Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Microsoft word - 039 - 041 cavanagh.doc
EXERCISE AND PHARMACOLOGICAL COUNTERMEASURES FOR BONE LOSS DURING LONG-
DURATION SPACE FLIGHT
Peter R. Cavanagh1,2,3,5, Angelo A. Licata1,3,4, and Andrea J. Rice1,2
1Department of Biomedical Engineering, Lerner Research Institute,
2Center for Space Medicine,
3Department of Orthopaedic Surgery,
4Department of Endocrinology, Diabetes & Metabolism
5Orthopaedic Research Center, The Cleveland Clinic Foundation, Cleveland, OH
ABSTRACT
2003). This adaptation to microgravity renders the
skeleton "at risk" for fracture, increases the risk of renal
Bone loss in the lower extremities and lumbar spine is an
stones (Whitson et al., 1999), and poses potential long-
established consequence of long-duration human space flight.
term health risks for astronauts on their return to Earth
Astronauts typically lose as much bone mass in the proximal
with reduced bone mass.
femur in 1 month as postmenopausal women on Earth lose in 1
year. Pharmacological interventions have not been routinely
In this article, we will examine the evidence for loss of
used in space, and countermeasure programs have depended solely upon exercise. However, it is clear that the osteogenic
bone mass during long-duration space flight, discuss the
stimulus from exercise has been inadequate to maintain bone
mechanisms for such loss, review countermeasures that
mass, due to insufficient load or duration. Attention has
have been attempted to date, and examine the potential of
therefore been focused on several pharmacological interventions
pharmaceutical countermeasures in the future. The
that have been successful in preventing or attenuating
implications of recent findings regarding the genetic
osteoporosis on Earth. Anti-resorptives are the class of drugs
determinants of bone mass will also be discussed.
most commonly used to treat osteoporosis in postmenopausal
women, notably alendronate sodium, risedronate sodium,
BONE LOSS IN SPACE: THE EVIDENCE
zoledronic acid, and selective estrogen receptor modulators,
such as raloxifene. There has also been considerable recent interest in anabolic agents such as parathyroid hormone (PTH)
Bone loss during space flight has been a concern since the
and teriparatide (rhPTH [1-34]). Vitamin D and calcium
Gemini flights (1-14 day missions, 1962-1966). Mack
supplementation have also been used. Recent studies of
and colleagues (Mack et al., 1967; Mack and LaChance,
kindreds with abnormally high bone mineral density have
1967), reported what they called "small but significant"
provided insight into the genetic regulation of bone mass. This
bone loss. If one extrapolated their results to long-
has led to potential therapeutic interventions based on the LRP5,
duration flights, these changes would have been
Wnt and BMP2 pathways. Another target is the RANK-
alarming—ranging from 5.3% per month in the calcaneus
L/osteoprotegerin signaling pathway, which influences bone
during Gemini 7 to 89% per month in the finger
turnover by regulating osteoclast formation and maturation.
phalanges during Gemini 4. The observations were based
Trials using such therapies in space are being planned. Among the factors to be considered are dose-response relationships,
on the use of densitometry of plain X-rays, which is now
bone quality, post-use recovery, and combination therapies—all
regarded as an inaccurate methodology (Rambaut et al.,
of which may have unique characteristics when the drugs are
1975). It is, however, interesting to note that these
authors also measured urinary and fecal loss of calcium in
a group of bed rest subjects and reported a correlation of
-0.7 between loss of bone mass and mean calcium intake.
Soviet researchers (Biriukov and Krasnykh, 1970;
The human skeleton has evolved in an environment where
Krasnykh, 1969—reported by Rambaut et al., 1975)
the force of Earth's gravity has been a continual presence.
found average losses of 4.7% per month in the os calcis in
It is, therefore, not surprising that removal of gravity
subjects in a bed rest study, and they reported that net
during long-duration space flight results in a loss of
losses during 8-10 weeks of bed rest were equivalent to
homeostasis in the skeleton, which adapts to the new
those seen in the 18-day Soyuz 9 flight.
environment by shedding calcium (Lang et al., 2004) at a
rate that is almost 10 times greater than that in a
In the Apollo flights (6-14 day missions, 1968-1972),
postmenopausal woman (Iki et al., 1996; Sirola et al.,
neutron activation analysis of fecal specimens (Brodzinski
et al., 1971) (Apollo VII-XI; 6-11 days), densitometry from plain radiographs (Mack and Vogt, 1971) (Apollo
* Correspondence to: Peter R. Cavanagh, Ph.D., D.Sc.
