetheless, the pattern of incorporation of fatty acids
into chylomicrons may explain why postprandial glucose after meals with butter are higher than those
with olive and maize/safflower oils. Butter contains roughly 25% of its fat as short- and medium-chain
fatty acids, while olive oil has about 75% oleic acid and safflower oil about 75% linoleic acid.
The Effects of Fats on Postprandial Glycemia: Mechanisms of Action
Prolonging gastric emptying causes a reduced rate in carbohydrate absorption, resulting in lower
postprandial glycemia [116, 119, 126]. Although it is generally accepted that fat delays gastric
emptying, some researchers argue that this characteristic is not specific to fat. Instead, it is a product of
additional nutrients [86].
Fat is proposed to delay gastric emptying by stimulating gut hormones such as gastric inhibitory
polypeptide (GIP) and glucagon-like peptide-1 (GLP-1). However, carbohydrate and protein also
increase these hormones to the same extent. Furthermore, one study that used 13C-labelled
carbohydrate demonstrated that adding fat to a carbohydrate meal reduced postprandial glycemia
without a change in the rate of appearance of the labelled carbohydrate in the blood. Therefore, delayed
gastric emptying may not be an adequate explanation for fat's ability to lower postprandial glycemia
In one study by Collier
et al., butter was added to either potatoes or lentils [127]. The postprandial
insulin response did not differ significantly from the responses to the carbohydrates alone. However,
the glycemic responses to the fat + carbohydrate meals were significantly decreased. When fat was
consumed with the meal, a lower amount of glucose required the same amount of insulin, suggesting
the potentiation of the insulin response. On the other hand, a decrease in insulin sensitivity is also
Some researchers report that fat increases insulin secretion [124, 128]. For instance, Gannon
et al.
found that the insulin response area to a starch + fat meal was significantly higher than that of a starch-
only meal [117]. Yet, some research demonstrates that while ingestion of fat does not result in
significant differences in insulin secretion, differences in glucose levels are evident as mentioned
above. This suggests that the ability of insulin to instigate glucose absorption may be altered when fat
is added to a carbohydrate meal. More specifically, ingestion of fat causes insulin insensitivity [127].
Studies that don't show a difference in insulin responses between carbohydrate and carbohydrate + fat
meals often show lowered postprandial glycemia for the fat meal, suggesting that the same amount of
insulin was needed for less glucose. One study even hypothesized that the effectiveness of insulin
depends on the type of fat ingested [129]. Research in this area is inconsistent, however, as insulin
responses differ in different studies.
2.2.5.3 The Effects of Protein on Postprandial Glycemia
The results of experiments that add protein to carbohydrate meals are inconsistent: several studies show
a reduction in glycemic response, while others show an increase[86]. Wolever reasons that glycemic
responses vary to such a great degree because there are many possible mechanisms by which protein
alters glucose metabolism and because they depend on different the rates of digestion and absorption of
individual amino acids[86].
Nonetheless, Gannon
et al. measured the glucose and insulin responses to glucose alone versus glucose
and lean beef, turkey, gelatin, egg white, cottage cheese, fish or soy in individuals with Type 2
diabetes. All glucose and protein meals resulted in a significantly lower glucose AUC than the glucose
meal, except when egg white was used. Furthermore, the insulin AUC was greater for all protein meals.
A previous study done by the same authors also demonstrated a synergistic effect on insulin release
when glucose and protein are consumed together [130].
The Effects of Different Types of Protein on Postprandial Glycemia
Different types of protein affect insulin secretion to different degrees. For instance, Gannon
et al
reported that protein from cottage cheese stimulated maximum insulin secretion, while protein from
egg whites stimulated insulin secretion to the lowest extent[131]. It is presumed that egg white is very
poorly digested and therefore increases insulin only slightly compared to egg white. Although the
increase in insulin secretion does not occur in a linear manner, the reduction in postprandial glycemia
does. Therefore, insulin cannot completely account for the lower glucose levels. Indeed, Wolever
reports that the insulin response to protein is responsible for approximately 30-40% of the glucose
The Effects of Protein on Postprandial Glycemia: Mechanisms of Action
It is generally accepted that protein's ability to lower postprandial glycemia is caused by delayed
gastric emptying [132]. As well, the ability of amino acids to promote insulin secretion has been put
forth as the main cause of the increase in insulin seen after consumption of protein [133], and therefore
another reason why protein lowers postprandial glycemia. Yet, the ingestion of protein has also been
shown to increase postprandial glycemia when added to carbohydrate meals [134], and when added to
carbohydrate and fat meals as well [135]. There are a few mechanisms by which protein can increase
glycemic responses; namely, the stimulation of glucagon secretion, conversion of amino acids to
glucose and increase in insulin resistance caused by amino acids. It is important to note that when
protein is ingested alone, it has no or very little effect on blood glucose in people with or without Type
2 diabetes [136, 137].
Given the conflicting results from protein and carbohydrate meal studies, it is difficult to predict the
effects of the protein in Salba on postprandial glycemia. Regarding the control meals, it would be
expected the protein used (egg white) would not affect postprandial glycemia since this protein has
been shown to have no effects on blood glucose.
2.2.5.4 Antioxidants and Hyperglycemia
Antioxidants do not have a postprandial glycemia-reducing effect. However, they can help alleviate ill
effects of hyperglycemia by combating oxidative stress. As mentioned previously, hyperglycemia
increases the production of ROS [80, 138]. This, in turn, causes the body to be more susceptible to a
variety of diseases. For instance, a state of oxidative stress increases the amount of oxidized LDL
cholesterol, which is a risk factor for CVD. Therefore, although antioxidants cannot prevent
hyperglycemia, they can help reduce the detrimental effects by binding to and deactivating harmful
2.3 The Short-term Regulation of Appetite
With the current high rates of obesity and obesity-related complications, increasing attention has been
given to the control of food intake as a preventative measure. It is presumed that the effects of short-
term appetite reduction act cumulatively to decrease total food intake over a prolonged period of time,
in turn preventing excessive caloric consumption and weight gain [140]. Decreasing appetite is a very
topical issue, intriguing both the research community and general public alike.
This thesis focuses on postprandial feelings of appetite. In the research setting, the question remains as
to whether subjective feelings of appetite influence food intake. Some studies have shown that
intentions to eat and ratings of hunger correlate with actual food consumption, while others show no
correlation [141]. Nonetheless, the measurement of subjective appetite is widely used in nutrition
research and is accepted as a valid measure by the medical community [142-145].
The regulation of appetite is a multifaceted process that although extensively researched, is not yet
completely understood. Short-term hunger and satiation are affected by numerous psychological,
mechanical and physiological factors [145-151]. Of particular interest to this thesis are the anorectic
gastrointestinal hormones that are released in response to a meal, namely cholecystokinin (CCK),
pancreatic polypeptide, peptide YY and glucagon-like peptide-1 (GLP-1) that act as negative feedback
signals to the satiety centres of the brain [147]. When released from enteroendocrine cells in the gut
mucosa, these hormones can communicate with brain regions via the circulation and afferent nerve
fibers to signal food intake to the central nervous system [146-148]. CCK was the first anorectic
hormone to be discovered. It is released postprandially and its concentration in the circulation can
remain elevated for up to 5 hours after a meal. CCK inhibits food intake in both humans and rats and
initiates gallbladder contraction and secretion of pancreatic enzymes. GLP-1 is another anorectic
hormone that is released in response to a meal. The amount released is dependant on the amount of
calories ingested, and it modulates gastric emptying and acid secretion. Infusion of GLP-1 reduces food
intake and increases feelings of satiety.
Ingested food is sensed by vagal afferent fibers in the mucosa, while food volume is detected by vagal
afferent nerves in external muscle layers of the gut [146]. Food volume and intragastric pressure are
both important factors in the initiation of satiety signalling, and may be the main cause of the satiating
effects of soluble dietary fiber. As well, circulating glucose, lipids and amino acids all affect feelings of
appetite and food intake. The effects of protein and fat on appetite are particularly relevant to this
The Glucostatic Theory, although not fully validated or proven, is one of the most well-known and
researched hypotheses linking appetite and macronutrient intake. Proposed by Mayer in 1951, it is a
homeostatic theory of hunger and states that the body has mechanisms in place to regulate glucose
levels and feeding behaviour [152]. Although other nutrients affect eating behaviour as well, glucose is
deemed to be of particular importance due to its key role as an energy source for the central nervous
system. If the body detects hyper- or hypoglycemia, signals are initiated to bring the glucose level back
to an acceptable range. For instance, when blood sugar is low, signals are sent to the hunger centres of
the brain to initiate feeding in an attempt to raise plasma glucose [152-154].
Many studies support the Glucostatic Theory by demonstrating associations between glucose levels and
feeding behaviour or hunger in rats [155, 156] and humans [157]. Campfield and Smith found that
transient declines in blood glucose resulted in food seeking and meal initiation in rats [155]. Pittas
et
al. studied the associations between interstitial glucose and energy intake, desire for a meal, self-
reported hunger and satiety in free-living nonobese women. Absolute interstitial glucose values up to
35 mins before meal initiation were significant predictors of food intake such that low absolute glucose
values preceded meal initiation. Blood glucose lags interstitial glucose by 10 mins; thus, the authors
hypothesized that blood glucose levels up to 25 mins before meal initiation are negatively correlated
with subsequent food intake [158].
In a study by Gielkens
et al., subjects received intravenous infusions of glucose, insulin or saline
(control). The authors found that hyperglycemia (15 mmol/L) induced significantly lower prospective
intentions of feeding and feelings of hunger compared to control or hyperinsulinemic conditions [157].
In a study done by Anderson
et al., subjects consumed a drink containing polycose, sucrose, glucose, a
glucose-fructose mixture or sucralose control. Over the next hour, blood glucose and self-reported
hunger were measured at various time points. Food intake after 60 mins was measured via an
ad
libitum pizza meal. It was reported that glucose iAUC was negatively correlated with energy intake
However, Wolever
et al. found no correlation between blood glucose and satiety as measured by VAS
for 2 hours postprandially [142]. Participants consumed the same white bread meal on separate
occasions and the postprandial glucose iAUC was measured for each session. The satiety scores for the
meals eliciting the greatest and smallest AUC's were compared, and it was reported that they did not
differ significantly. The authors suggest that the increased satiety after low-GI foods must occur
through a mechanism that does not rely on blood glucose level. In another study, Freeland and Wolever
measured postprandial glycemia and food intake after low- and high-fiber cereals and found that blood
glucose responses were not correlated with energy intake [159]. Other studies have also failed to
demonstrate a link between blood glucose and satiety [160, 161].
There is still debate as to whether it is a change in blood glucose or the absolute blood glucose level
that influences eating behaviour. Pittas
et al.'s study provides support for absolute levels being the
determinant of food intake, while Campfield and Smith reported that it was changes in glucose level
that affected it. This issue will be discussed further in a following section.
The Components of Salba potentially affecting Appetite
Salba's components may exist in favourable proportions and amounts to increase satiety. Firstly, Salba
is composed of 34% dietary fiber, a nutrient implicated in reduced feelings of hunger and lower risk of
obesity. As well, the type of fat in this grain has been reported to be satiating. In addition, with a
calcium content of 770mg per 100g, this grain is exceptionally high in calcium. Intracellular calcium
has a regulatory role in fat metabolism by influencing lipolysis, fat oxidation, and lipogenesis; all
processes that may influence appetite regulation [162]. Lastly, Salba contains a significant proportion
of protein, the most satiating macronutrient [163]. We thus hypothesize that all these factors may act
additively to promote satiety, in particular Salba' fiber, polyunsaturated fat and vegetable protein
2.3.1.1 The Effects of Carbohydrates and Dietary Fiber on Appetite
Both soluble and insoluble fiber have been shown to reduce appetite and/or subsequent food intake
[100, 164, 165]. Howarth
et al. analyzed the results of over 20 studies in which the effects of fiber on
hunger and satiety were measured [115]. In the majority of these studies, the addition of fiber to the
diet caused either a significant or nonsignificant increase in satiety between meals and/or a decrease in
hunger relative to control. No differences were found for the effects of soluble versus insoluble fibers.
The two types of fiber decrease hunger by different mechanisms. Unlike insoluble fiber, soluble fiber
becomes viscous when mixed with liquid, leading to mainly mechanical effects on appetite. Increased
gastric distention due to the swelling of soluble fiber creates a feeling of fullness by activating stretch
receptors within the gut walls and initiating afferent vagal signals [115]. Soluble fiber also delays
gastric emptying, meaning food remains in the stomach longer, which creates a feeling of fullness for a
longer amount of time [115].
Soluble fiber-rich foods are exceptionally low density due the fact that fiber is not digested and that the
soluble component swells in the stomach. Many studies done by Rolls
et al have proven that high
volume foods are more satiating than low volume foods [166-168]. For instance, incorporating air into
a beverage resulted in less subsequent energy intake than the beverage alone. Soluble fiber influences
satiety by metabolic pathways as well, by possibly stimulating the secretion of gut hormones that signal
Lowered postprandial glycemia, a metabolic effect, is yet another mechanism that has been suggested
to increase feelings of fullness. Soluble fiber slows gastric emptying and traps carbohydrates, slowing
their digestion and absorption. This, in turn, is hypothesized to increase satiety by preventing sudden
drops and wide swings in blood sugar levels [28, 169], signals that would normally trigger hunger as
explained in the Glucostatic Theory. In line with these hypotheses, it was found that subjective feelings
of appetite were greater after a greater consumption of pectin, a soluble fiber, than the same meal
without it [170].
On the other hand, insoluble fiber increases food transit time in the small intestine, which may result in
decreased food absorption. Thus more undigested food particles reach the distal intestine, which is
proposed to increase the secretion of satiety hormones [171]. It has also been reported that increased
levels of insoluble fiber cereal decreased food consumed at an
ad libitum meal in men [102].
Low GI Carbohydrates
Salba does not have a GI value
per se, as it contains no carbohydrate. Yet, when added to a
carbohydrate meal, it has the ability to lower the meal's glycemic response. Low Glycemic Index (GI)
foods have received much attention in recent years for their purported effects on hunger. Researchers
theorize that a consistently stable, moderate level of plasma glucose signals an adequate supply of
glucose and as a result, hunger signals are not initiated. However, high-GI foods cause an immediate
and large increase in blood glucose levels. In an attempt to normalize the blood glucose level, a great
amount of insulin is released, leading to the rapid removal of glucose from the blood. The counter-
regulatory response may overcompensate, resulting in hypoglycemia. Hunger signals are then initiated.
It follows that since low-GI foods promote stable blood glucose levels and prevent precipitous declines
into hypoglycemia, they would also prevent hunger [28].
Results from studies evaluating the effects of GI on satiety have been inconsistent. Roberts evaluated
the results of studies measuring satiety, hunger and/or food intake after low-GI and high-GI preloads
[172]. These studies controlled for caloric value, energy density and palatability of the test meals.
Although there were no significant differences for satiety and hunger as measured by visual analog
scales, all five studies demonstrated lower subsequent energy intake following the low-GI meals (three
were significant). Roberts also reported that a meta-analysis of these experiments revealed an 81%
larger energy intake after high-GI meals than after low-GI meals.
It is postulated that since low-GI foods are digested more slowly, more undigested starch reaches the
ileum [173]. It is believed that the presence of starch in this region promotes the release of satiety-
signalling hormones such as GLP-1[171]. Another theory to explain the effect of low-GI carbohydrates
on appetite is the change of fuel source availability depending on the GI-value. Consumption of a high
GI-meal may promote the uptake of glucose and fatty acids while decreasing lipolysis [174, 175]. Thus
levels of these macronutrients decrease in the circulation, and since this represents a decrease in
circulating metabolic fuels, hunger is initiated.
Ludwig concluded from 20 GI studies that low-GI meals consistently lower appetite and/or food intake
[176]. Unfortunately, many of these studies failed to control for confounding factors [86]. For instance,
in some experiments the test meals contained different amounts of protein or fiber, two components
that can affect hunger. Another study included in the meta-analysis tested meals with different
glycemic loads, but not GI values. Therefore, although one cannot dismiss the theory, more research
must be conducted. With this in mind, it can be theorized that the consumption of Salba may help
increase satiety since its components promote the moderate and stable release of glucose into the
circulation and the induction of satiety signals.
2.3.1.2 The Effects of Fat on Appetite
Researchers consider fat to be the least satiating nutrient in the short-term [140], mostly due to its high
energy density and its palatability [87, 177]. Studies have shown that high-fat foods lead to passive
overconsumption compared to lower-fat, less energy-dense foods [178]. Due to its effects on gastric
emptying, however, fat may be satiating for a longer period of time than the other macronutrients.
Also, it has been demonstrated that dietary fat or the products of its digestion are more effective
stimulators of CCK release than carbohydrate [179].
Salba contains mainly polyunsaturated fat, which has been reported to induce greater satiety than other
types of fat. For instance, the degree of saturation of fat appears to affect satiety. Two studies
conducted by Lawton
et al. tested the effects of three types of fat incorporated into a meal. Fat A
consisted of mainly monounsaturated fat (oleic blends), Fat B polyunsaturated fat (linoleic blends) and
Fat C saturated fat (stearic-oleic blends). Satiety was measured with visual analog scores, food intake
during an
ad libitum meal and 24-hr food intake diaries[180]. The results showed that the
polyunsaturated fat meal reduced appetite to the greatest extent. The authors hypothesized that the
effects of fats on satiety depend on whether they are oxidized or stored. Friedman has suggested that
when a fat is oxidized, it causes more satiety signals [181]. Saturated fat is not oxidized to fuel sources
as readily as polyunsaturated fat [182], which would lend support to the results of Lawton
et al.
2.3.1.3 The Effects of Protein on Appetite
Protein is the most satiating of macronutrients, and research has demonstrated that consumption of
protein reduces both appetite and food intake compared to consumption of carbohydrate [140, 183].