VII and VIII; 11 and 6 days, respectively), and single-
The Cleveland Clinic Foundation
photon absorptiometry of calcaneus and forearm
9500 Euclid Avenue / ND-20 Cleveland, OH 44195
(Rambaut et al., 1975) (Apollo XIV-XVI; 9, 12, and 11
Email:
[email protected]
days, respectively) were used to assess changes in bone
Phone: 216-445-6980; Fax: 216-445-6083
status. Brodzinski et al. (1971) called the Gemini findings of Mack and LaChance (1967) "dubious," and their own
Gravitational and Space Biology 18(2) June 2005 39
P.R. Cavanagh - Preventing Bone Loss in Space
measurements of calcium loss in Apollo VII-XI
changes was not correlated with flight time, presumably
crewmembers suggested less substantial changes. They
due to individual differences in rates of bone loss.
estimated that loss of total body calcium could be as little
as 7.5% per year of space flight, but they suggested that a
McCarthy et al. (2000) used three techniques (dual energy
calcium balance experiment should be conducted on
X-ray absorptiometry [DXA], ultrasonic measurements of
Skylab, and this was in fact accomplished (see below).
velocity [SOS], and broadband attenuation [BUA] of the
calcaneus) to evaluate changes in bone during two
Using similar methodology to their earlier studies, Mack
missions, of 180 and 20 days, to the Mir space station,
and Vogt (1971) reported average losses of 11.6% per
involving three subjects. DXA measurements resulted in
month in the lower extremity and 22.6% losses in the
significant variation between different sites in the body
upper extremity of six Apollo VII and VIII crew
for changes in BMD, with the greatest losses occurring in
members. As discussed above, in retrospect, these
the lumbar spine and proximal femur.
changes in "bone density" measured from plain
radiographs were clearly erroneous; control subjects on
Earth did not show such large changes. The single gamma photon absorptiometry of Rambaut et
al. (1975) found a loss of 5.1% per month in the lower extremity, a gain of 0.6% per month in the radius, and a loss of 4.6% per month in the ulnae of nine Apollo XIV-
XVI crew members.
D/Month (%)
One of the most complete series of calcium balance studies in space was conducted during the Skylab
missions (Skylab 2, 28 days, 1973; Skylab 3, 59 days,
1973; Skylab 4, 84 days, 1973-1974—Rambaut and Johnston, 1979; Smith et al., 1977; Smith et al., 1998; Smith et al., 1999; Tilton et al., 1980; Whedon et al.,
1977). Commencing 21 days prior to flight, during flight, and for 18 days post flight, the intake of 30 nutrients were
Figure 1a: Change in bone mineral density in different
monitored, 24-hour pooled urine collections were made,
anatomical regions (in percent change per month; negative
and fecal samples were vacuum dried for analysis.
values represent loss) during Mir missions and bed rest. Data
Weekly plasma samples were also taken. A 56-day
adapted from LeBlanc et al. (2000).
ground-based control experiment was also conducted.
During the Skylab 4 mission, average negative calcium
The application of modern imaging techniques to bone
balances of -100 (+25), -180 (+36), -229 (+60), -223
changes during space flight was first accomplished by
(+42), -88 (+52) were reported for the pre-flight, flight
LeBlanc and colleagues (LeBlanc et al., 1996; LeBlanc et
days 1-24, 25-56, 57-84, and post-flight measurements,
al., 1998; LeBlanc et al., 2000). In 1989, they installed a
respectively. In addition, using single-photon absorp-
Hologic 1000W dual X-ray absorptiometry (DXA)
tiometry, Smith et al. (1977) indicated that a mean loss of
scanner at the cosmonaut training center in Star City,
0.4% per month occurred in the calcanei of all nine
Moscow, in the former USSR. Between 1990 and 1995,
Skylab 2-4 crew members, while the investigators
they studied 18 cosmonauts who had flown for between
detected negligible losses in the radii (0.06% per month)
126 and 438 days (LeBlanc et al., 1996; LeBlanc et al.,
and a gain in the ulnae (0.4% per month).