Although carbohydrates, and especially simple carbohydrates, provide the most satiation immediately
after consumption [184], protein provides feelings of fullness for prolonged periods of time. Thus in
theory, Salba consumption may reduce feelings of satiety and food intake for a period of time beyond
those of the effects seen from dietary fiber and carbohydrate when added to a carbohydrate meal.
Evidence exists for the short-term, 24-hour and long-term satiating effect of protein [163]. For instance,
greater satiety and GLP-1 levels were seen after a high-protein dinner compared to an adequate-protein
dinner [185]. In one long-term study by Johnston
et al., participants were put on either a high-
protein/low-fat diet or a high-carbohydrate/low fat diet. Those in the high-protein group reported
feeling more satiated during the first 4 weeks of the study [186]. Lejeune
et al. studied the effects of a
high protein versus an adequate protein diet on healthy females [187]. The HP diet contained 30%
protein, 40% carbohydrate and 30% fat, while the AP diet contained 10% protein, 60% carbohydrate
and 30% fat. The experiments were conducted in a respiration chamber, and diet-induced
thermogenesis, sleeping metabolic rate and activity-induced energy expenditure were monitored.
Twenty-four hour satiety was also measured via VAS. The authors reported that the high protein diet
resulted in significantly greater satiety AUC. Diet-induced thermogenesis was also significantly greater
in the HP condition. Subjects in another study reported siginificantly higher feelings of satiety 30 and
120 mins after consuming a lunch with 25% protein versus one with 10%. Satiety AUC over 2 hours
was significantly higher in the former condition, as well [188].
Westerterp-Plantenga
et al. suggest that diet-induced thermogenesis may be a factor in causing satiety
[185]. It is theorized that increased oxygen consumption occurs during increased energy expenditure,
and that this oxygen deprivation creates feelings of satiety [163]. Additionally, there is evidence that
different protein sources elicit different levels of energy expenditure, and therefore perhaps satiety. For
instance, it was reported that animal protein caused a 2% higher energy expenditure than vegetable
protein [163]. Thus, the protein in Salba may not promote as much satiety as would be expected from
an animal source. Nonetheless, it can be hypothesized that the addition of vegetable protein to a
carbohydrate-rich meal would be expected to lower appetite to a greater extent than an equicaloric
amount of added carbohydrate.
3 Rationale and Objectives
3.1 Summary and Rationale
Salvia hispanica L., also known as Salba, is an ancient oil-rich grain with a unique and superior
composition. Although consumed for centuries, little clinical research has been conducted to examine
its potential effects on health. Research by Vuksan
et al. suggests that Salba may decrease risk factors
for CVD in Type 2 diabetes. Diabetic individuals who supplemented their CDA-recommended diet
with 37g of Salba a day for a month experienced lower blood pressure, coagulation and low-grade body
inflammation. As well, results from two studies demonstrate a possible role for Salba in the reduction
of visceral adiposity, and perhaps, therefore, the risk of obesity. Vertommen
et al. found a significant
reduction of waist circumference in healthy individuals consuming Salba for one month. That there was
no significant decrease in body weight is a possible indication that fat mass was preferentially lost.
Salba has also been shown to reduce visceral adiposity in rats. Animals fed a sucrose-rich diet
supplemented with Salba experienced lower visceral adiposity than those on a maize control diet in a 5-
month study by Chicco
et al. [5]
.
The promising results of these studies with Salba suggest a possible ability of this grain to reduce risk
factors for CVD and obesity. More research is thus warranted to determine both the acute and long-
term effects of this grain. With regards to Salba's acute effects, the reduction of postprandial glycemia
may explain the results seen in aforementioned long-term studies, as hyperglycemia has been shown to
trigger biochemical cascades related to atherosclerosis and CVD.
We theorize that the smaller blood glucose responses from consuming Salba may have promoted lower
blood pressure, coagulation factors and body inflammation by the reduction of physiological pathways
triggered by excess glucose. Postprandial hyperglycemia has been demonstrated to promote oxidative
stress, which in turn affect endothelial function and LDL oxidation [71, 80, 138]. As well, we
hypothesize that a reduction of appetite may have contributed to the decrease in waist circumference
seen in Vertomment
et al.We thus investigated the acute effects of Salba in various dosages on
postprandial glycemia and subjective appetite. We also employed whole and ground forms of this
grain, as it is commercially available as ground and whole yet a comparison of the effects of these
forms has not been assessed specifically. We began examining the possible relationship of acute and
long-term effects in a series of acute studies measuring the effects of a Salba-enriched carbohydrate
meal on postprandial glycemia and appetite, two factors that may play a role in the development and
severity of obesity and CVD.
3.2 Hypothesis and Objectives
The main objective of this thesis is to evaluate the effects of Salba on postprandial hyperglycemia and
appetite, two factors that have been suggested to play a role in the development of Type 2 diabetes,
CVD and obesity.
3.2.1 Specific Objectives
1. To measure the effects escalating doses of Salba added to white bread in reducing postprandial
glycemia in healthy individuals
2. To measure the effects escalating doses of Salba added to white bread in decreasing subjective
appetite in healthy individuals
1. To determine the effects of escalating doses of ground vs. whole forms of Salba added to white
bread on postprandial glycemia in healthy individuals
2. To determine the effects of escalating doses of ground vs. whole forms of Salba added to white
bread on subjective appetite in healthy individuals
3.2.2 Hypotheses
1. Salba will lower postprandial glycemia in a dose-dependent manner when added to white bread
2. Salba will decrease subjective appetite in a dose-dependent manner when added to white bread
1. Salba-enriched white bread will lower postprandial glycemia to a significantly greater extent
than equicaloric fat-, protein- and carbohydrate-matched white bread.
2. Salba-enriched white bread will decrease subjective appetite to a significantly greater extent
than equcaloric fat-, protein- and carbohydrate-matched white bread.
3. Ground and whole forms of Salba will have comparable effects on both postprandial glycemia
and subjective appetite.
4 Study 1: The Effects of Escalating Doses of Salvia
hispanica L. (Salba) on Postprandial Glycemia and
Subjective Appetite in Healthy Individuals
Objective: To assess the effects of escalating doses of the whole grain Salba on postprandial glycemia
Methods Using an acute randomized, double-blind, crossover design, 12 healthy individuals
(7M;5F;BMI 22.2 ± 1.3 kg/m2) received 0, 7, 15 or 24 g of Salba baked into white bread. The control
(0g) was repeated twice. All meals contained 50 g of available carbohydrates. Thus, subjects consumed
5 different meals with at least 2 days between visits. Fingerprick blood samples and ratings of appetite
scores on a 100 mm visual analog scale were taken at fasting and 15, 30, 45, 60, 90 and 120 mins post-
Results iAUC was reduced by approximately 2% for one gram of Salba. Glucose iAUC was negatively
correlated with dose of Salba (
r=-0.47, p=0.001). The highest and intermediate doses of Salba resulted
in a reduction in blood glucose iAUC of 44% and 25% (p=0.002), respectively. Subjective appetite was
also negatively correlated with dose of Salba (
r=-0.3, p=0.043). The appetite iAUC for low,
intermediate and high doses were lower than control by 54.0%, 62.1% and 66.8%, respectively, but
these reductions did not reach significance. However, appetite for the high and intermediate doses at 90
mins (p = 0.044) and intermediate dose at 120 mins (p = 0.044) were significantly lower than control.
Conclusions Addition of the whole grain Salba to white bread lowers postprandial glycemia and
subjective appetite, two possible factors that may help reduce the risk of obesity and CVD. Further
research is warranted to assess the effects of Salba on long-term reduction of cardiovascular risk
factors, carbohydrate metabolism and control of body weight.
4.2 Introduction
Recommendations for various dietary interventions have been attempted to reduce the risk of obesity,
Type 2 diabetes and CVD. One suggestion is the reduction of postprandial hyperglycemia. Growing
evidence on the independent predictive role of 2-hour postprandial glycemia for cardiovascular disease
suggests that interventions should target this risk factor. Explanations of the risk of cardiovascular
disease and diabetes linked to postprandial glycemia suggest that endothelial and beta cells, both freely
permeable to glucose via GLUT- 2 and 1, respectively, are susceptible to the excessive oxidative stress
resulting from increased glucose uptake. Oxidative stress, in turn, increases risk factors for CVD such
body inflammation and coagulation. Supplementing the diet with certain functional foods, such as
whole grains, has been suggested as a dietary intervention to lower postprandial glycemia.
Due to the key role of obesity in the development of both cardiovascular disease and Type 2 diabetes,
nutrition research has increasingly focused attention on appetite regulation. Researchers theorize that
certain functional foods may help reduce the risk of obesity. With the presumption that lowered
appetite would translate to less food intake [140], investigation of the ability of certain foods to lower
appetite could be valuable in the prevention of obesity and CVD.
Evidence suggests that phytochemicals naturally-occurring in whole grains have the ability to lower
postprandial glycemia and appetite. Phytochemicals such as fiber, minerals, unsaturated fat and
antioxidants and nutrients such as vegetable protein may separately promote such effects. Here we
investigate the ability of the novel whole grain
Salvia hispanica L. (Salba) to lower postprandial
glycemia and reduce appetite when added to a carbohydrate meal. Salba has a composition superior to
commonly-consumed whole grains and oily seeds, as it contains comparable amounts or more fiber,
omega-3 fatty acids, minerals, antioxidants and protein. In addition, the possible beneficial effects of
Salba consumption such as decreased blood glucose and appetite may provide mechanisms to explain
the results of previous Salba studies showing decreased blood pressure, low-grade body inflammation,
coagulation factors and adiposity. These represent possible risk factors for Type 2 diabetes and CVD.
4.3.1 Participants
A total of 12 healthy individuals (7M; 5F; Age: 30.2 ± 3.6 years; BMI 22.2±1.3 kg/m2) participated in
the study, as typical G.I. studies employ from 10-12 participants [86]. All participants were healthy,
between 18-66 years old and clinically euthyroid with normal hepatic and renal function. Those who
were pregnant, suffered from gastrointestinal or metabolic diseases, or regularly ingested fiber
supplements were excluded. All participants gave written informed consent, and the study was
approved by the St. Michael's Hospital Research Ethics Board.
4.3.2 Treatments
There were five experimental meals consisting of 50g carbohydrate servings of white bread with 0, 7.3,
15.6 or 24g of ground Salba added. The lowest dose, 7.3g, contains the American Heart Association's
daily minimum recommended intake of omega-3 fatty acids. The bread without any Salba comprised
the control meal and was served twice. All breads were prepared on-site with a Black & Decker® All-
In-One Pro™ Breadmaker (Towson, MD, USA). Two-hundred and fifty millilitres of water was
served with each meal. Please see Table 4.1-4.3 for percentage of calories per macronutrient, bread
ingredients and caloric values of experimental breads.
Table 4.1 Nutritional Information for Experimental Breads
4.3.3 Experimental Design
The study consisted of a double-blind, placebo-controlled, randomized block design in which subjects
underwent five 2.5-hour sessions separated by a washout period of at least 48 hours. Subjects visited
the Risk Factor Modification Centre in the morning after having fasted for 10-12 hours overnight and
engaging in normal eating and exercise habits the preceding day. They had an initial fasting finger
prick blood sample taken and completed a subjective appetite questionnaire in the form of a 100mm
visual analog scale (VAS). The experimental meal was then served and subjects were required to finish
the meal within 15 mins. Finger prick blood samples were taken at 15, 30, 45, 60, 90 and 120 mins
postprandially. Subjects also completed the appetite VAS at these times. For the duration of the study
session, subjects remained at the clinic and were instructed not to eat or drink and to keep physical
activity to a minimum.
4.3.4 Blood Glucose Analysis
Capillary blood samples were obtained using sterile single-use lancets. Two to three drops of capillary
blood were collected in plastic flat-bottomed 5ml tubes with a push cap containing a small amount of
sodium fluoride and potassium oxalate as an anticoagulant and preservative. The blood samples were
placed in a -20ºC freezer for a maximum of 5 days until the analysis of whole blood glucose. Capillary
blood glucose was measured by the glucose oxidase method using a YSI 2300 STAT Plus Glucose &
Lactate Analyzer™ (Yellow Springs Instruments, Yellow Springs, OH, USA).
4.3.5 Measurement of Appetite
Four unipolar visual analogue scales were used for each appetite assessment. For each of the four
questions, subjects indicated their response by drawing a vertical line along a 100 mm horizontal line
that was anchored by two statements. The questions were "How strong if your desire to eat?", "How
hungry do you feel?", "How full do you feel?" and "How much do you think you could eat now?"
Subjects' ratings were converted to numerical values by measuring the distance between the left anchor
and their drawing. A combined appetite score for each appetite assessment was computed with the
following formula:
4.3.6 Study Variables
The primary variables for this study were the mean iAUC's for blood glucose and the mean
incremental change from baseline in blood glucose at 15, 30, 45, 60, 90 and 120 min postprandially.
The secondary variables were the mean iAUC's of satiety scores and the incremental change in appetite
from baseline at the same time points.
4.3.7 Statistical Analysis
Statistical analysis was performed using NCSS 2000 (NCSS, Kaysville, UT) and SPSS release 16.0
(SPSS Inc., Chicago, IL). Incremental areas under the blood glucose response curves and appetite
scores (iAUC) were calculated by applying the trapezoid rule and analyzed by one-way ANOVA using
the Neuman-Keuls method to adjust for multiple comparisons. We performed Pearson correlations and
conducted linear regression analysis to determine dose-response relationships for glucose and appetite
iAUCs. Two-factor ANOVA was performed on blood glucose levels and appetite scores at each time
point to test for a time x treatment interaction. When an interaction was statistically significant, a one-
factor ANOVA using a GLM procedure was followed by Newman-Keuls post hoc test to identify mean
differences among treatments at each time of measurement. Significance was set at p < 0.05.
4.4.1 Postprandial Blood Glucose Responses
Blood glucose iAUC was negatively correlated with dose of Salba (
r=-0.47 p=0.001) (Data not
shown). Thus, approximately 22% of the variation in blood glucose could be accounted for by the dose
of Salba. The mean glucose incremental AUC (± s.e.m.) was 156.6 ± 20, 135.6 ± 18.3, 114.5 ± 8.5 and
88.4 ± 5.7 min.mmol/L for control, low dose, intermediate dose and high dose breads, respectively.
(Please see Table 4.1). Glucose iAUC was significantly reduced in both high (44%) and intermediate
(25%) doses compared to control (p = 0.002). The low dose reduced iAUC by 13%, which did not
reach significance. The incremental blood glucose value was significantly lower for the high dose
compared to control at 30 mins (p=0.016) and for both high and intermediate doses at 60 mins
postprandially (p=0.007).
Graph 4.1 The Effects of Salba on Blood Glucose iAUC (n=12)
) 180
l/L
o 160
p = 0.002; 1-way ANOVA; Newman-Keuls
Graph 4.2 The Effects of Salba on Incremental Blood Glucose (n=12)
Time (mins)
* High < Control; p = 0.016
* High and Int < Control; p = 0.007
1-way ANOVA; Newman-Keuls
Graph 4.2 The mean incremental glucose plasma glucose level at each time point. * indicates that High and Int are less than Control at 60 mins mins (p= 0.007) and * indicates that High is less than Control at 30 mins (p 0.016)
4.4.2 Appetite Scores
Appetite scores were measured via four visual analog scales (VAS), and a combined score was
calculated at fasting and for each of the following time points: 15, 30, 45, 60, 90 and 120 mins
postprandially. Appetite score was inversely correlated with dose of Salba (r=-0.3, p=0.043). The
satiety iAUC for low, intermediate and high doses were higher than control by 54.0%, 62.1% and
66.8% respectively (all NS, p= 0.108), with the mean satiety iAUC (± s.e.m.) of control, low,
intermediate and high breads being -2400.2 ± 719.8, -3695.8 ± 950.1, -3890.3 934.5 ± 69.0 and -
4003.6± 689.1 mm.min, respectively. Two-way ANOVA revealed a significant time by treatment
interaction (p = 0.027). Analyzing incremental appetite scores over time, it was found that the appetite
scores for the high and intermediate doses at 90 mins (p = 0.044) and intermediate dose at 120 mins (p
= 0.044) were significantly lower than control.
Graph 4.3 The Effects of Salba on Incremental Subjective Appetite (n=12)
Time (min)
*High and Int < Control; p= 0.044
Graph 4.3 The mean incremental subjective appetite scores at each time point. * indicates that High and Int are less than Control at 120 mins (p= 0.044) and * indicates that Int is less than Control at 90 mins (p 0.045)
Graph 4.4 The Effects of Salba on Satiety Score iAUC's (n=12)
(m
C
U -3000
tite -4000
p= 0.108; 1-way ANOVA
Results showed that high (24g) and intermediate (15.6g) doses of Salba added to white bread
containing 50g of available carbohydrate significantly reduced glucose iAUC by 44% and 24%,
respectively. This is equivalent to an average iAUC reduction of 2% for one gram of Salba We cannot
determine exactly which components of Salba reduced postprandial glycemia, as the breads differed in
caloric value. In this case, we hypothesize that the added protein, fat and/or fiber from the Salba most
likely contributed to the lowered blood glucose responses. The effects of Salba on postprandial
glycemia may help explain the reduction in blood pressure, low-grade body inflammation and
coagulation factors observed in Vuksan
et al.'s long-term study. Postprandial hyperglycemia promotes
oxidative stress, which in turn can cause or exacerbate risk factors for CVD such as those assessed. It
follows, then, that reduction of postprandial glycemia may suppress the cascade of effects normally
triggered by oxidative stress.
Appetite score was significantly decreased at 90 (high and intermediate doses) and 120 mins
(intermediate dose) compared to control. However, there were no significant differences between the
appetite iAUC's of all three Salba doses. Apparently the effectiveness of the appetite-suppressing
component does not act proportionally to its amount. The fact that the control and low dose breads
elicited significantly higher glucose iAUC's than the other two breads but did not promote lower
appetite appears to contradict the Glucostatic Theory at first glance. However, it is possible that since
hypoglycemia was never reached (i.e. glucose level did not fall below baseline value) for either control
or low dose bread, hunger signals were not triggered. On the other hand, we believe that appetite was
marginally lower for intermediate and high dose breads despite eliciting lower blood glucose responses
because of their greater fiber, protein, mineral and/or fat contents. Such components are proposed to
promote certain satiety signals, such as the release of GLP-1.