2000). These measurements showed regional losses
during flight of between 1.06% and 1.56% per month in
Rambaut et al. (1979) pointed out that "the chain of
the spine, pelvis and proximal femur, but no significant
events leading ultimately to bone loss inflight remains
changes in the upper extremities (Figure 1a). Losses were
elusive." In an article almost 20 years later, in which the
parallel, but smaller, during bed rest, except in the arms,
urine of Skylab crew members was re-analyzed, Smith et
where losses were greater than during flight. These data
al. (1998) shed light on this mechanism by demonstrating
showed, for the first time, a pattern of lower-extremity
that urinary excretion of collagen breakdown products
loss and upper-extremity preservation during flight. The
during the Skylab 4 mission was 40-45% higher than pre-
authors concluded that the in-flight exercise programs
flight values, indicating that space flight is associated
were not sufficient to completely ameliorate bone loss
with increased bone resorption.
during flight (no countermeasures were used during bed
Using computer tomography (CT), Oganov et al. (1990)
measured mineral density of lumbar vertebrae in four
Lang et al. (2004) provided data from DXA, volumetric
Salyut-7 crew members before and after extended flights
quantitative computer tomography (vQCT), and
(5-7 months' duration). These authors reported that bone
quantitative ultrasound (QUS) on crew members from the
mineral density (BMD) diminished only in some of the
Expeditions 2-6 to the International Space Station (ISS;
test subjects and emphasized that the magnitude of
2001-2003, 130-197 days). The authors' data confirmed
40
Gravitational and Space Biology 18(2) June 2005
P.R. Cavanagh - Preventing Bone Loss in Space
that little progress had been made in preventing loss of
PRIOR COUNTERMEASURES
bone mineral in the 30 years since Skylab. Notably,
vQCT allowed an examination of the loss in both
The only countermeasure that has so far been used in
trabecular and cortical fractions of bone and also
space for bone loss, albeit unsuccessfully, is exercise.
estimates of the volumetric BMD (vBMD) as well as the
Astronaut-physician William E. Thornton was a tireless
conventional areal BMD (aBMD). These data confirmed
proponent of exercise countermeasures, and his accounts
the large losses in the spine and proximal femur (Figure
of exercise countermeasures and devices (Thornton,
1b), and indicated that the rate of loss of bone mineral
1989a; Thornton, 1989b; Thornton, 1989c) are required
content (BMC) in trabecular bone in the proximal femur
reading in order to understand the history of use of this
was approximately twice that of the cortical loss. Since
modality. There is also a good description of exercise and
trabecular bone cannot be replaced after loss of trabecular
other countermeasures in Nicogossian et al. (1994). The
continuity (Langton et al., 2000), this later finding is of
countermeasure tradition began in the confined space of
particular concern. The authors also found that calcaneal
the Gemini capsule, where a bungee cord held by a loop
estimates are not good surrogates for central or upper
to the feet was pulled to exercise the arms and legs
extremity skeletal measures and concluded that there was
(Dietlein, 1965). There is no record of its efficacy,
a continuing need to improve countermeasures to bone
although measurements of heart rate, blood pressure, and
loss, as it has become clear that current efforts are
respiration rate were taken during exercise to record
cardiac response to exercise in space (Dietlein and Rapp,
The Soyuz 9 flight (18 days, 1970), on which bungee and
expanders were also used for exercise (Nicogossian et al., 1994), highlighted the need for more effective
countermeasures to combat the general loss of
conditioning (Yegorov et al., 1972). Subsequently, some
of the Salyut Space orbital stations (Salut 1, 1971 to
Salyut 6, 1985) were equipped with a passive treadmill, a bicycle ergometer, and a gravity simulation suit for long
wear (Gazenko et al., 1976). The efficacy of this
"Penguin Suit" (Nicogossian et al., 1994) has not been
Figure 1b: Data showing change in regional bone (in percent
change per month; negative values represent loss) from 13 crew
The Skylab astronauts used several on-board exercise
members on the International Space Station. Data adapted from
devices, including a bicycle ergometer and a Teflon®
Lang et al. (2004).
plate, not available until Skylab 4, on which they
performed an unusual form of tethered locomotion
It is interesting to note that long-duration space flight
(Figure 2a; Thornton and Rummel, 1977; Thornton,
continues to be a male bastion, and thus we do not have
1989a). They also had a Mini Gym exercise device,
adequate data on gender differences in bone loss in space.
which allowed concentric muscular exercise to be
For the 32 subjects for whom DXA data are available,
performed, primarily benefiting the arms and trunk.
there are only two women: one in the LeBlanc et al.
Although this device probably transmitted higher forces
(2000) series, who was reported to have similar responses
to the legs than those from the bicycle ergometer, the
to the mean of the group, and one in the Lang et al. (2004)
force levels were still considered inadequate (Thornton
series, whose data were not uniquely identifiable.
and Rummel, 1977). No systematic record of the use of
Presumably, privacy issues prevented this disclosure, but
these devices by Skylab crew members is available in the
one would hope that all crew members would make such
literature, although it is likely that such records were kept.
data available in the future in the interest of science.
Gravitational and Space Biology 18(2) June 2005 41
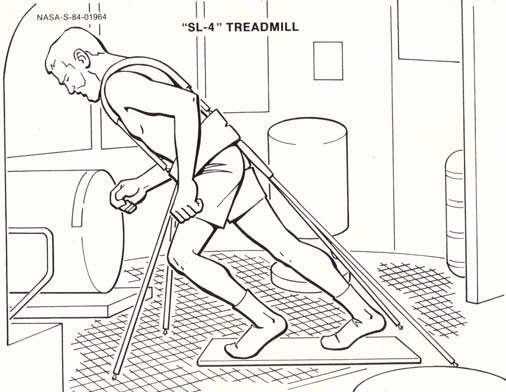
P.R. Cavanagh - Preventing Bone Loss in Space
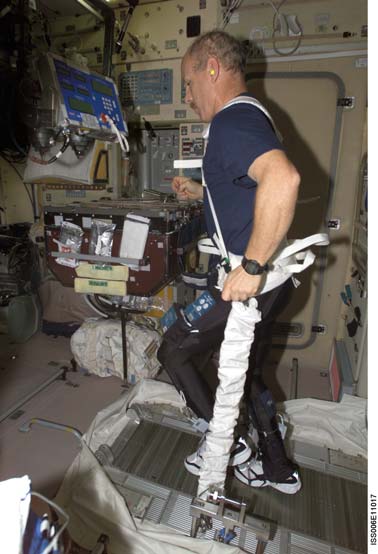
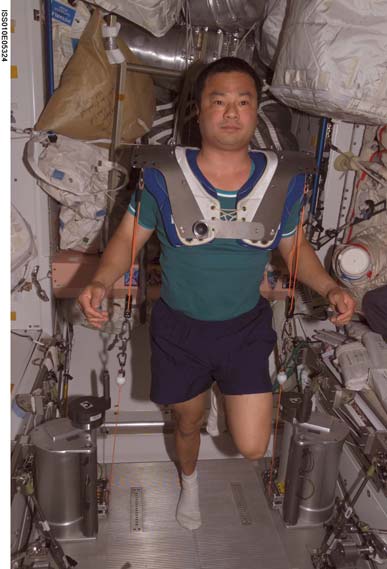
Cosmonauts on Mir have been said to perform exercise "up to 3 hours per day" (Nicogossian et al., 1994), while others believe that the exercise was 2-3 hours on 3 of 4 days (LeBlanc et al., 2000). The passive treadmill was considered the "stadium" from which exercise was performed. While the subject was tethered in place using bungees, he not only walked and ran, but also performed calisthenics and upper-body exercises using additional bungee cords for resistance (Figure 3). The data from LeBlanc et al. (2000) showed clearly that this protocol, even if faithfully performed, is not an effective countermeasure for bone loss. The exercise facilities available on the ISS through Expedition 12 consist of a Treadmill Vibration Isolation and Stabilization System (TVIS; Figure 2c; McCrory et
Figure 2a: A Teflon® plate on which Skylab astronauts
al., 1999), a cycle ergometer with vibration isolation
exercised in an unusual form of locomotion (Thornton and
(CEVIS; Figure 2d), and the Interim Resistive Exercise
Rummel, 1977; Thornton, 1989a). Artwork courtesy of NASA.