As for the marginally greater effects on appetite for the intermediate dose bread compared to the high
dose bread, we speculate that the high dose lowered postprandial glycemia to such a great degree that
the blood glucose level was not high enough to elicit as strong satiety signals as the intermediate dose.
Such a hypothesis would be in line with The Glucostatic Theory which states that satiety and blood
glucose concentration are linearly correlated. As well, we theorize that the low dose and control breads
did not elicit significantly decreased appetite despite causing greater blood glucose responses because
they had lower fiber, protein, mineral and fat contents than the intermediate and high dose Salba
We cannot determine specifically which aspect of the enriched breads lowered appetite, as the breads
were not equicaloric. Thus, variables such as increased caloric value might have been responsible for
the effect seen. Yet the changes in satiety were most likely not caused by the unequal caloric values of
the preloads because the differences were too small. Referring to past research on energy intake and
satiety, it is improbable that the differences of 29 and 59 kcal as used in our study would be sufficient
to elicit a difference in appetite. For instance, Hulsof
et al. found no difference in energy intake after
two preloads differing by 700kcal. In the majority of studies that do find differences in subjective
and/or objective satiety after experimental preloads, the preloads differed in calories by anywhere from
150 to 600 kcal. Nonetheless, future studies should address this issue by investigating the effects of
equicaloric experimental meals on satiety.
In conclusion, this study demonstrates that the addition of Salba to a carbohydrate meal lowers the
glycemic response and subjective appetite. Controlling these factors may help decrease the risk of
certain diseases such as Type 2 diabetes and CVD. Future studies could assess the effectiveness of
Salba in reducing postprandial glycemia in individuals with Type 2 diabetes. Furthermore, they should
address the effectiveness of Salba to lower appetite by comparing equicaloric test meals and assessing
subsequent food intake.
5 Study 2: The Effects of Escalating Doses of Whole vs.
Ground Salvia hispanica L. (Salba) on Postprandial
Glycemia and Subjective Appetite in Healthy Individuals
Objectives: To assess if the effects of escalating doses of whole versus ground forms of the whole
grain Salba on postprandial glycemia and subjective appetite compared to energy-matched white bread
Methods Using an acute randomized controlled design, 20 healthy individuals (8M;12F; BMI 25.7 ±
2.4 kg/m2) received on nine occasions either 7, 15 or 24 g of ground or whole Salba baked into white
bread. There was one energy-matched control bread for each of the three doses of Salba. All meals
contained 50 g of available carbohydrates. Fingerprick blood samples and ratings of satiety scores on a
100 mm visual analog scale were taken at fasting and 15, 30, 45, 60, 90 and 120 mins post-
Results. Blood glucose iAUC was negatively correlated to the dose of Salba of whole and ground
forms combined (
r=
-0.17, p=0.035). There was a difference between iAUC's for the high doses only,
with blood glucose responses to the two Salba breads significantly lower than that of the high dose
control (p=0.02). Average percent reductions in appetite for whole and ground Salba breads combined
relative to equicaloric controls were -5.3%, -9.5% and -14.8%, respectively.
Conclusions Salba's ability to lower postprandial glycemia is not fully due to its macronutrient
proportion. As well, both ground and whole Salba are equally effective in lowering postprandial
glycemia when added to white bread.
5.2 Introduction
The novel oil-rich whole grain
Salvia hispanica L. (Salba) has been shown to reduce postprandial
glycemia and subjective appetite when added to white bread. Such effects may help explain the
lowered blood pressure, coagulation, low-grade body inflammation and adiposity seen in a previous
long-term Salba studies. Postprandial glycemia has been shown to have predictive and diagnostic value
for both Type 2 diabetes and cardiovascular disease. Postprandial hyperglycemia triggers harmful
effects on endothelial cell function, blood coagulation and body inflammation. Pharmacological and
lifestyle interventions show that adequate control of 2-hour postprandial glycemic rises in individuals
with diabetes or pre-diabetes can reverse the risk for and progression of cardiovascular disease and
metabolic morbidities.
Recent data has shown that as little as 15g of Salba added to a 50g carbohydrate portion of white bread
significantly lowers blood glucose iAUC and subjective appetite in the 2 hours postprandially. Since
this represents the addition of only 55kcal, the incorporation of Salba to high glycemic index meals
may prove to be a simple and effective measure to prevent postprandial hyperglycemia and the
resulting detrimental atherosclerotic changes. As well, past research suggests that the addition of only
55kcal of Salba to white bread significantly decreases subjective appetite, which in turn could reduce
overconsumption. However, equicaloric test meals were not used. Here, we investigated the effects of
calorie-, protein- and fat-matched white bread controls versus Salba-enriched white breads to elucidate
possible factors that contributed to the previous findings, especially those concerning appetite.
In addition, Salba is commercially available in both whole grain and ground forms, yet any differences
in effects on postprandial glycemia and appetite have not been assessed. Few studies have investigated
the differences of oily grains or seeds in ground and whole form. In this study we compared the
effectiveness of both forms in reducing glycemic response and subjective appetite.
5.3.1 Participants
A total of 20 healthy individuals (8M; 12F; Age: 39.4 ± 3.4 years BMI 25.7 ± 2.4 kg/m2) participated
in the study. All participants were healthy, between 18-65 years old and clinically euthyroid with
normal hepatic and renal function. Those who were pregnant, suffered from gastrointestinal or
metabolic diseases or regularly ingested fiber supplements were excluded. All participants gave written
informed consent, and the study was approved by the St. Michael's Hospital Research Ethics Board.
5.3.2 Treatments
The test meals consisted of servings of bread (served with 250 millilitres of water) enriched with 7, 15
or 24 g of ground or whole Salba. The lowest dose, 7.3g, contains the American Heart Association's
daily minimum recommended intake of omega-3 fatty acids. The three calorie-, protein- and fat-
matched controls consisted of white bread baked with egg whites and margarine to match the three
doses of Salba. All 9 experimental breads contained 50g available carbohydrate and were prepared on-
site with a Black & Decker® All-In-One Pro™ Breadmaker (Towson, MD, USA)
Table 5.1 Nutritional Facts of Salba-enriched Breads
Intermediate Dose
Table 5.2 Nutritional Facts of Control Breads
Intermediate Dose
5.3.3 Experimental Design
The study consisted of a double-blind, placebo-controlled, randomized crossover design in which
subjects underwent nine 2.5-hour sessions separated by a washout period of at least 48 hours. Subjects
visited the Risk Factor Modification Centre in the morning after having fasted for 10-12 hours
overnight and engaging in normal eating and exercise habits the preceding day. They had an initial
finger prick blood sample taken and completed a subjective appetite questionnaire in the form of a
100mm visual analog scale (VAS). The experimental meal was then served and subjects were required
to finish the meal within 15 mins. Finger prick blood samples were taken at 15, 30, 45, 60, 90 and 120
mins postprandially. Subjects also completed the appetite questions at these times. For the duration of
the study session, subjects remained at the clinic and were instructed not to eat or drink and to keep
physical activity to a minimum.
5.3.4 Blood Glucose Analysis
Capillary blood samples were obtained using sterile single-use lancets. Two to three drops of capillary
blood were collected in plastic flat-bottomed 5ml tubes with a push cap containing a small amount of
sodium fluoride and potassium oxalate as an anticoagulant and preservative. The blood samples were
placed in a -20ºC freezer for a maximum of 3 days until the analysis of whole blood glucose. Capillary
blood glucose was measured by the glucose oxidase method using a YSI 2300 STAT Plus Glucose &
Lactate Analyzer™ (Yellow Springs Instruments, Yellow Springs, OH, USA).
5.3.5 Measurement of Appetite
Four unipolar visual analogue scales were used for each appetite assessment. For each of the four
questions, subjects indicated their response by drawing a vertical line along a 100 mm horizontal line
that was anchored by two statements. The questions were "How strong if your desire to eat?", "How
hungry do you feel?", "How full do you feel?" and "How much do you think you could eat now?"
Subjects' ratings were converted to numerical values by measuring the distance between the left anchor
and their drawing. A combined appetite score for each appetite assessment was computed with the
following formula:
5.3.6 Study Variables
The primary variables for this study were the mean incremental AUC's for blood glucose and the mean
incremental change from baseline in blood glucose at 15, 30, 45, 60, 90 and 120 min postprandially.
The secondary variables were the mean iAUC's of appetite scores and the incremental change in
appetite from baseline at the same time points.
5.3.7 Statistical Analysis
Statistical analysis was performed using NCSS 2000 (NCSS, Kaysville, UT) and SPSS release 16.0
(SPSS Inc., Chicago, IL). Incremental areas under the blood glucose response curves and appetite
scores (iAUC) were calculated by applying the trapezoid rule and analyzed by one-way ANOVA using
the Neuman-Keuls method to adjust for multiple comparisons. We performed Pearson correlations and
conducted linear regression analysis to determine dose-response relationships for glucose and appetite
iAUCs. Two-factor ANOVA was performed on blood glucose levels and appetite scores at each time
point to test for a time x treatment interaction. When an interaction was statistically significant, a one-
factor ANOVA using a GLM procedure was followed by Newman-Keuls post hoc test to identify mean
differences among treatments at each time of measurement. Significance was set at p < 0.05.
5.4.1 Postprandial Blood Glucose Responses
The means of glucose iAUC's for all nine experimental breads are shown in Table 5.3 and Graph 5.1.
Glucose iAUC was negatively correlated to dose of Salba of ground and whole forms combined
(
r=0.17, p=0.035). There was no significant difference found between the iAUC's of all three calorie-
matched control breads (HC = 154.6 ±13.0, IC = 154.3 ±17.1 and LC = 160.8 ± 16.8 min.mmol/L, p =
0.87). The iAUC's for the HG, IG and LG were 126.5 ± 12.7, 142.7 ± 15.2 and 157.9±16.7
mmol.min/L, respectively. The iAUC's for the HW, IW and LW were 130.5 ± 15.6, 139.2 ± 15.4 and
151.6±16.1 mmol.min/L, respectively.
Table 5.3 Glucose iAUC (min.mmol/L) values (± sem) for all Experimental Breads
Graph 5.1 The Effects of Salba on the Blood Glucose iAUC of all Salba Breads (n=20)
m 180
.m 160
When comparing the breads within each dose group, it was found that there was a difference between
the AUC's of the three high dose breads only. One-way ANOVA of the iAUC's of the high dose
breads indicated a significant difference between both HG and HW with respect to HC (p = 0.021).
Analysis by two-factor ANOVA identified a time by treatment interaction within the high dose breads
(p=0.024). However, 1-way ANOVA revealed no significant differences in high dose incremental
glucose values at any of the time points.
Graph 5.2 The Effects of the High Dose Breads on Blood Glucose iAUC (n=20)
in 120
(m
C 100
p=0.02; 1-way ANOVA, Newman-Keuls
A mild dose-response effect was seen within the ground breads, with the iAUC of HG significantly
lower than LG (p=0.033) (data not shown). In regards to the whole breads, IW tended to be
significantly lower than LW but did not reach significance (p= 0.06). Analysis of incremental glucose
values also indicated a dose-response effect. Within the 3 doses of ground breads, the HG and IG were
significantly less than LG at 60 mins postprandially (data not shown).
5.4.2 Appetite Scores
Appetite scores were measured via four visual analog scales (VAS), and a mean combined score was
calculated for each of the following time points: fasting, 15, 30, 45, 60, 90 and 120 mins postprandial.
No correlation was found between appetite score and dose of Salba (p=0.29). The appetite score
iAUC's were not significantly different within each dose (and thus within equicaloric breads), as
p=0.29, 0.27 and 0.48 for high, intermediate and low doses, respectively. See Table 5.5 for iAUC
values and Table 5.6 for percent reductions in appetite of Salba breads.
Table 5.4 Appetite iAUC's of Experimental Breads
Intermediate Dose
-4258 ± 689.9 mm.min
-4438 ± 612.6 mm.min
-4315 ± 599.6 mm.min
-5064 ± 669.7 mm.min
-4662 ± 594.8 mm.min
-4274 ± 666.3 mm.min
-4420 ± 691.2 mm.min
-4049 ± 625.9 mm.min
-3827 ± 675.0 mm.min
Table 5.5 Percent Reductions of Appetite iAUC's of Salba Breads with Respect to Calorie-, protein- and
fat-matched Control Breads
Intermediate Dose
However, two-factor ANOVA of the appetite scores of the high dose breads revealed a significant time
x treatment interaction (p = 0.032). Intermediate whole bread had a lower mean appetite score than
intermediate control bread at 120 mins postprandially, yet this did not reach significance (p = 0.056)
Results indicated that Salba is most effective at reducing postprandial glycemia at the highest dose,
which was 24g per 50g available carbohydrate. Perhaps the differences in nutrient composition
between the other doses of test and control breads were too small to be seen with this sample size.
Nonetheless, a significant linear correlation between Salba dose and postprandial glycemia was found
(
r=0.17, p=0.035).
The proportion of fat and protein in Salba cannot completely account for the iAUC reduction for both
HW and HG compared to HC as they were constant across the treatments, yet postprandial glycemia
differed. Put differently, adding Salba to white bread does not reduce postprandial glycemia solely
because of the added fat and protein. It can thus be concluded that there is some aspect of the grain
that is partly responsible for its ability to lower postprandial glycemia other than its protein and fat
contents. It is possible that the specific types of fat and protein in Salba may act synergistically to
lower blood glucose. As well, the amount of fiber and/or the proportion of soluble vs. insoluble fiber
are possible contributing factors, as is the degree of saturation of the fat. Future acute studies could
employ controls that contain the same type of protein, same type of fat and/or an equal amount of fiber
as Salba to adjust for these factors.
However, past research has demonstrated that the differences in energy from fat and protein in our
experimental breads were insufficient to elicit a difference in postprandial glycemic response. Studies
investigating the addition of fat to a carbohydrate meal use a substantially greater amount of fat than
the present study. For instance, Gatti
et al. added 35g of fat to 75g carbohydrate to produce 70-80%
reductions in glucose iAUC, while Collier
et al. reported reductions of 10-20% when 37.5g of fat were
added to 75g of carbohydrate [120, 127]. Owen and Wolever added 0, 5, 10, 20 and 40g of fat in the
form of non-hydrogenated margarine and found that although the lowest amounts of fat lowered
postprandial glycemia to a proportionally larger degree, only the highest amount of fat elicited a
significant decrease in iAUC [118].
The studies investigating the addition of protein to carbohydrate meals show that protein does not elicit
a consistent effect on postprandial glycemia. Some studies conclude that added protein lowers the
glycemic response, while others show no difference [134]. One even reported an increase in glucose
AUC when protein was added to a carbohydrate and fat meal [135]. Yet, when protein is ingested alone
it has no or very little effect on blood glucose in people with or without Type 2 diabetes [136, 137].
Thus, it is very unlikely that the observed differences in iAUC in our study were due to the small
variations in calories from protein and fat. Again, we speculate that an aspect of Salba such as its high
soluble fiber content was responsible for lowering postprandial glycemia.
The reductions in postprandial glycemia were not as pronounced as the first study. We speculate that
the addition of fat and protein to the control meals in the present study reduced the blood glucose
response compared to white bread control to such an extent that the differences in effects of Salba and
control breads were noticeably smaller. Therefore, the proportion of fat and/or protein did have an
effect on postprandial glycemia; however, it was not completely responsible for the changes in blood
glucose response.
To our knowledge, there have been very few studies examining the effects of oily foods such as flax
and sesame seed in ground and whole forms on postprandial glycemia. One study assessed postprandial
glycemia elicited by white bread enriched with flax. Only ground flax was used, however [189]. Most
nutrition experts recommend the consumption of ground flax as opposed to whole flax to maximize the
its health benefits [190]. They contend that with its tough husk, whole flax can pass through the body
undigested, and thus fewer nutrients are absorbed. Salba, on the other hand, has a very permeable husk
which in theory would allow digestive enzymes easier access to its nutrients. There has also been
research determining the effects of different food forms of legumes on postprandial glycemia; however,
given their relatively high starch contents, we feel that this research is not applicable to Salba [191].
Regarding research on other whole grains and postprandial glycemia, the typical effects of whole grain
particle size were not seen with Salba. Extensive research demonstrates that a grain's ability to lower
postprandial glycemia is positively correlated with increasing particle size [192]. Researchers theorize
that since bigger particles have a smaller surface-to-volume ratio, their contents are less accessible to
digestive enzymes. As such, the contents of bigger particles are released and digested more slowly,
leading to a flattened and/or prolonged glucose response [193].
However, we expected the opposite effect with Salba, and we attribute this to the grain's extremely low
carbohydrate content and high content of components capable of reducing postprandial glycemia.
Firstly, carbohydrate is the primary macronutrient responsible for raising blood glucose, but there is a
negligible amount of carbohydrate in the grain. In addition, the contents of the intact Salba grain are
mainly fat, protein and fiber – all components reported to lower glycemic responses. Therefore adding
Salba to a carbohydrate meal contributes only an inconsequential amount of carbohydrate while
providing components known to lower glycemia. When whole Salba is consumed, we would expect
some interaction between digestive enzymes and its contents because the grain's soft husk is easily
broken, which results in a modest reduction of blood glucose. In contrast, there would be a much
greater surface area for enzyme-food interactions with ground Salba, allowing the contents to interact
with digestive enzymes more easily. Thus, it would be expected that ground Salba would lower
postprandial glycemia to a greater extent than whole Salba.
With regards to appetite, it appears that Salba has a very modest ability to lower subjective appetite.
The iAUC's for appetite scores did not differ within each dose. However, there is evidence to suggest
that whole Salba bread is more satiating than control bread. When the IG, IW and IC were analyzed
together by 2-way ANOVA, it was found that IW tended to have a lower mean appetite score than IC
at 120 mins postprandially (p =0.056). It is possible that the amount of calories from Salba and
differences in calories tested in this thesis were too little to see greater effects on satiety. Future studies
could employ larger differences in Salba to determine the extent of its appetite-lowering abilities.
One possible explanation for why whole Salba elicited lower appetite than control and why the ground
form did not is the difference in effort during its consumption. The increased "crunchiness" of the
whole Salba requires more force during mastication, which in turn may create greater feelings of
satiety. Haber
et al, 1977 noted that apple pieces reduced hunger to a greater extent than an equicaloric
amount of apple purée and suggested that the disruption of fiber increases subjective appetite, partly
due to the less mastication required [194]. In contrast, a high fiber food requires more mastication,
which results in greater satiety. Chewing solid or viscous food as has been reported to increase satiety
signals and hormones compared to simply swallowing liquid, which requires no chewing [195].
6 General Discussion and Conclusion
This thesis sought to investigate the effectiveness of the novel oily whole grain
Salvia hispanica L.
(Salba) on postprandial glycemia and subjective appetite via two acute studies. Study 1 evaluated the
effects of three doses of ground Salba added to white bread on the postprandial glycemic response and
subjective appetite. Results showed that Salba reduced both measures in a dose-dependent manner in
healthy individuals. Study 2 examined the effects of whole versus ground Salba on postprandial
glycemia and subjective appetite. We demonstrated that the ground and whole forms are approximately
equal in effectiveness. As well, Study 2 sought to determine if Salba's protein and fat contents are
responsible for its ability to lower postprandial glycemia and appetite. We thus employed equicaloric
protein- and fat-matched white bread controls by adding margarine and egg white to white bread.
6.2 The Effects of Salba on Postprandial Glycemia
The addition of 7, 15 and 24g of Salba to white bread containing 50g available carbohydrate lowered
postprandial glycemia in a dose-dependent fashion. Linear regression analysis showed a significant
dose-response decrease in blood glucose incremental area under the curve with increasing doses of
Salba (iAUC). In other words, blood glucose iAUC was negatively correlated with dose of Salba (
r=-
0.47). The amount of Salba therefore accounts for approximately 22% of the variation in postprandial
glycemia. For the ground Salba-enriched breads, glucose iAUC reductions of 44%, 25% and 13% were
seen for the high, intermediate and low doses compared to white bread, respectively. Only the
reductions for the high and intermediate doses were significant. The incremental blood glucose level
was significantly lower for the high dose compared to control at 30 mins and for both high and
intermediate doses at 60 mins postprandially.
For Study 2, each dose category was analyzed separately. Glucose iAUC was negatively correlated to
dose of Salba of ground and whole forms combined (
r=0.17). It was found that there was a significant
difference between iAUC's for the three high dose breads only. Incremental blood glucose levels to
HG and HW were significantly lower than that of HC (p=0.024). Further analysis by 1-way ANOVA
yielded no significant differences for incremental glucose values within each dose category.
Data from Study 2 suggest that the ground form of Salba is marginally more effective at reducing the
postprandial glycemic response than the whole form. Although 1-way ANOVA of glucose values
indicated no significant differences within each dose, 2-way ANOVA revealed that the glucose values
for the HG and HC differed significantly, with that of the former being lower. We cannot dismiss the
possibility that these results occurred due to random variation, and thus suggest future studies with
greater doses of ground and whole Salba.
6.3 The Effects of Salba on Subjective Appetite
The results of our studies suggest that Salba has a very modest ability to lower subjective appetite. In
Study 1, linear regression analysis of appetite demonstrated a significant dose-response decrease in
appetite iAUC with increasing doses of Salba (
r=-0.3, p=0.043). Dose of Salba can therefore account
for approximately 9% of the variation in appetite iAUC. The appetite score iAUC for low, intermediate
and high doses were lower than control by 54.0%, 62.1% and 66.8%, respectively. Although these
reductions occurred in a dose-dependent manner, no significant differences in satiety score iAUC's
were found between high, intermediate and low Salba breads. Appetite scores were significantly higher
for the high dose at 90 mins and for the intermediate dose at 90 and 120 mins.
These results were unexpected and appear to be counterintuitive, as the intermediate dose contains
fewer calories than does the high dose. It is therefore possible that there exists an optimal amount of
Salba to add to a carbohydrate meal in order to achieve maximum satiety. More likely however, is that
there is an optimal ratio of calories from Salba to total calories from the entire carbohydrate meal to
elicit the greatest reduction in appetite. Both scenarios would require producing the optimal
postprandial glycemic response for lowering appetite: a rise in blood glucose sufficiently low and
prolonged to prevent hyperglycemia, while maintaining a concentration high enough to trigger satiety
Results from Study 2 also provide evidence for Salba's modest ability to decrease appetite. Although
appetite score iAUC's were not significantly different within each dose (and thus within equicaloric
breads), intermediate whole bread tended to have a lower appetite score than intermediate control bread
at 120 mins (p = 0.056). Unlike Study 1, however, no linear correlation was found between appetite
score and dose of Salba.
It is noteworthy that such a small addition of calories in the form of Salba reduced subjective appetite.
As mentioned previously, the majority of appetite studies require differences of more than 100kcal for
an effect to be apparent. The results of the intermediate breads suggest that Salba could be more
satiating than the same macronutrient proportion and amount of calories as another food source (in this
study, white bread baked with egg white and margarine).
6.4 Relevance of Findings
Epidemiological evidence suggests that postprandial glycemic rises are an independent and modifiable
predictor of Type 2 diabetes and cardiovascular disease. The whole grain Salba has the ability to lower
postprandial glycemia when incorporated into a carbohydrate-rich meal, which represents a possible
method in reducing risk factors for such diseases. Salba may even prove to be sufficiently effective in
some cases to replace medications designed to lower postprandial glycemia, such as acarbose. Our
finding that one gram of Salba decreased glucose iAUC by approximately 2% could be useful in
determining the amounts required per carbohydrate-rich meal.
Our data suggest that consumption of Salba may also target another possible risk factor for the
development of Type 2 Diabetes and cardiovascular disease: obesity. Salba has a fairly small ability to
lower subjective appetite, which we hypothesize would lead to less energy consumption. At 120 mins
postprandially, the appetite score of the intermediate whole Salba bread was tended to be lower than
intermediate control. When added to a portion of white bread, whole Salba may increase satiety more
than an equal amount of calories from another source. Thus, adding Salba to one's diet could be a
valuable and simple dietary modification for individuals with excess bodyweight. Most people
attempting to lose weight consume calorie-restricted diets. Unfortunately, many of these diets lack one
or more essential macro- or micronutrients, especially unsaturated fats, minerals and proteins. Salba
would be an ideal complementary food for a calorie-restricted weight loss diet as its nutrient-dense
composition would help alleviate any deficiencies.
Additionally, this thesis has clarified the effects of a range of Salba doses and the efficacy of both
ground and whole forms. These results could be applied to the manufacturing of baked products with
oily seeds such as flax and sunflower.
The findings of this project should be considered in light of the limitations pertaining to the study
concept. Equicaloric breads were not used in Study 1, rendering it difficult to compare the subjective
appetite results from each experimental bread. This issue was addressed in Study 2 with the use of a
calorie-matched white bread control for each dose of Salba. However, the addition of fat and protein to
the white bread controls caused the blood glucose responses to be lower than white bread alone since
both macronutrients have been shown to reduce postprandial glycemia. In doing so, we were only
examining the ability of the fiber and mineral contents of the Salba to lower postprandial glycemia.
This may thus account for the smaller decreases in postprandial glycemia in Study 2 compared to Study
Additionally, a slight difference in procedure between Studies 1 and 2 may also have contributed to the
fact that the decrease in blood glucose response was less drastic in the latter. For Study 1, bread baked
less than 48 hours previously was given to participants. In contrast, bread was frozen and thawed in
Study 2, due to temporal and logistical restraints. Research has suggested than freezing and thawing
fiber-rich foods can destruct the physical structures of the fibers, resulting in diminished effects on
postprandial glycemia [196].
With respect to both studies, appetite was measured via visual analog scales, and thus represents solely
subjective feelings. Past research has shown that desire and intentions to eat do not consistently
correlate with actual food intake [141, 177, 197]. Thus, our research would have benefited from
measuring subsequent food intake via an
ad libitum meal or 24-hour food diaries. Furthermore, we
could not adjust for differences in density of the experimental breads. The breads enriched with ground
Salba had a denser texture than both the whole and control breads. The calorie- and macronutrient-
matched control breads had a significantly fluffier texture, which we attribute to the addition of egg
whites. Burton and Lightowler reported that denser breads elicited greater feelings of satiety and lower
peak glucose levels than less dense breads [198]. Yet research by Rolls
et al. has demonstrated that
food volume is negatively correlated to subsequent food intake [167, 168]. As these findings are
contradictory, it is difficult to suggest how the different bread densities in our study affected satiety. As
well, the density of a food is by nature known by the individual consuming it; thus, equalizing densities
may not be relevant.
Lastly, it was impossible to keep the whole Salba breads completely double-blinded. Participants can
obviously perceive differences between the breads with whole Salba versus those without, and it is
possible that the perception of whole Salba grains in the bread may influence subjective appetite. It
would be difficult, however, to distinguish between low, intermediate and high dose whole Salba
breads. As well, the knowledge of the whole Salba bread's texture is a legitimate characteristic that
may affect appetite and is therefore simply another property of whole Salba that promotes appetite
6.6 Future Research
This thesis paves the way for many future avenues of research. Further investigations should address
the limitations of this project and the research questions that arose from it. The effects of Salba on
appetite could be more thoroughly examined with acute studies measuring objective satiety with an
ad
libitum meal and/or 24-hr food diaries. In addition, acute studies measuring key regulatory gut
hormones such as CCK and GLP-1 would be valuable in determining the metabolic effects of this grain
and elucidating the mechanisms by which it increases satiety and decreases food intake.
Our research demonstrated that the amount of protein and fat in Salba-enriched bread is not an
important factor in reducing postprandial glycemia and appetite. It may be Salba's physical structure
and/or its fiber, antioxidant or unsaturated fat contents, among others. Future studies should focus on
matching the content of such components in control meals to isolate which nutrients are responsible for
the health benefits seen in this study.
The results from our research dovetail with those of Vuksan
et al.'s research as they both demonstrated
Salba's potential to reduce possible CVD risk factors. Further studies should be undertaken to
determine if these results are replicable, and if so, examine other criteria such as the daily amount of
Salba required and effects on additional risk factors. Our research provides another mechanism by
which Salba can promote weight loss; namely, by lowering appetite. As such, randomized, parallel
studies could examine both of these effects by measuring anthropometrics and food intake to explore
this issue in further detail.
Furthermore, Salba may directly affect body composition by decreasing fat mass. Research by both
Chicco and Vertommen suggest that consumption of Salba has the potential to lower adiposity. Chicco
et al. studied the effects of a diet including
Salvia hispanica L. in rats after a 5-month high-sucrose diet
[5]. They found that visceral adiposity (epididymal and retroperitoneal fat) was significantly decreased
relative to body weight for the rats on the
Salvia hispanica L. diet versus rats on a maize diet. In a
fluidity study with healthy individuals, Vertommen
et al. demonstrated a significant decrease in waist
circumference while maintaining body weight after merely 50g Salba per day for one month [20].
Thus, given the significant evidence showing that Salba may specifically target the loss of fat mass,
future long-term studies analyzing body composition are warranted.
This thesis has helped determine a range of optimal amounts of Salba to consume and the efficacy of
both ground and whole forms. Further research could be performed with Salba in ground and whole
forms in other solid foods and beverages to investigate whether food forms have similar health effects
in different food matrices. Salba is sometimes consumed as a drink in South America and some North
American companies have expressed interest in developing a beverage.
In conclusion, further studies are needed to elucidate the mechanisms by which Salba reduces
postprandial glycemia and appetite, and if doing so reduces risk factors for Type 2 diabetes and CVD.
Metabolic studies will further our understanding of the effects of Salba on risk factors such as glucose
and insulin response, blood lipids, clotting factors and low-grade body inflammation. As well,
examining the effects of Salba on satiety and body weight regulation is of particular interest as a
preventative measure in these diseases.
The oily whole grain Salba has the ability to significantly reduce postprandial glycemia when added to
a carbohydrate meal. Salba-enriched white bread also elicits lower, non-significant ratings of subjective
appetite than equicaloric protein- and fat-matched white bread. Prevention of both postprandial
hyperglycemia and overconsumption may reduce the risk of diseases such as Type 2 diabetes and CVD
in the long-term. Incorporating Salba in the diet could be both an excellent method of attaining the
recommended daily servings of fiber and omega-3 fatty acids and of obtaining essential nutrients
lacking from calorie-reduced diets.
Ayerza, R. and W. Coates,
Chia: Rediscovering a Forgotten Crop of the Aztecs. 2005, Arizona,
USA: University of Arizona Press.
Riccardi, G., B. Capaldo, and O. Vaccaro,
Functional foods in the management of obesity and
type 2 diabetes. Current Opinion in Clinical Nutrition & Metabolic Care, 2005.
8(6): p. 630-5.
Anonymous,
Bibliography. Current world literature. Functional foods. Current Opinion in
Clinical Nutrition & Metabolic Care, 2007.
10(6): p. 778-90.
Vuksan, V., et al.,
Supplementation of conventional therapy with the novel grain Salba (Salvia
hispanica L.) improves major and emerging cardiovascular risk factors in type 2 diabetes:
results of a randomized controlled trial. Diabetes Care, 2007.
30(11): p. 2804-10.
Chicco, A., et al.,
Dietary chia seed (Salvia hispanica L.) rich in a-linolenic acid improves
adiposity and normalises hypertriacylglycerolaemia and insulin resistance in dyslipaemic rats.
British Journal of Nutrition, 2008: p. 1-10.
Vuksan, V.,
Salba vs. Chia. 2006: Toronto.
Anderson, J.W., et al.,
Whole grain foods and heart disease risk. Journal of the American
College of Nutrition, 2000.
19(3 Suppl): p. 291S-299S.
Anderson, J.W.,
Whole grains protect against atherosclerotic cardiovascular disease.
Proceedings of the Nutrition Society, 2003.
62(1): p. 135-42.
de Munter, J.S., et al.,
Whole grain, bran, and germ intake and risk of type 2 diabetes: a
prospective cohort study and systematic review. PLoS Medicine / Public Library of Science,
2007.
4(8): p. e261.
Jacobs, D.R., Jr, et al.,
Fiber from Whole Grains, but not Refined Grains, Is Inversely
Associated with All-Cause Mortality in Older Women: The Iowa Women's Health Study. Journal
of the American College of Nutrition J Am Coll Nutr, 2000.
19(90003): p. 326S-330.
Koh-Banerjee, P. and E.B. Rimm,
Whole grain consumption and weight gain: a review of the
epidemiological evidence, potential mechanisms and opportunities for future research.
Proceedings of the Nutrition Society, 2003.
62(1): p. 25-9.
Pereira A, J.D., Slattery M, Ruth K, Van Horn L, Hilner J & Kushi L,
The association of whole
grain intake and fasting insulin in a biracial cohort of young adults: the CARDIA study. CVD
Prevention, 1998.
1: p. 231-242.
Pereira, M.A., et al.,
Effect of whole grains on insulin sensitivity in overweight
hyperinsulinemic adults. American Journal of Clinical Nutrition, 2002.
75(5): p. 848-55.
Steffen, L.M., et al.,
Whole grain intake is associated with lower body mass and greater insulin
sensitivity among adolescents. American Journal of Epidemiology, 2003.
158(3): p. 243-50.
Jacobs, D.R., et al.,
Fiber from whole grains, but not refined grains, is inversely associated with
all-cause mortality in older women: the Iowa women's health study. Journal of the American
College of Nutrition, 2000.
19(3 Suppl): p. 326S-330S.
Salba: Nature's Most Powerful Whole Food. 2007, SourceSalba.
Nutrient Composition of Salba Seeds. 2001, Dr. David Jenkins Lab [Report, Personnal
Communication], University of Toronto: Toronto.
Wu, X., et al.,
Lipophilic and hydrophilic antioxidant capacities of common foods in the United
States. Journal of Agricultural & Food Chemistry, 2004.
52(12): p. 4026-37.
Beck, L.,
It's time to cut the fat and go for the whole grain, in
Globe and Mail. 2008: Toronto.
Vertommen, J., et al.
Efficacy and Safety of 1 Month Supplementation of SALBA (Salvia
Hispanica Alba)Grain to Diet of Normal Adults on Body Parameters, Blood Pressure, Serum
Lipids,Minerals Status and Haematological Parameters. Results of a Pilot Study. in
24th
International Symposium on Diabetes and Nutrition. 2006. Salerno, Italy.
USDA Nutrient Data Laboratory. 2008, United States Department of Agriculture.
NutritionData.com,
Nutrition Data, Condé Nast Publications.
Faulkner, H.,
Nutritional Labeling Analysis (Salba seeds). 2001, [Report, Personal
Communication] University of Guelph: Guelph.
Fatty Acid Profile of Salba Seeds. 2001, Dr. Steven Cunnane's Lab, University of Toronto:
Fiber Content of Salba Seeds. 2001, [Report, Personal Communication] Maxxan Analytics.
Welcome to AACCNet. 2008, American Association of Cereal Chemists.
Sungsoo Cho, S.,
Handbook of Dietary Fiber. 2001, Maryland: CRC Press. 894.
Schneeman, B. and T. J,
Modern Nutrition in Health and Disease. 8 ed. ed, ed. M. Shike, M.
Shills, and J. Olsen. 1994, Philadelphia: Lea & Febiger.
Physiological effects and health consequences of dietary fiber, ed. S.M. Pilch, Bethesda, Md.