Device (iRED; Figure 2e; Schneider et al., 2003). There
is also a bicycle ergometer available in the Russian
Thornton is thought to be the first man to run around the
segment. None of these devices has a force measurement
world in low Earth orbit. This feat was performed during
capability, and there is very little published information
one complete orbit of STS-8 (1983) on a treadmill that
about their performance characteristics. When running on
Thornton helped to design. Because the mid-deck of the
the treadmill, a subject must be tethered using a subject
Space Shuttle was not particularly spacious, the passive
load device (SLD) to restrain him on the treadmill
treadmill had to be stowable in a locker, a fact that
surface, and, optionally, a subject position device (SPD)
severely limited its belt length (Figure 2b). The subject
is used to keep the subject in an area of the treadmill
was tethered by bungee cords, which applied an unknown
where a pitch oscillation of the treadmill will not be
tension to return the crew member to the treadmill
initiated. Each crew member is assigned a period of 2.25-
surface. Kinematic analysis of on-orbit film taken during
2.5 hours every day for exercise—including set-up and
running on the treadmill (Thornton et al., 1998) indicated
break-down time, which can consume more than 50% of
that there was restricted range of motion at the lower-
the assigned period. The work of Lang et al. (2004)
extremity joints and a plantar-flexed "tip-toe" gait. No
showed that these devices as they are presently used are
measurements of the foot forces were made.
not effective as a countermeasure for bone loss during
long-duration flights. As we shall discuss below, prolonged bed rest is considered to be a viable analog of space flight.
Shackelford et al. (2004) conducted a program of vigorous resistance training (averaging 74% of one repetition maximum) in nine individuals during a 17-week confinement. The exercise was found to have a beneficial effect on BMD during bed rest compared to controls, specifically in the lumbar spine (+3% vs. -1%), total hip (+1% vs. -3%), heel (+1% vs. -3%), total body (0% vs. -1%), and pelvis (-0.5% vs. -3%). However, the high levels of load imposed on the muscle groups studied have never been achieved in space, and it is unlikely that in-flight exercise devices currently in use will permit such loads to be achieved.
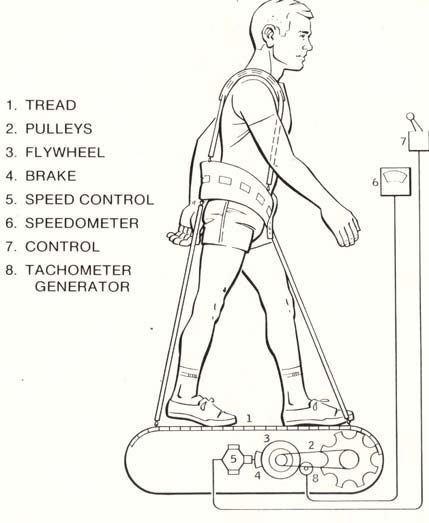
Figure 2b: The passive Shuttle treadmill designed by
Astronaut-Physician William Thornton (Thornton, 1989c).
Artwork courtesy of NASA.
42 Gravitational and Space Biology 18(2) June 2005


P.R. Cavanagh - Preventing Bone Loss in Space
Figure 2c: The International Space Station Treadmill with a
Figure 2d: The Cycle Ergometer with Vibration Isolation and
Vibration Isolation and Stabilization System (TVIS). (NASA
Stabilization System (CEVIS) in use on the International Space
photography)
Station. (NASA photography)
Figure 2e: The Interim Resistive Exercise Device (iRED) in use on the International Space Station (ISS). (NASA photography)
Gravitational and Space Biology 18(2) June 2005 43
P.R. Cavanagh - Preventing Bone Loss in Space
Figure 3: A page from the Mir cosmonaut exercise instruction manual showing a 24-stage exercise session performed on the treadmill.
44 Gravitational and Space Biology 18(2) June 2005
P.R. Cavanagh - Preventing Bone Loss in Space
WHY HAVE EXERCISE COUNTERMEASURES IN
BONE REMODELING
SPACE NOT BEEN EFFECTIVE?
Bone is an active tissue that is constantly being
Since exercise has been the only countermeasure to bone
remodeled, principally by the action of two cell types:
loss so far attempted in space, and since considerable
osteoclasts, which resorb bone, and osteoblasts, which
bone loss has occurred on all flights to date, it would be
build new bone (Figure 4). It is estimated that all of the
tempting to conclude that exercise is not an appropriate
bone in the adult skeleton is replaced every 10 years
countermeasure. There are, however, several reasons why
(Marx, 2004). Homeostasis of bone is only maintained if
such a conclusion may be premature: (1) There has never
the opposing—or perhaps complementary—actions of
been a controlled study of exercise, either in space or
osteoblasts and osteoclasts are balanced. A defect in
during bed rest. The lack of such a study in the more than
either process can result in accumulation of bone (as in
40 years that this problem has been recognized is highly
osteopetrosis) or in a net loss of bone (as in osteoporosis)
perplexing to the current authors and perhaps reflects the
(Helfrich, 2003; Phan et al., 2004). The mineral phase of
fact that NASA has traditionally been an engineering
bone is of primary importance to density, and therefore
rather than a science agency; (2) The loads applied to the
BMD has been used in the past as a main indicator of
body by any piece of exercise equipment were not
bone status (Kanis, 2002). However, there is now
measured prior to 2003, so it is not known whether or not
increasing interest in measures of bone "quality" that
equipment exerted 1-g-like loads; (3) Exercise adherence
include structural as well as compositional information
may have been less than optimal and, contrary to common
(Ammann and Rizzoli, 2003; Turner, 2002), and it is
belief, the ISS program was the first time a mandatory
likely that a composite measure will eventually replace
exercise program was instituted as part of a flight plan;
BMD as the parameter of choice.
(4) It is not known if a single daily concentrated "dose" of
exercise in 0-g can effectively replace a "dose" that in 1-g
The majority of current therapeutic interventions could be
is distributed throughout the day; (5) The duration of
classed as resorption-prevention drugs. A number of
exercise programmed to date may not have been adequate
advances in understanding how osteoclasts differentiate,
to achieve the desired result; (6) There is considerable
mature, and are activated have recently been made (Boyle
debate in the scientific community about the optimal
et al., 2003; Marx, 2004). Prevention of osteoclast
loading strategy that will provide an osteogenic stimulus
formation (osteoclastogenesis) and development has been
to bone (Turner, 1998; Turner and Pavalko, 1998).
a prime target through a number of different pathways
Evidence in the literature ranges from a few intermittent
(see below). On the formation side of the equation,
large loads per day (Lanyon, 1996) to 18,000 small-
preventing osteoblast cell death (apoptosis) is also of
amplitude vibrations in a 10-minute period (Rubin et al.,
interest, and a number of other "anabolic" or bone-
2002a; Rubin et al., 2002b). There is also debate
building drugs with uncertain mechanisms are also being
regarding the relative role of force and rate of change of
explored (Bisello et al., 2004; Deal and Gideon, 2003).
force (Cullen et al., 2001; Linde et al., 1991; Mosley and
Our own experiments using force-measuring insoles
Traditionally, supplementation of daily intake of vitamin
during exercise on the ISS (Rice et al., 2004) have
D and calcium (current recommended daily allowances
suggested that neither the load nor the duration of
[RDAs] 400 IUs and 1500 mg, respectively) have been
treadmill exercise in the current ISS exercise program is
considered mainstays of osteoporosis prevention.
adequate to replace 1-g exercise.
Adequate calcium is needed for mineralization, and
vitamin D plays a role in the regulation of calcium
Only when all six issues noted above have been carefully
deposition for bone mineralization. Both of these agents
examined can the role of exercise as a countermeasure to
have weak antiresorptive properties (compared, for
in-flight bone loss be determined. Until such time, it is
example, to bisphosphonates [Reginster, 2004]—see
reasonable that the flight medicine community is looking
below), but combined therapy for 18 months (1200 mg
to explore the use in space of pharmacological options
calcium plus 800 IU vitamin D3 [cholecalciferol]) has
that are being used on Earth to prevent postmenopausal
been shown to be effective in reducing hip fracture in
elderly women who were Vitamin D deficient (Chapuy et
al., 1992). The bioavailability of the various forms of
The remainder of this review will examine the cellular
calcium used in supplementation (calcium carbonate,
and molecular targets for such therapy, present the
citrate, phosphate, lactate, and formate) have been shown
currently available options, and discuss the limitations of
to be different (Hanzlik et al., 2005).
knowledge required for the implementation of these
therapies in space.