(USA): Life Sciences Research Office, Federation of American Societies for Experimental
Dietary Reference Intakes Proposed Definition of Dietary Fiber: A Report of the Panel on the
Definition of Dietary Fibre and the Standing Committee on the Scientific Evaluation of Dietary
Reference Intakes Food and Nutrition Board. 2001, Institute of Medicine: Washington, D.C.
Vuksan, V., et al.,
Konjac-mannan (glucomannan) improves glycemia and other associated risk
factors for coronary heart disease in type 2 diabetes. A randomized controlled metabolic trial.
Diabetes Care, 1999.
22(6): p. 913-9.
Jenkins, D.J., et al.,
Improved glucose tolerance four hours after taking guar with glucose.
Diabetologia, 1980.
19(1): p. 21-4.
Anderson, J.W.,
Dietary fiber, lipids and atherosclerosis. American Journal of Cardiology,
1987.
60(12): p. 17G-22G.
Ausman, L.M.,
Fiber and colon cancer: does the current evidence justify a preventive policy?
Nutrition Reviews, 1993.
51(2): p. 57-63.
Lands, W.,
Biochemistry and physiology of n-3 fatty acids. FASEB J., 1992.
6(8): p. 2530-
Lunn, J. and H.E. Theobald,
Briefing Paper: The health effects of dietary unsaturated fatty
acids. 2006, Brisith Nutrition Foundation: London. p. 178-224.
Stark, A.H., M.A. Crawford, and R. Reifen,
Update on alpha-linolenic acid. Nutrition Reviews,
2008.
66(6): p. 326-332.
Simopoulos, A., A. Leaf, and N. Salem Jr.
Statement on the essentiality of and recommended
dietary intakes for n-6 and n-3 fatty acids. 2000. The Center for Genetics, Nutrition and Health.
Bazinet, R.P., H. Douglas, and S.C. Cunnane,
Whole-body utilization of n-3 PUFA in n-6
PUFA-deficient rats. Lipids, 2003.
38(2): p. 187-9.
DeFillipis, A. and L. Sperling,
Understanding omega-3's. American Heart Journal, 2006.
Burdge, G.C. and S.A. Wootton,
Conversion of alpha-linolenic acid to EPA, DPA and DHA in
your women. British Journal of Nutrition, 2002.
88: p. 411-420.
Burdge, G.C., A.E. Jones, and S.A. Wootton,
Eicosapentanaeoic and docosapentanaenoic acid
are the principal products of alpha-linolenic acid metabolism in young men. British Journal of
Nutrition, 2002.
88: p. 355-363.
Bemelmans, W.J., et al.,
Increased alpha-linolenic acid intake lowers C-reactive protein, but
has no effect on markers of atherosclerosis. European Journal of Clinical Nutrition, 2004.
58(7): p. 1083-9.
Kim, H.K., S. Choi, and H. Choi,
Suppression of hepatic fatty acid synthase by feeding alpha-
linolenic acid rich perilla oil lowers plasma triacylglycerol level in rats. Journal of Nutritional
Biochemistry, 2004.
15(8): p. 485-92.
Brody, T.,
Nutritional Biochemistry. 2 ed. 1999, New York: Academic Press. 1006.
Hopkins, D.T. and F.H. Steinke,
Effect of Water of Hydration on the Measurement of the
Protein Efficiency Ratio of Casein and Soybean Protein with Rats. J. Nutr., 1976.
106(10): p.
Prior, R.L. and G. Cao,
In vivo total antioxidant capacity: comparison of different analytical
methods1. Free Radical Biology and Medicine, 1999.
27(11-12): p. 1173-1181.
Pellegrini, N., et al.,
Total antioxidant capacity of plant foods, beverages and oils consumed in
Italy assessed by three different in vitro assays. Journal of Nutrition, 2003.
133(9): p. 2812-9.
Bruckdorfer, K.R.,
Antioxidants and CVD. Proceedings of the Nutrition Society, 2008.
67(2): p.
Beaglehole, R., et al.,
Decreased blood selenium and risk of myocardial infarction.
International Journal of Epidemiology, 1990.
19(4): p. 918-22.
Riemersma, R.A., et al.,
Plasma antioxidants and coronary heart disease: vitamins C and E,
and selenium. European Journal of Clinical Nutrition, 1990.
44(2): p. 143-50.
Gresch, E.E.M.D.,
Vitamin E Consumption and the Risk of Coronary Disease in Women.
Journal of Occupational Medicine November, 1993.
35(11).
Gresch, E.E.M.D.,
Vitamin E Consumption and the Risk of Coronary Heart Disease in Men.
Journal of Occupational Medicine November, 1993.
35(11).
Stampfer, M.J., et al.,
Vitamin E consumption and the risk of coronary disease in women.[see
comment]. New England Journal of Medicine, 1993.
328(20): p. 1444-9.
Rimm, E.B., et al.,
Vitamin E consumption and the risk of coronary heart disease in men.[see
comment]. New England Journal of Medicine, 1993.
328(20): p. 1450-6.
Yochum, L.A., A.R. Folsom, and L.H. Kushi,
Intake of antioxidant vitamins and risk of death
from stroke in postmenopausal women.[see comment]. American Journal of Clinical Nutrition,
2000.
72(2): p. 476-83.
Stephens, N.G., et al.,
Randomised controlled trial of vitamin E in patients with coronary
disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet March, 1996.
347(9004): p.
Hennekens, C.H., et al.,
Lack of effect of long-term supplementation with beta carotene on the
incidence of malignant neoplasms and cardiovascular disease.[see comment]. New England
Journal of Medicine, 1996.
334(18): p. 1145-9.
Gissi-Prevenzione Investigators,
Dietary Supplementation With n-3 Polyunsaturated Fatty
Acids and Vitamin E After Myocardial Infarction: Results of the Gissi-Prevenzione Trial.
Journal of Cardiopulmonary Rehabilitation March/April, 2000.
20(2): p. 131.
Gissi-Prevenzione Investigators,
Dietary supplementation with n-3 polyunsaturated fatty acids
and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet
August, 1999.
354(9177): p. 447-455.
Salba: a superior source of antioxidants, Salba Group.
American Diabetes, A.,
Postprandial blood glucose. American Diabetes Association.[see
comment]. Diabetes Care, 2001.
24(4): p. 775-8.
American Diabetes, A.,
Diagnosis and classification of diabetes mellitus. Diabetes Care, 2005.
28 Suppl 1: p. S37-42.
Alberts, B., et al.,
Molecular Biology of the Cell. 4 ed. 2002, New York: Garland Science.
Ferraris, R.P.,
Dietary and developmental regulation of intestinal sugar transport. Biochemical
Journal, 2001.
360(Pt 2): p. 265-76.
Raben, A., et al.,
Decreased postprandial thermogenesis and fat oxidation but increased
fullness after a high-fiber meal compared with a low-fiber meal. American Journal of Clinical
Nutrition, 1994.
59(6): p. 1386-94.
Murray, R.K., et al.,
Harper's Illustrated Biochemistry. 26 ed ed. 2003, New York, USA:
McGraw-Hill companies.
DeFronzo, R.A.,
Pathogenesis of type 2 diabetes mellitus. Medical Clinics of North America.
88(4): p. 787-835.
Dietary Guidelines for Americans. 2006, US Department of Health and Human Services.
Aroda, V.R. and R.R. Henry,
Incretin hormones in diabetes and metabolism. 2004, Medscape.
Giugliano, D., A. Ceriello, and K. Esposito,
Glucose metabolism and hyperglycemia. American
Journal of Clinical Nutrition, 2008.
87(1): p. 217S-222S.
Brownlee, M.,
Biochemistry and molecular cell biology of diabetic complications. Nature,
2001.
414(6865): p. 813-20.
Unwin, N., et al.
Impaired glucose tolerance and impaired fasting glycemia: the current status
on definition and intervention. in
International Diabetes Federation IGT/ IFG Consensus
Statement. Report of an Expert
Consensus Workshop 1-4. 2001. Stoke Poges, UK.
Decode Study Group, t.E.D.E.G.,
Glucose tolerance and cardiovascular mortality: comparison
of fasting and 2-hour diagnostic criteria. Archives of Internal Medicine, 2001.
161(3): p. 397-
Coutinho, M., et al.,
The relationship between glucose and incident cardiovascular events. A
metaregression analysis of published data from 20 studies of 95,783 individuals followed for
12.4 years.[see comment]. Diabetes Care, 1999.
22(2): p. 233-40.
Hanefeld, M., et al.,
Risk factors for myocardial infarction and death in newly detected
NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia, 1996.
39(12): p.
Chiasson, J.L., et al.,
Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM
randomised trial.[see comment]. Lancet, 2002.
359(9323): p. 2072-7.
Chiasson, J.L., et al.,
Acarbose treatment and the risk of cardiovascular disease and
hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial.[see
comment]. Jama, 2003.
290(4): p. 486-94.
Definition and diagnosis of diabetes mellitus
and intermediate hyperglycemia: report of a WHO/IDF consultation. 2006, World Health
Organization: Geneva.
Yorek, M.A.,
The role of oxidative stress in diabetic vascular and neural disease. Free Radical
Research, 2003.
37(5): p. 471-80.
Brownlee, M.,
The pathobiology of diabetic complications: a unifying mechanism. Diabetes,
2005.
54(6): p. 1615-25.
Ceriello, A., et al.,
Meal-induced oxidative stress and low-density lipoprotein oxidation in
diabetes: the possible role of hyperglycemia. Metabolism: Clinical & Experimental, 1999.
48(12): p. 1503-8.
Anderson, J.W., et al.,
Effects of psyllium on glucose and serum lipid responses in men with
type 2 diabetes and hypercholesterolemia. American Journal of Clinical Nutrition, 1999.
70(4):
Jenkins, D.J., et al.,
The glycaemic index of foods tested in diabetic patients: a new basis for
carbohydrate exchange favouring the use of legumes. Diabetologia, 1983.
24(4): p. 257-64.
Brouns, F., et al.,
Glycaemic index methodology. Nutrition Research Reviews June, 2005.
18(1): p. 145-171.
Wolever, T.M.S.,
The Glycaemic Index: A Physiological Classification of Dietary
Carbohydrate. 2006: Cabi Publishing. 272.
Kamphuis, M.M., M.S. Westerterp-Plantenga, and W.H. Saris,
Fat-specific satiety in humans
for fat high in linoleic acid vs fat high in oleic acid. European Journal of Clinical Nutrition,
2001.
55(6): p. 499-508.
Chau, C.F., Y.L. Huang, and M.H. Lee,
In vitro hypoglycemic effects of different insoluble
fiber-rich fractions prepared from the peel of Citrus sinensis L. cv. Liucheng. Journal of
Agricultural & Food Chemistry, 2003.
51(22): p. 6623-6.
Brand-Miller, J., et al.,
Low-glycemic index diets in the management of diabetes: a meta-
analysis of randomized controlled trials.[see comment]. Diabetes Care, 2003.
26(8): p. 2261-7.
Stevens, J., et al.,
Dietary fiber intake and glycemic index and incidence of diabetes in African-
American and white adults: the ARIC study. Diabetes Care, 2002.
25(10): p. 1715-21.
Hodge, A.M., et al.,
Glycemic index and dietary fiber and the risk of type 2 diabetes.[see
comment]. Diabetes Care, 2004.
27(11): p. 2701-6.
Schulze, M.B., et al.,
Glycemic index, glycemic load, and dietary fiber intake and incidence of
type 2 diabetes in younger and middle-aged women.[see comment]. American Journal of
Clinical Nutrition, 2004.
80(2): p. 348-56.
Kelly, S., et al.,
Low glycaemic index diets for coronary heart disease. Cochrane Database of
Systematic Reviews, 2004(4): p. CD004467.
Opperman, A.M., et al.,
Meta-analysis of the health effects of using the glycaemic index in
meal-planning. British Journal of Nutrition, 2004.
92(3): p. 367-81.
Aller, R., et al.,
Effect of soluble fiber intake in lipid and glucose levels in healthy subjects: a
randomized clinical trial. Diabetes Research & Clinical Practice, 2004.
65(1): p. 7-11.
McIntosh, M. and C. Miller,
A diet containing food rich in soluble and insoluble fiber improves
glycemic control and reduces hyperlipidemia among patients with type 2 diabetes mellitus.
Nutrition Reviews, 2001.
59(2): p. 52-5.
Nishimune, T., et al.,
Glycemic response and fiber content of some foods. American Journal of
Clinical Nutrition, 1991.
54(2): p. 414-9.
Edwards, C.A.,
The Physiological Effects of Dietary Fiber, in
Dietary Fiber in Health and
Disease, D. Kritchevsky and C. Bonfield, Editors. 1995, Eagan Press: St. Paul. p. 58-71.
Jenkins, D.J., et al.,
Unabsorbable carbohydrates and diabetes: Decreased post-prandial
hyperglycaemia. Lancet, 1976.
2(7978): p. 172-4.
Pick, M.E., et al.,
Oat bran concentrate bread products improve long-term control of diabetes:
a pilot study. Journal of the American Dietetic Association, 1996.
96(12): p. 1254-61.
Anderson, J.W.,
Cholesterol-Lowering Effects of Soluble Fiber in Humans, in
Dietary Fiber in
Health and Disease, D. Kritchevsky and C. Bonfield, Editors. 1995, Eagan Press: St. Paul. p.
Samra, R.A. and G.H. Anderson,
Insoluble cereal fiber reduces appetite and short-term food
intake and glycemic response to food consumed 75 min later by healthy men. American Journal
of Clinical Nutrition, 2007.
86(4): p. 972-9.
Schenk, S., et al.,
Different glycemic indexes of breakfast cereals are not due to glucose entry
into blood but to glucose removal by tissue. American Journal of Clinical Nutrition, 2003.
78(4): p. 742-8.
Weickert, M.O., et al.,
Cereal fiber improves whole-body insulin sensitivity in overweight and
obese women. Diabetes Care, 2006.
29(4): p. 775-80.
Weickert, M.O., et al.,
Impact of cereal fibre on glucose-regulating factors. Diabetologia, 2005.
48(11): p. 2343-53.
Jenkins, D.J., et al.,
Dietary fibres, fibre analogues, and glucose tolerance: importance of
viscosity. British Medical Journal, 1978.
1(6124): p. 1392-4.
Jenkins, D.J. and T.M. Wolever,
Slow release carbohydrate and the treatment of diabetes.
Proceedings of the Nutrition Society, 1981.
40(2): p. 227-35.
Benini, L., et al.,
Gastric emptying of a solid meal is accelerated by the removal of dietary fibre
naturally present in food. Gut, 1995.
36(6): p. 825-30.
Holt, S., et al.,
Effect of gel fibre on gastric emptying and absorption of glucose and
paracetamol. Lancet, 1979.
1(8117): p. 636-9.
Ximenes, H.M., et al.,
Propionate inhibits glucose-induced insulin secretion in isolated rat
pancreatic islets. Cell Biochemistry & Function, 2007.
25(2): p. 173-8.
Boillot, J., et al.,
Effects of dietary propionate on hepatic glucose production, whole-body
glucose utilization, carbohydrate and lipid metabolism in normal rats. British Journal of
Nutrition, 1995.
73: p. 241-251.
Asplund, J.M., et al.,
The effect of intragastric infusion of glucose, lipids or acetate on fasting
nitrogen excretion and blood metabolites in sheep. British Journal of Nutrition, 1985.
54(1): p.
Jenkins, D.J., W.M. Hunter, and D.V. Goff,
Ketone bodies and evidence for increased insulin
secretion. Nature, 1970.
227(5256): p. 384-5.
Vuksan, V., J. Blom, and A.S. Lee,
Measurement of viscosities of ground flax and Salba. 2007,
University of Toronto: Toronto.
Howarth, N.C., E. Saltzman, and S.B. Roberts,
Dietary fiber and weight regulation. Nutrition
Reviews, 2001.
59(5): p. 129-39.
Welch, I.M., et al.,
Duodenal and ileal lipid suppresses postprandial blood glucose and insulin
responses in man: possible implications for the dietary management of diabetes mellitus.
Clinical Science, 1987.
72(2): p. 209-16.
Gannon, M., et al.,
Effect of added fat on plasma glucose and insulin response to ingested
potato in individuals with NIDDM 10.2337/diacare.16.6.874. Diabetes Care, 1993.
16(6): p.
Owen, B. and T.M. Wolever,
Effect of fat on glycaemic responses in normal subjects: a dose-
response study. Nutrition Research, 2003.
23: p. 1341-1347.
Cunningham, K.M. and N.W. Read,
The effect of incorporating fat into different components of
a meal on gastric emptying and postprandial blood glucose and insulin responses. British
Journal of Nutrition, 1989.
61(2): p. 285-90.
Gatti, E., et al.,
Differential effect of unsaturated oils and butter on blood glucose and insulin
response to carbohydrate in normal volunteers. European Journal of Clinical Nutrition, 1992.
46(3): p. 161-6.
Richieri, G.V., et al.,
Fatty acid binding proteins from different tissues show distinct patterns of
fatty acid interactions. Biochemistry, 2000.
39(24): p. 7197-204.
Rasmussen, O., et al.,
Differential effects of saturated and monounsaturated fat on blood
glucose and insulin responses in subjects with non-insulin-dependent diabetes mellitus.
American Journal of Clinical Nutrition, 1996.
63(2): p. 249-53.
MacIntosh, C.G.H.S.H.B.-M., J.C.,
The degree of fat saturation does not alter glycemic,
insulinemic or satiety responses to a starch staple in healthy men. Journal of Nutrition, 2003.
133(8): p. 2577-80.
Joannic, J.L., et al.,
How the degree of unsaturation of dietary fatty acids influences the glucose
and insulin responses to different carbohydrates in mixed meals. American Journal of Clinical
Nutrition, 1997.
65(5): p. 1427-33.
Sigalet, D.L. and G. Martin,
Lymphatic absorption of glucose and fatty acids as determined by
direct measurement. Journal of pediatric surgery, 1999.
34(1): p. 39-43.
Gentilcore, D., et al.,
Effects of fat on gastric emptying of and the glycemic, insulin, and
incretin responses to a carbohydrate meal in type 2 diabetes. Journal of Clinical Endocrinology
& Metabolism, 2006.
91(6): p. 2062-7.
Collier, G. and K. O'Dea,
The effect of coingestion of fat on the glucose, insulin, and gastric
inhibitory polypeptide responses to carbohydrate and protein. American Journal of Clinical
Nutrition, 1983.
37(6): p. 941-4.
Collier, G.R., et al.,
The acute effect of fat on insulin secretion. Journal of Clinical
Endocrinology & Metabolism, 1988.
66(2): p. 323-6.
Dworatzek, P.D., R.A. Hegele, and T.M. Wolever,
Postprandial lipemia in subjects with the
threonine 54 variant of the fatty acid-binding protein 2 gene is dependent on the type of fat
ingested. American Journal of Clinical Nutrition, 2004.
79(6): p. 1110-7.
Ercan, N., M.C. Gannon, and F.Q. Nuttall,
Effect of added fat on the plasma glucose and
insulin response to ingested potato given in various combinations as two meals in normal
individuals. Diabetes Care, 1994.
17(12): p. 1453-9.
Gannon, M.C., et al.,
The insulin and glucose responses to meals of glucose plus various
proteins in type II diabetic subjects. Metabolism: Clinical & Experimental, 1988.
37(11): p.
Karamanlis, A., et al.,
Effects of protein on glycemic and incretin responses and gastric
emptying after oral glucose in healthy subjects. American Journal of Clinical Nutrition, 2007.
86(5): p. 1364-8.
Floyd, J.C., Jr., et al.,
Secretion of insulin induced by amino acids and glucose in diabetes
mellitus. Journal of Clinical Endocrinology & Metabolism, 1968.
28(2): p. 266-76.
Simpson, R.W., et al.,
Macronutrients have different metabolic effects in nondiabetics and
diabetics. American Journal of Clinical Nutrition, 1985.
42(3): p. 449-53.
Peters, A. and M. Davidson,
Protein and fat effects on glucose responses and insulin
requirements in subjects with insulin-dependent diabetes mellitus. Am J Clin Nutr, 1993.
58(4):
Nuttall, F.Q. and M.C. Gannon,
Metabolic response to egg white and cottage cheese protein in
normal subjects. Metabolism: Clinical & Experimental, 1990.
39(7): p. 749-55.
Nuttall, F.Q., M.A. Khan, and M.C. Gannon,
Peripheral glucose appearance rate following
fructose ingestion in normal subjects. Metabolism: Clinical & Experimental, 2000.
49(12): p.
Rask-Madsen, C. and G.L. King,
Mechanisms of Disease: endothelial dysfunction in insulin
resistance and diabetes. Nature Clinical Practice Endocrinology & Metabolism, 2007.
3(1): p.
Josse, A.,
Almonds, Glycemic Excursions, Oxidative Stress and Risk Factors for Coronary
Heart Disease, in
Nutritional Sciences. 2006, University of Toronto: Toronto. p. 170.
Poppitt, S.D., D. McCormack, and R. Buffenstein,
Short-term effects of macronutrient preloads
on appetite and energy intake in lean women. Physiology & Behavior, 1998.
64(3): p. 279-285.
Flint, A., et al.,
Reproducibility, power and validity of visual analogue scales in assessment of
appetite sensations in single test meal studies. International Journal of Obesity & Related
Metabolic Disorders: Journal of the International Association for the Study of Obesity, 2000.
24(1): p. 38-48.
Wolever, T., et al.,
Day-to-day variation in glycaemic response elicited by white bread is not
related to variation in satiety in humans. 2008: Toronto.
Akhavan, T. and G.H. Anderson,
Effects of glucose-to-fructose ratios in solutions on subjective
satiety, food intake, and satiety hormones in young men. American Journal of Clinical
Nutrition, 2007.
86(5): p. 1354-63.
Anderson, G.H., et al.,
Inverse association between the effect of carbohydrates on blood
glucose and subsequent short-term food intake in young men. American Journal of Clinical
Nutrition, 2002.
76(5): p. 1023-30.
Cardello, A.V., et al.,
Development and testing of a labeled magnitude scale of perceived
satiety. Appetite, 2005.
44(1): p. 1-13.
Berthoud, H.R.,
Vagal and hormonal gut-brain communication: from satiation to satisfaction.
Neurogastroenterology & Motility, 2008.
20 Suppl 1: p. 64-72.
Bloom, S.,
Hormonal regulation of appetite. Obesity Reviews, 2007.
8 Suppl 1: p. 63-5.
Chaudhri, O., C. Small, and S. Bloom,
Gastrointestinal hormones regulating appetite.
Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences,
2006.
361(1471): p. 1187-209.
Dhillo, W.S.,
Appetite regulation: an overview. Thyroid, 2007.
17(5): p. 433-45.
Porrini, M., et al.,
Evaluation of satiety sensations and food intake after different preloads.
Appetite, 1995.
25(1): p. 17-30.
Naslund, E. and P.M. Hellstrom,
Appetite signaling: from gut peptides and enteric nerves to
brain. Physiology & Behavior, 2007.
92(1-2): p. 256-62.
Mayer, J.,
The glucostatic theory of regulation of food intake and the problem of obesity.
Bulletin, 1952.
14(2): p. 43-9.
Mayer, J.,
Bulletin of the New England Medical Center, Volume XIV, April-June 1952: The
glucostatic theory of regulation of food intake and the problem of obesity (a review). Nutrition
Reviews, 1991.
49(2): p. 46-8.
VanderWeele, D.A., D.R. Skoog, and D. Novin,
Glycogen levels and peripheral mechanisms of
glucose-induced spppression of feeding. American Journal of Physiology, 1976.
231(6): p.
Campfield, L.A. and F.J. Smith,
Functional coupling between transient declines in blood
glucose and feeding behavior: temporal relationships. Brain Research Bulletin, 1986.
17(3): p.
Louis-Sylvestre, J. and J. Le Magnen,
Fall in blood glucose level precedes meal onset in free-
feeding rats. Neuroscience & Biobehavioral Reviews, 1980.
4 Suppl 1: p. 13-5.
Gielkens, H.A.J.V., Marco; Lam, Wai F.; Lamers,Cornelis B.H.W.; Masclee, Ad A.M.,
Effects
of hyperglycemia and hyperinsulinemia on satiety in humans. Metabolism, 1998.
47(3): p. 321-
Pittas, A.G., et al.,
Interstitial glucose level is a significant predictor of energy intake in free-
living women with healthy body weight. Journal of Nutrition, 2005.
135(5): p. 1070-4.
Freeland, K. and T. Wolever,
Effects of cereal fiber Compared to Glycemic Carbohydrate on
Appetite, Short-term Food Intake, Orocecal Transit Time and Blood Glucose [Abstract]. Am J
Clin Nutr, 2002.
75(2): p. 339S-439.
Holt, S.H., et al.,
A satiety index of common foods. European Journal of Clinical Nutrition,
1995.
49(9): p. 675-90.
Holt, S.H., J.C. Brand Miller, and P. Petocz,
Interrelationships among postprandial satiety,
glucose and insulin responses and changes in subsequent food intake. European Journal of
Clinical Nutrition, 1996.
50(12): p. 788-97.
Zemel, M.B., et al.,
Regulation of adiposity by dietary calcium. FASEB Journal, 2000.
14(9): p.
Westerterp-Plantenga, M.S., et al.,
Dietary protein, metabolism, and body-weight regulation:
dose-response effects. International Journal of Obesity December, 2006.
30(3): p. S16-S23.
Grimes, D.S. and C. Gordon,
Satiety value of wholemeal and white bread. Lancet, 1978.
2(8080): p. 106.
Turnbull, W.H., J. Walton, and A.R. Leeds,
Acute effects of mycoprotein on subsequent energy
intake and appetite variables. American Journal of Clinical Nutrition, 1993.
58(4): p. 507-12.
Rolls, B.J.B., Elizabeth A.; Thorwart,Michelle L.,
Water incorporated into a food but not
served with a food decreases energy intake in lean women. American Journal of Clinical
Nutrition, 1999.
70(4): p. 448-455.
Rolls, B.J., E.A. Bell, and B.A. Waugh,
Increasing the volume of a food by incorporating air
affects satiety in men. American Journal of Clinical Nutrition, 2000.
72(2): p. 361-8.
Rolls, B.J.B., E A.Castellanos,V H.Chow,M.Pelkman,C L.Thorwart,M L.,
Energy density but
not fat content of foods affected energy intake in lean and obese women. American Journal of
Clinical Nutrition, 2008.
Papazian, R.,
Bulking up fiber's healthful reputation: more benefits of "roughage" are
discovered. 1998, U.S. Food and Drug Administration.
Kay, R.M. and S. Stitt,
Food form, postprandial glycemia, and satiety. American Journal of
Clinical Nutrition, 1978.
31(5): p. 738-9.
Qualmann, C., et al.,
Insulinotropic actions of intravenous glucagon-like peptide-1 (GLP-1) [7-
36 amide] in the fasting state in healthy subjects. Acta Diabetologica, 1995.
32(1): p. 13-6.
Roberts, S.B.,
High-glycemic index foods, hunger, and obesity: is there a connection? Nutrition
Reviews, 2000.
58(6): p. 163-9.
McMillan-Price, J. and J. Brand-Miller,
Low-glycaemic index diets and body weight regulation.
International Journal of Obesity December, 2006.
30(3): p. S40-S46.
Holt, S., et al.,
Relationship of satiety to postprandial glycaemic, insulin and cholecystokinin
responses. Appetite, 1992.
18(2): p. 129-41.
Pereira, M.A. and D.S. Ludwig,
Dietary fiber and body-weight regulation. Observations and
mechanisms. Pediatric Clinics of North America, 2001.
48(4): p. 969-80.
Ludwig, D.S.,
Dietary Glycemic Index and Obesity. J. Nutr., 2000.
130(2): p. 280-.
Blundell, J.E. and R.J. Stubbs,
High and low carbohydrate and fat intakes: limits imposed by
appetite and palatability and their implications for energy balance. European Journal of
Clinical Nutrition, 1999.
53 Suppl 1: p. S148-65.
Blundell, J.E., et al.,
The fat paradox: fat-induced satiety signals versus high fat
overconsumption. International Journal of Obesity & Related Metabolic Disorders: Journal of
the International Association for the Study of Obesity, 1995.
19(11): p. 832-5.
Liddle, R.A., et al.,
Cholecystokinin bioactivity in human plasma. Molecular forms, responses
to feeding, and relationship to gallbladder contraction. Journal of Clinical Investigation, 1985.
75(4): p. 1144-52.
Lawton, C.L., et al.,
The degree of saturation of fatty acids influences post-ingestive satiety.
British Journal of Nutrition, 2000.
83(5): p. 473-82.
Chavez, M., et al.,
Effect of a high-fat diet on food intake and hypothalamic neuropeptide gene
expression in streptozotocin diabetes. Journal of Clinical Investigation, 1998.
102(2): p. 340-6.
Jones, P., P. Pencharz, and M. Clandinin,
Whole body oxidation of dietary fatty acids:
implications for energy utilization. Am J Clin Nutr, 1985.
42(5): p. 769-777.
Erlanson-Albertsson, C. and J. Mei,
The effect of low carbohydrate on energy metabolism.
International Journal of Obesity, 2005.
29 Suppl 2: p. S26-30.
Paddon-Jones, D., et al.,
Protein, weight management, and satiety. American Journal of Clinical
Nutrition, 2008.
87(5): p. 1558S-1561S.
Westerterp-Plantenga, M.S., et al.,
Satiety related to 24 h diet-induced thermogenesis during
high protein/carbohydrate vs high fat diets measured in a respiration chamber. European
Journal of Clinical Nutrition, 1999.
53(6): p. 495-502.
Johnston, C.S., S.L. Tjonn, and P.D. Swan,
High-protein, low-fat diets are effective for weight
loss and favorably alter biomarkers in healthy adults. Journal of Nutrition, 2004.
134(3): p.
Lejeune, M.P., et al.,
Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and
energy and substrate metabolism during a high-protein diet and measured in a respiration
chamber. American Journal of Clinical Nutrition, 2006.
83(1): p. 89-94.
Smeets, A.J., et al.,
Energy expenditure, satiety, and plasma ghrelin, glucagon-like peptide 1,
and peptide tyrosine-tyrosine concentrations following a single high-protein lunch. Journal of
Nutrition, 2008.
138(4): p. 698-702.
Dahl, W.J., et al.,
Effects of flax fiber on laxation and glycemic response in healthy volunteers.
Journal of Medicinal Food, 2005.
8(4): p. 508-11.
Zeratsky, K.,
Ground flaxseed: Better than whole? 2008, Mayo Clinic.
Wong, S., K. Traianedes, and K. O'Dea,
Factors affecting the rate of hydrolysis of starch in
legumes. American Journal of Clinical Nutrition, 1985.
42(1): p. 38-43.
Jenkins, D.J., et al.,
Wholemeal versus wholegrain breads: proportion of whole or cracked
grain and the glycaemic response. Bmj, 1988.
297(6654): p. 958-60.
Behall, K.M., D.J. Scholfield, and J. Hallfrisch,
The effect of particle size of whole-grain flour
on plasma glucose, insulin, glucagon and thyroid-stimulating hormone in humans. Journal of
the American College of Nutrition, 1999.
18(6): p. 591-7.
Haber, G.B., et al.,
Depletion and disruption of dietary fibre. Effects on satiety, plasma-glucose,
and serum-insulin. Lancet, 1977.
2(8040): p. 679-82.
DiMeglio, D.P. and R.D. Mattes,
Liquid versus solid carbohydrate: effects on food intake and
body weight. International Journal of Obesity & Related Metabolic Disorders: Journal of the
International Association for the Study of Obesity, 2000.
24(6): p. 794-800.
Lan-Pidhainy, X.,
The effects of freezing and thawing on fiber-rich carbohydrate foods, A.S.
Lee,[Personal Communication]. 2008: Toronto.
Campfield, L.A., et al.,
Human eating: evidence for a physiological basis using a modified
paradigm. Neuroscience & Biobehavioral Reviews, 1996.
20(1): p. 133-7.
Burton, P. and H.J. Lightowler,
Influence of bread volume on glycaemic response and satiety.
British Journal of Nutrition, 2006.
96(5): p. 877-82.
Consent to Participate in a Research Study
Before agreeing to participate in this research study, it is important that you read and
understand this research consent form. This form provides all the information we think you will need to
know in order to decide whether you wish to participate in the study. If you have any questions after
you read through this form, please address your study doctor or study personnel. You should not sign
this form until you are certain that you understand everything on this form. You may also wish to
discuss your participation in this study with your family doctor, a family member or a close friend. It is
important that you are completely truthful with study personnel with respect to your health history and
any medications you may be taking in order to prevent unnecessary harm to you if you decide to
participate in this study.
Title of Research Study: The Effects of
Salvia hispanica-enriched Foods on
Glycemic Responses and Subjective Satiety
Vladimir Vuksan, PhD Professor, Department of Nutritional Sciences and Medicine Faculty of Medicine, University of Toronto; Associate Director, Risk Factor Modification Centre, St. Michael's Hospital Phone: (416) 864-5525 E-mail:
[email protected] Amy S. Lee BSc, MSc Candidate (under the supervision of Dr. Vuksan) Department of Nutritional Sciences Faculty of Medicine, University of Toronto Phone: (416) 864-6060 ext. 3364 E-mail:
[email protected]
Study Sponsor:
Salba Nutritional Solutions, Inc.
Purpose of the Research
The purpose of this study is to determine if breads containing the whole grain
Salvia hispanica
lower blood glucose responses and decrease hunger compared to breads without the grain but with the same amount of carbohydrate. The study will also determine which amount and which form (whole or ground) of
Salvia hispanica causes the greatest feelings of fullness. Furthermore, we will investigate if there is a relationship between blood insulin levels and fullness or satiety. This study will comprise a portion of Amy Lee's research for her Master's thesis.
Salvia hispanica is a whole grain grown in South America that has been consumed as early as
3500 B.C. It has also been recently demonstrated to be completely safe for human consumption. Its composition is similar to that of the common flaxseed.
Description of the Research
Approximately 30 subjects will be enrolled in this study and this study will be conducted at the
Risk Factor Modification Centre, St. Michael's Hospital (70 Richmond St. East, Main Floor, Toronto, Ontario, M5C 1N8).
You will be asked to visit the clinic on 11 separate occasions to eat 11 different meals. The
visits will be approximately 2 hours long. The meals will consist of a serving of bread 9 containing either no
Salvia hispanica or various amounts of it. Visits must be at least 48 hours apart. Therefore, your participation should last from between 5-10 weeks.
For each visit, you will come to the clinic in the morning after having fasted for 10-12 hours.
You will first have a finger prick blood sample taken and will fill out a questionnaire, then will be
served a meal. At 15, 30, 45, 60, 90 and 120 minutes after the meal you will have additional finger
prick blood samples taken and will complete a questionnaire at each of these times.
Potential Harms (Injury, Discomforts or Inconvenience)
Participation in this study carries a very low risk of injury. You may experience a fleeting
stinging sensation when you receive a finger prick for a blood sample, and may experience some minor
swelling at the sample site for one or two hours after the sample is taken. The risk of infection from
having a finger prick blood sample taken is extremely low. It is possible that you could have an allergic
reaction to the test meals; this, however, has never been documented.
Potential Benefits
There may be no direct benefit to you for participating in this study. However, the knowledge
gained from this study may benefit others in the future.
Confidentiality and Privacy
The study investigators, (hereby referred to as "study personnel") are committed to respecting
your privacy. No other persons will have access to your personal health information or identifying information without your consent, unless required by law. Any medical records, documentation, laboratory samples, or information related to you will be coded by study numbers to ensure that persons outside of the study (i.e., sponsors) will not be able to identify you. No identifying information about you will be allowed off site. All information that identifies you will be kept confidential and stored and locked in a secure place that only the study personnel will have access to. In addition, electronic files will be stored on a secure hospital or institutional network and will be password protected. It is important to understand that despite these protections being in place, experience in similar studies indicates that there is the risk of unintentional release of information. The principal investigator will protect your records and keep all the information in your study file confidential to the greatest extent possible. The chance that this information will accidentally be given to someone else is small.
National and Provincial Data Protection regulations, including the Personal Information
Protection and Electronic Documents Act (of Canada) or PIPEDA and the Personal Health Information Protection Act (PHIPA) of Ontario, protect your personal information. They also give you the right to control the use of your personal information, including personal health information, and require your written permission for your personal information (including personal health information) to be collected, used or disclosed for the purposes of this study, as described in this consent form. You have the right to review and copy your personal information. However, if you decide to be in this study or chose to withdraw from it, your right to look at or copy your personal information related to this study will be delayed until after the research is completed.
Publication of Results
We may present this study at scientific conferences and we intend to write an article about this
study for a scientific journal. You can ask us to send you a copy of the article when it is published by
contacting Dr. Vladimir Vuksan, the principal investigator.
Reimbursement
You will be compensated $30 per session, for a total of $330 for all 11 sessions.
Compensation for Injury
If you suffer a physical injury as a direct result of the administration of study foods or study
procedures, you may obtain medical care in the same manner as you would ordinarily obtain any other
medical treatment. In no way does signing this form waive your legal rights nor relieve the
investigator, sponsors or involved institutions from their legal and professional responsibility.
Participation and Withdrawal
Participation in any research study is voluntary. If you choose not to participate, you and your
family will continue to have access to customary care at St. Michael's Hospital. If you decide to participate in this study you can change your mind at any time without giving a reason, and you may withdraw from the study at any time without any effect on the care you and your family will receive at St. Michael's Hospital.
Research Ethics Board Contact
If you have any questions about your rights as a research subject, you may contact:
Dr. Julie Spence Chair, St. Michael's Hospital Research Ethics Board Telephone (416) 864-6060 ext. 2557
The Effects of Salvia hispanica-enriched Foods on Glycemic Responses Responses and
Subjective Satiety
I acknowledge that the research study described above has been explained to me and that any
questions that I have asked have been answered to my satisfaction. I have been informed of the alternatives to participation in this study, including the right not to participate and the right to withdraw without compromising the quality of medical care at St. Michael's Hospital for me and for other members of my family. As well, the potential risks, harms and discomforts have been explained to me and I also understand the benefits (if any) of participating in the research study.
I understand that I have not waived my legal rights nor released the investigators, sponsors or
involved institutions from their legal and professional duties. I know that I may ask now, or in the future, any questions I have about the study or the research procedures. I have been assured that records relating to me and my care will be kept confidential and that no information will be released or printed that would disclose personal identity without my permission unless required by law. I have been given sufficient time to read and understand the above information.
I hereby consent to participate, and I have been told I will be given a signed copy of this
Participants Name
Participants Signature
I, the undersigned, have fully explained the study to the above participant.
Investigator or Designate Name
Investigator or Designate Signature
If Signed by Designate:
Position of Designate
Investigator's Signature
INFORMATION FORM
Salvia hispanica /ACUTE STUDY
All information provided in this questionnaire will be kept confidential
and released only for the purposes of the present study
First name and initials:
Mailing address:
Office use only:
DOB (dd/mm/yyyy):
Family Physician:
High blood sugar
Has your doctor ever told you that
you have high blood sugar, high
blood pressure? If yes, then please
give details: when, how high,
Fasting glucose: _mmolL
sBP/dBP: _/ _ mmHg
medications (Rx), complications, etc.
Post-meal glucose: _mmol/L
HbA1c (glycosolated haemoglobin) %
Complications: _
Does anyone in your family have
diabetes, high blood pressure, or
heart disease? If yes, then please
describe, indicating how long they
have had it and their relationship to
Grandmother/grandfather
Grandmother/grandfather
Do you take medications, herbs or
supplements? If yes, then please
describe, indicating types, brand
names, doses, and times.
Have you been diagnosed with any of the following? (If yes, please indicate onset date, treatment and current status- recovered/ active condition)
Present status
CONDITION
Recovered
(please indicate treatment)
Malabsorption syndrome
Ulcerative colitis
Stomach (gastric) ulcer
Duodenal ulcer
Intestinal parasites
Diarrhea (> 2 liquid
stools/day)
Constipation (≥
3 days
duration)
Heart disease
Heart attack
Arrhythmia
Uncontrolled hypertension
Systolic BP ≥
140
Diastolic BP ≥
90
Blood clotting disorders
Kidney disease
Psychiatric conditions
Present status
CONDITION
Recovered
(please indicate treatment)
Infectious hepatitis
Recently diagnosed
infectious hepatitis A, E
HIV/ AIDS
Thyroid disease
Do you experience any of
the following
:
Fatigue
Unexplained weight gain
Dry skin and hair
Depressed mood
Cold intolerance
Constipation
Increased cholesterol?
Nervousness/irritability
Palpitations
Heat intolerance
Increased sweating
Unexplained weight loss
Insomnia
Pancreatic disease
Diabetes
Any food allergies
Allergies to ginseng or
wheat bran powder
Any food intolerance
Any other health problems? No
Yes (please describe)
_
Lifestyle and diet
Are you following a special diet?
Yes (please describe)
If yes, how many cigarettes per day?
< 10 cigarettes/ day
> 10 cigarettes /day
If you are a past smoker, how many cigarettes did you smoke per day and when did you quit? Please list type, duration and frequency of any regular exercise (including walking): Please indicate the number of alcoholic beverages (spirit 1.5 oz, beer 1 bottle, wine 1 200 ml glass) consumed per day:
Please indicate the number of coffee drinks per day (1 cup = 1.5 fl.oz.) indicating the type of coffee consumed (filtered, espresso, boiled, etc.)
≥ 9 cups/ day
Type of coffee: WOMEN ONLY: Are you post-menopausal?
Did you recently experience any of the following symptoms?
Severity (mild/
Frequency
Duration
moderate/ severe)
Bloating
Belching
Flatulence
Diarrhoea
Excessive urination
Headache
Dizziness
Insomnia
Poor wound healing
Excessive bleeding after
Impaired vision
Heart flutters
Joint pain
Numbness
Have you participated in a clinical trial within the last 3 months?
Salvia hispanica/ Acute
CLINICAL ASSESSMENT
Subject #:
_
Treatment Code:
Anthropometry
Waist:Hip (cm:cm):
START TIME: _ FINISH TIME: _ Time taken to consume test meal: _
Preclinical information
Did you consume at least
150g (6oz.) of carbohydrate on
each of the
Time Food item Quantity
three days previous to this test? This amount is equivalent to
3 servings
of any of the following alone or in combination: 2 slices of bread, 1 cup
of cooked rice/pasta, 1 medium potato, 1 bowl of cereal with milk, 1
glass of juice/soft-drink, 3 oranges/apples, or 1 bowl of ice cream.
Are you fasting this morning? If yes, then please describe the last meal
you consumed before beginning your fast.
Did you take
any medications (prescription, OTC, etc.), remedies, or
supplements last night or this morning? If yes, then please describe
Type
Dose
: Time: _
Yes No How long ago did you last (1) empty your bladder and/or (2) have a
(1) Last urination: hrs ago (2) Last Bowel movement: _hrs ago
Did you do anything last night that is not part of your regular routine?
This may include social activities, exercise, or use of alcohol,
medications, or supplements. If yes, then please describe.
How many hours of sleep did you have last night? Does this represent a
Did you do anything before the test this morning that is not part of your
regular routine? This may include exercise or use of alcohol,
medications, or supplements. If yes, then please describe.
What was your mode of transportation to the clinic this morning? Is this
different from other clinic mornings?
How would you rate your current level of health/well-being. Please
comment on anything unusual.
Excellent Good Fair Poor
Time: FASTING (0 min)
PHYSICAL QUESTIONNAIRE
These questions relate to your physical assessment at this time. Please rate your feelings by
placing a vertical line across the line at the point which best reflects your present feelings.
1. How strong is your desire to eat?
Very weak _ Very strong
2. How hungry do you feel?
Not hungry _ As hungry
at all
as I have ever felt
3. How full do you feel?
Not full at all _ As full as I have
4. How much do you think you could eat now?
Nothing at all _ A large amount
SYMPTOMS
PRESENCE
SEVERITY
Bloating
Low 1---------2---------3---------4---------5---------6---------7 High
Belching
Low 1---------2---------3---------4---------5---------6---------7 High
Diarrhoea
Low 1---------2---------3---------4---------5---------6---------7 High
Flatulence
Low 1---------2---------3---------4---------5---------6---------7 High
Excessive urination
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Headache
Low 1---------2---------3---------4---------5---------6---------7 High
Dizziness
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Poor wound healing
Low 1---------2---------3---------4---------5---------6---------7 High
Excessive bleeding after cuts
Low 1---------2---------3---------4---------5---------6---------7 High
Other (specify):
Low 1---------2---------3---------4---------5---------6---------7 High
PHYSICAL QUESTIONNAIRE
These questions relate to your physical assessment at this time. Please rate your feelings by
placing a vertical line across the line at the point which best reflects your present feelings.
1. How strong is your desire to eat?
Very weak _ Very strong
2. How hungry do you feel?
Not hungry _ As hungry
at all
as I have ever felt
3. How full do you feel?
Not full at all _ As full as I have
4. How much do you think you could eat now?
Nothing at all _ A large amount
SYMPTOMS
PRESENCE
SEVERITY
Bloating
Low 1---------2---------3---------4---------5---------6---------7 High
Belching
Low 1---------2---------3---------4---------5---------6---------7 High
Diarrhoea
Low 1---------2---------3---------4---------5---------6---------7 High
Flatulence
Low 1---------2---------3---------4---------5---------6---------7 High
Excessive urination
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Headache
Low 1---------2---------3---------4---------5---------6---------7 High
Dizziness
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Poor wound healing
Low 1---------2---------3---------4---------5---------6---------7 High
Excessive bleeding after cuts
Low 1---------2---------3---------4---------5---------6---------7 High
Other (specify):
Low 1---------2---------3---------4---------5---------6---------7 High
PHYSICAL QUESTIONNAIRE
These questions relate to your physical assessment at this time. Please rate your feelings by
placing a vertical line across the line at the point which best reflects your present feelings.
1. How strong is your desire to eat?
Very weak _ Very strong
2. How hungry do you feel?
Not hungry _ As hungry
at all
as I have ever felt
3. How full do you feel?
Not full at all _ As full as I have
4. How much do you think you could eat now?
Nothing at all _ A large amount
SYMPTOMS
PRESENCE
SEVERITY
Bloating
Low 1---------2---------3---------4---------5---------6---------7 High
Belching
Low 1---------2---------3---------4---------5---------6---------7 High
Diarrhoea
Low 1---------2---------3---------4---------5---------6---------7 High
Flatulence
Low 1---------2---------3---------4---------5---------6---------7 High
Excessive urination
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Headache
Low 1---------2---------3---------4---------5---------6---------7 High
Dizziness
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Poor wound healing
Low 1---------2---------3---------4---------5---------6---------7 High
Excessive bleeding after cuts
Low 1---------2---------3---------4---------5---------6---------7 High
Other (specify):
Low 1---------2---------3---------4---------5---------6---------7 High
PHYSICAL QUESTIONNAIRE
These questions relate to your physical assessment at this time. Please rate your feelings by
placing a vertical line across the line at the point which best reflects your present feelings.
1. How strong is your desire to eat?
Very weak _ Very strong
2. How hungry do you feel?
Not hungry _ As hungry
at all
as I have ever felt
3. How full do you feel?
Not full at all _ As full as I have
4. How much do you think you could eat now?
Nothing at all _ A large amount
SYMPTOMS
PRESENCE
SEVERITY
Bloating
Low 1---------2---------3---------4---------5---------6---------7 High
Belching
Low 1---------2---------3---------4---------5---------6---------7 High
Diarrhoea
Low 1---------2---------3---------4---------5---------6---------7 High
Flatulence
Low 1---------2---------3---------4---------5---------6---------7 High
Excessive urination
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Headache
Low 1---------2---------3---------4---------5---------6---------7 High
Dizziness
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Poor wound healing
Low 1---------2---------3---------4---------5---------6---------7 High
Excessive bleeding after cuts
Low 1---------2---------3---------4---------5---------6---------7 High
Other (specify):
Low 1---------2---------3---------4---------5---------6---------7 High
PHYSICAL QUESTIONNAIRE
These questions relate to your physical assessment at this time. Please rate your feelings by
placing a vertical line across the line at the point which best reflects your present feelings.
1. How strong is your desire to eat?
Very weak _ Very strong
2. How hungry do you feel?
Not hungry _ As hungry
at all
as I have ever felt
3. How full do you feel?
Not full at all _ As full as I have
4. How much do you think you could eat now?
Nothing at all _ A large amount
SYMPTOMS
PRESENCE
SEVERITY
Bloating
Low 1---------2---------3---------4---------5---------6---------7 High
Belching
Low 1---------2---------3---------4---------5---------6---------7 High
Diarrhoea
Low 1---------2---------3---------4---------5---------6---------7 High
Flatulence
Low 1---------2---------3---------4---------5---------6---------7 High
Excessive urination
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Headache
Low 1---------2---------3---------4---------5---------6---------7 High
Dizziness
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Poor wound healing
Low 1---------2---------3---------4---------5---------6---------7 High
Excessive bleeding after cuts
Low 1---------2---------3---------4---------5---------6---------7 High
Other (specify):
Low 1---------2---------3---------4---------5---------6---------7 High
PHYSICAL QUESTIONNAIRE
These questions relate to your physical assessment at this time. Please rate your feelings by
placing a vertical line across the line at the point which best reflects your present feelings.
1. How strong is your desire to eat?
Very weak _ Very strong
2. How hungry do you feel?
Not hungry _ As hungry
at all
as I have ever felt
3. How full do you feel?
Not full at all _ As full as I have
4. How much do you think you could eat now?
Nothing at all _ A large amount
SYMPTOMS
PRESENCE
SEVERITY
Bloating
Low 1---------2---------3---------4---------5---------6---------7 High
Belching
Low 1---------2---------3---------4---------5---------6---------7 High
Diarrhoea
Low 1---------2---------3---------4---------5---------6---------7 High
Flatulence
Low 1---------2---------3---------4---------5---------6---------7 High
Excessive urination
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Headache
Low 1---------2---------3---------4---------5---------6---------7 High
Dizziness
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Poor wound healing
Low 1---------2---------3---------4---------5---------6---------7 High
Excessive bleeding after cuts
Low 1---------2---------3---------4---------5---------6---------7 High
Other (specify):
Low 1---------2---------3---------4---------5---------6---------7 High
Time: 120 minutes (your clock may say 2:00)
PHYSICAL QUESTIONNAIRE
These questions relate to your physical assessment at this time. Please rate your feelings by
placing a vertical line across the line at the point which best reflects your present feelings.
1. How strong is your desire to eat?
Very weak _ Very strong
2. How hungry do you feel?
Not hungry _ As hungry
at all
as I have ever felt
3. How full do you feel?
Not full at all _ As full as I have
4. How much do you think you could eat now?
Nothing at all _ A large amount
SYMPTOMS
PRESENCE
SEVERITY
Bloating
Low 1---------2---------3---------4---------5---------6---------7 High
Belching
Low 1---------2---------3---------4---------5---------6---------7 High
Diarrhoea
Low 1---------2---------3---------4---------5---------6---------7 High
Flatulence
Low 1---------2---------3---------4---------5---------6---------7 High
Excessive urination
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Headache
Low 1---------2---------3---------4---------5---------6---------7 High
Dizziness
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Low 1---------2---------3---------4---------5---------6---------7 High
Poor wound healing
Low 1---------2---------3---------4---------5---------6---------7 High
Excessive bleeding after cuts
Low 1---------2---------3---------4---------5---------6---------7 High
Other (specify):
Low 1---------2---------3---------4---------5---------6---------7 High
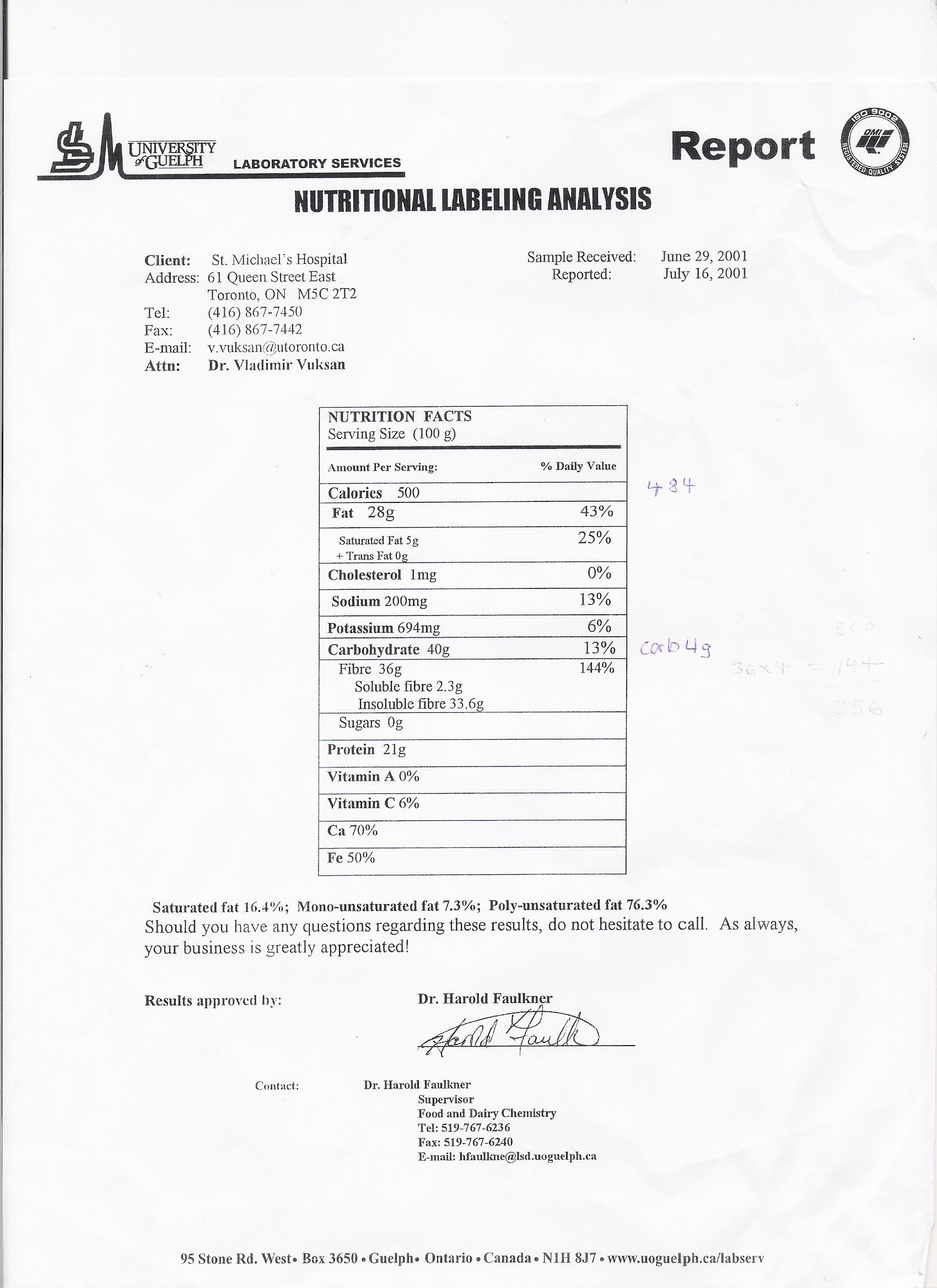
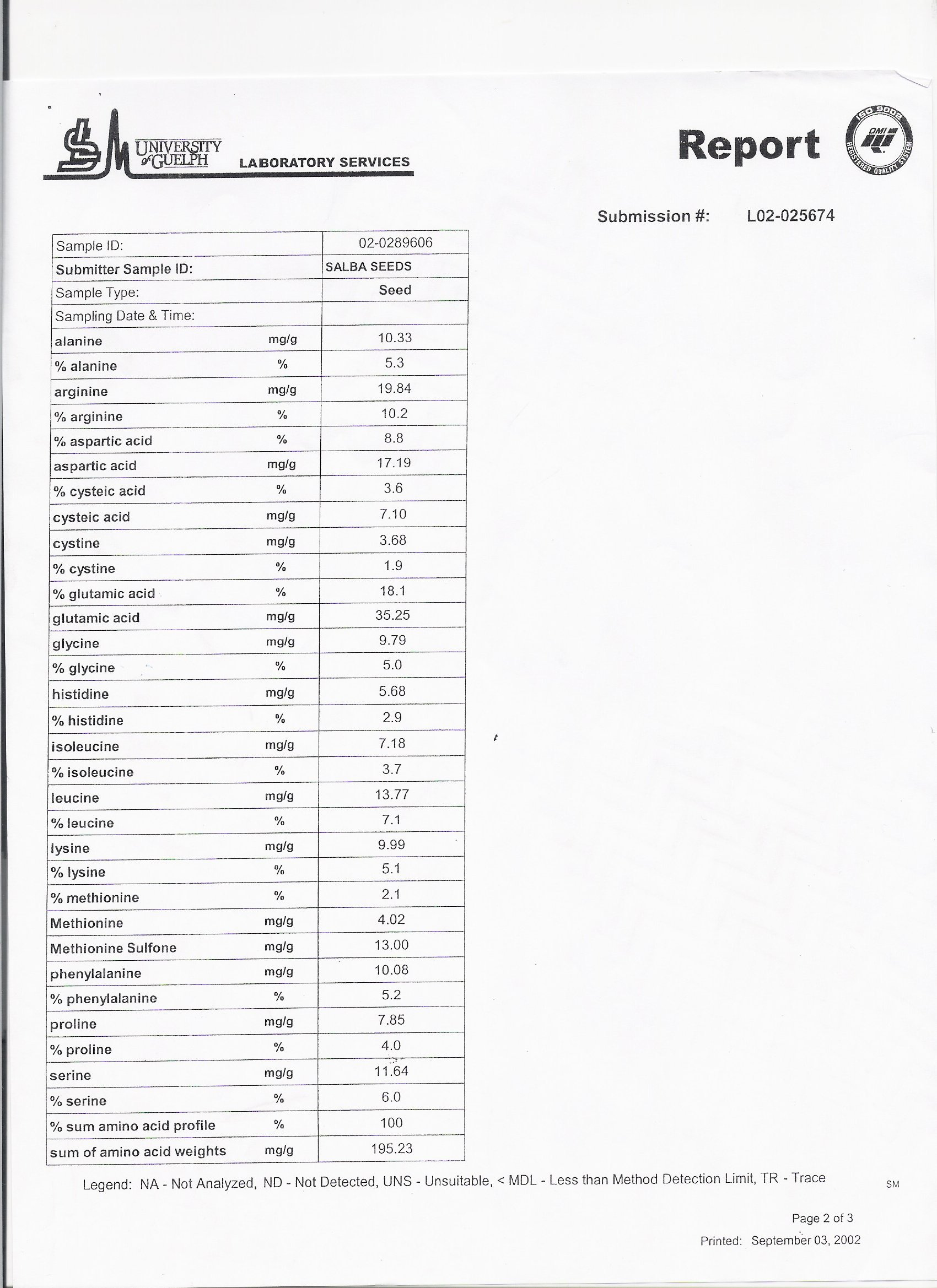
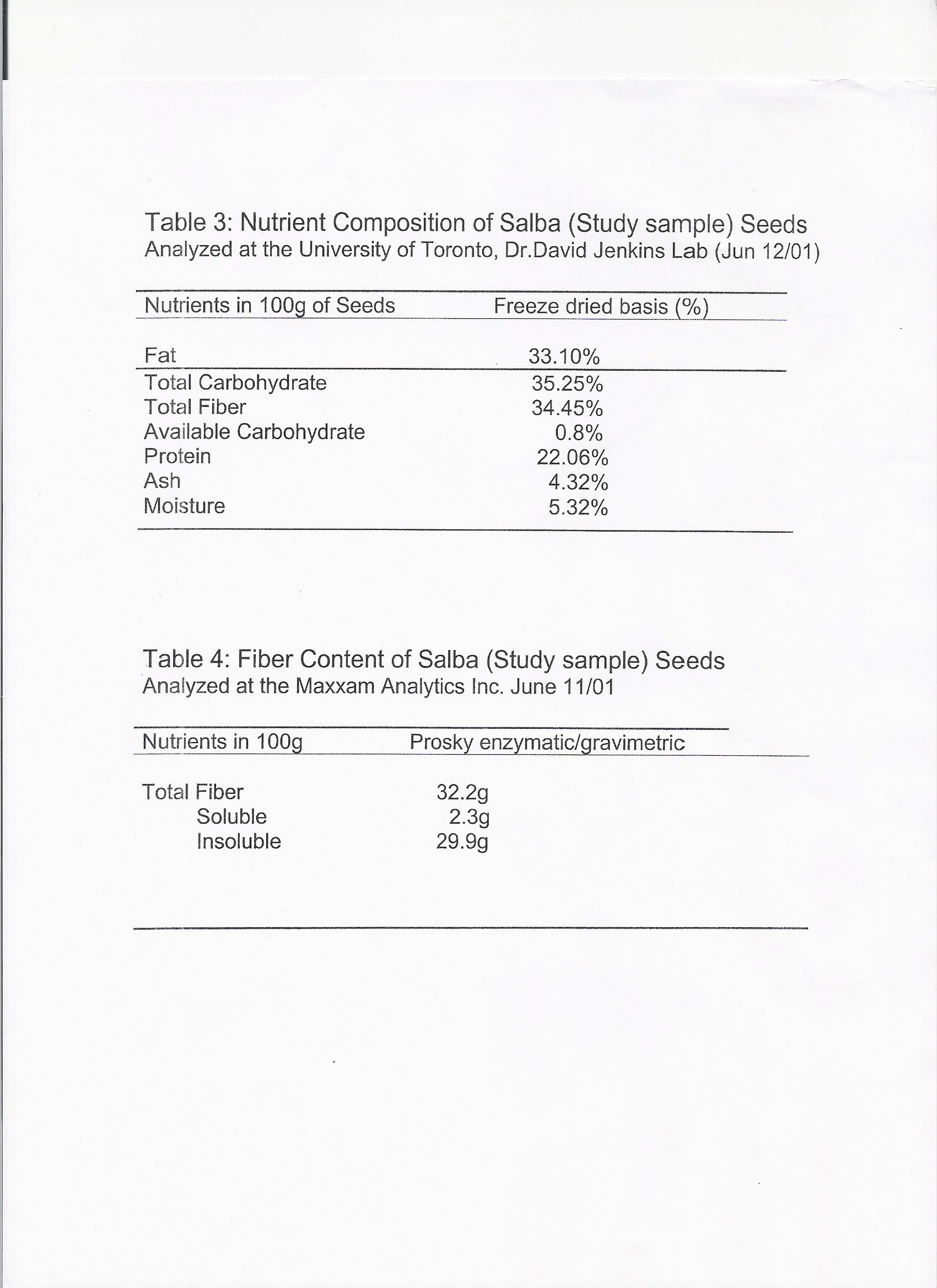
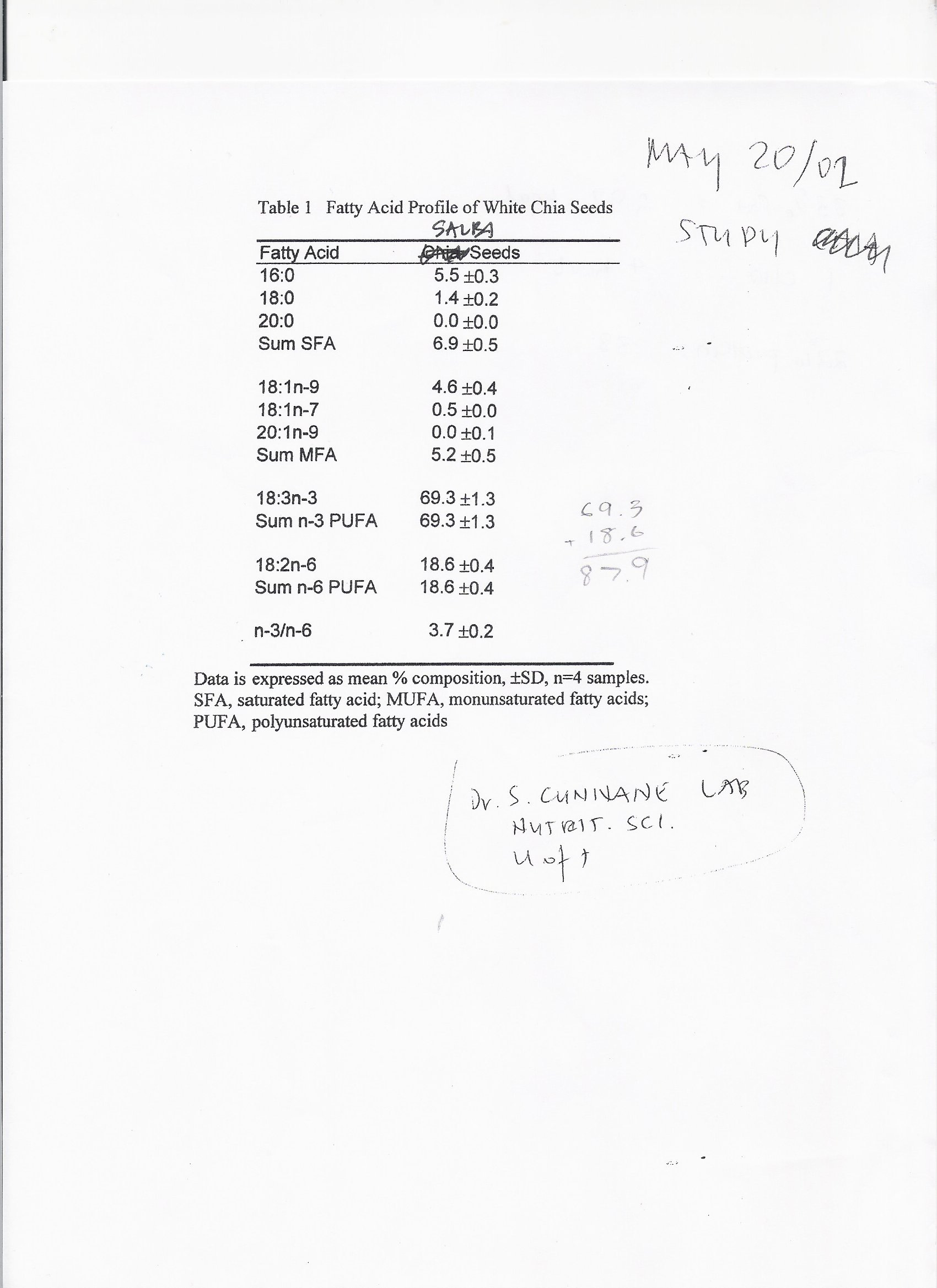
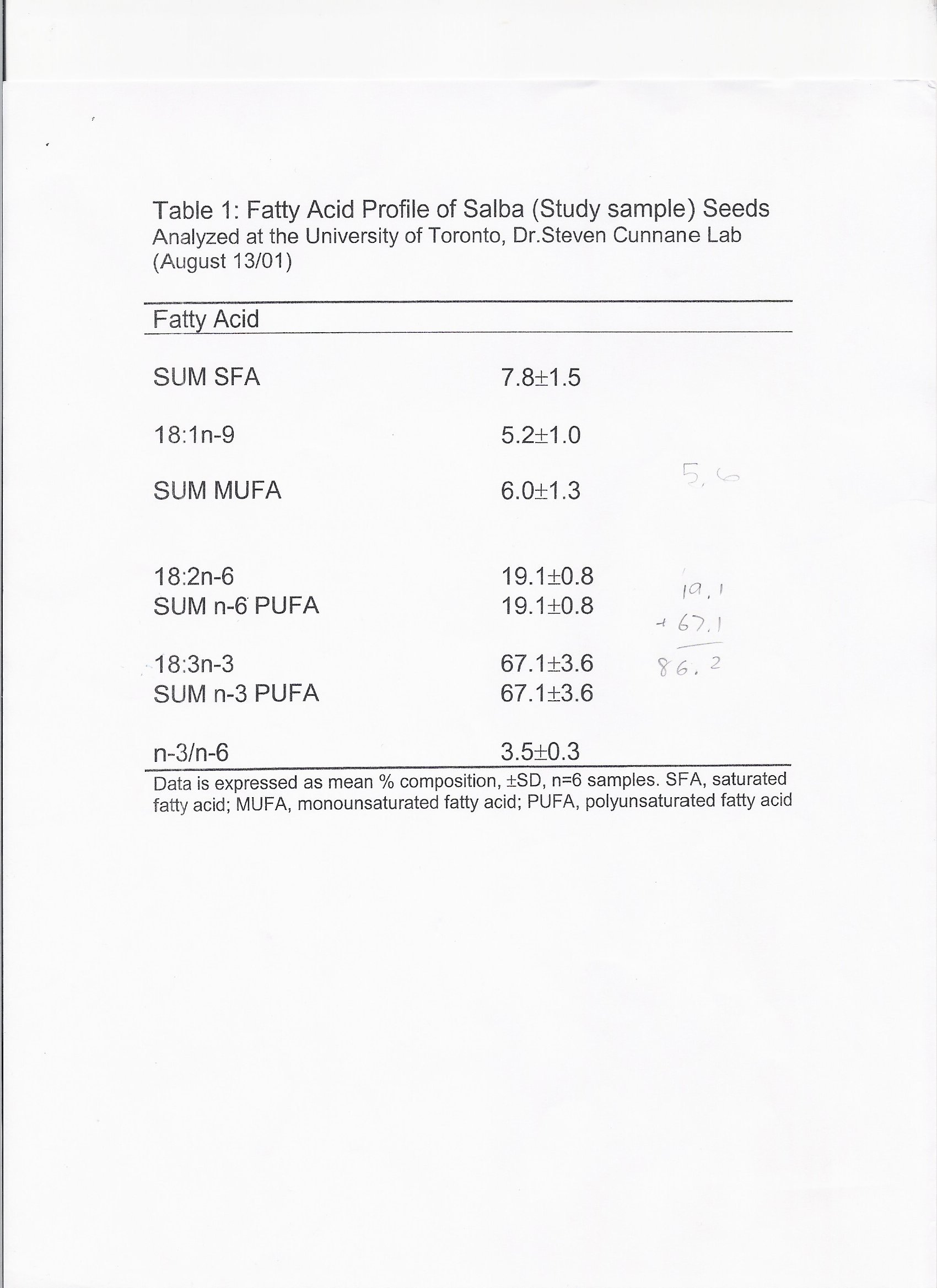
Source: http://chiaseeds.ru/files/Lee_Amy_S_2009.pdf
Making Education Easy Issue 7 - 2014 Welcome to the 7th issue of Fertility Research Review. This issue begins with the miracle of 2014 – a live birth after uterus transplantation from a postmenopausal donor. This is the culmination of a 20-year research programme and an extraordinary achievement. Live birth after uterus
A BRAZILIAN NEGLECTED DISEASE Jansen Fernandes Medeiros1, Felipe Arley Costa Pessoa2 and Luis Marcelo Aranha Mansonelliasis is a filariasis whose etiological agents are Mansonella ozzardi, Mansonella perstans and Mansonella streptocerca. Only the first two cited species occur in Brazil. M. ozzardi is widely distributed in Amazonas state and it is found along the rivers Solimões, Purus, Negro and their