Since it is not always clear whether or not dietary intake
of these agents is adequate, most drug trials routinely
include calcium and vitamin D supplementation in
control, placebo, and treatment arms. Astronaut diets can
Gravitational and Space Biology 18(2) June 2005 45
P.R. Cavanagh - Preventing Bone Loss in Space
Figure 4: Schematic of a bone multi-cellular unit (BMU). Osteoclasts resorb a cavity that is later occupied by osteoblasts that lay down new bone in the form of osteoid that subsequently undergoes mineralization (Deal and Gideon, 2003). Reprinted with the permission of The Cleveland Clinic Foundation.
be closely controlled, so inclusion of RDA and
considered the regulator of the female skeleton and
supplementation in diet can be easily accomplished.
testosterone the male regulator. The discovery of
mutations in the aromatase gene in men and concurrent
HORMONE REPLACEMENT THERAPY
abnormalities in skeletal metabolism (osteopenia and
unfused epiphyses) have focused attention on the
Estrogen, in the form of 17β-estradiol, has a complex
importance of estrogen physiology. Aberrations in
agonistic action on estrogen receptors (ERs) in the
osteoclast acivity due to deficiency of inhibitors may
nucleus of osteoblastsic cells (Riggs and Hartmann,
attend the loss of estrogen with aging in both men and
2003), which in turn affect estrogen receptor elements
women and cause increased bone turnover (Carani et al.,
(EREs) in target genes. In estrogen deficiency, resorption
1997; Khosla et al., 2002; Khosla et al., 2004).
outpaces formation, resulting in net bone loss. Estrogen
also stimulates breast epithelial cell production and has
SELECTIVE ESTROGEN RECEPTOR
been implicated in breast cancer risk (Riggs and
MODULATORS (SERMS)
Hartmann, 2003).
Because of the side effects of HT, there has been
Hormone replacement therapy (HT) using estrogen
increased interest in this class of nonhormonal drugs that
(unopposed HT) or estrogen-progestin (opposed HT) was
target the ER. SERMs can have both agonist and
widely recommended for postmenopausal women until
antagonist effects in different tissues (e.g., tamoxifen
the landmark Women's Health Initiative (WHI) study
[Tamofen], used in the treatment of ER-positive breast
(Rossouw et al., 2002) demonstrated a number of adverse
cancer, is an antagonist that slows the proliferation of
responses (increased risk of coronary artery disease,
tumor cells, whereas raloxifene [Evista] is a bone agonist
stroke, thromboembolism, and breast cancer) in subjects
that has an antiresorptive effect) (Riggs and Hartmann,
using opposed HT. Riggs and Hartmann (2003) stated
2003). Different SERMs that have similar effects on bone
that estrogen was the most widely prescribed drug in the
(e.g., raloxifene and idoxifene [investigational]) appear to
world and that it was taken by 38% of postmenopausal
have their modes of action through different molecular
women in the United States. In addition to reducing the
pathways (Nuttall et al., 2000). Because raloxifene,
risk for nonvertebral fractures (Torgerson and Bell-Syer,
which is administered orally once per day, has a
2001), HT also had the added advantage of relieving a
preferential effect on vertebral fracture risk reduction
number of perimenopausal symptoms. Because of the
(Ettinger et al., 1999), it is possible that there are
increased risk of adverse side effects, HT is no longer
differences between the action of SERMs on trabecular
recommended for prevention or treatment of osteoporosis
vs. cortical bone.
There are some indications that raloxifene therapy
Estrogen also has significant effects on skeletal
decreases cardiovascular events in women with risk
metabolism in men. Traditionally, estrogen was
factors at baseline (Barrett-Connor et al., 2002) but carries
46 Gravitational and Space Biology 18(2) June 2005
P.R. Cavanagh - Preventing Bone Loss in Space
with it a small increase in the risk for thromboembolism
ANTIRESORPTIVE DRUGS
(Daly et al., 1996). SERMs do not appear to alleviate
postmenopausal symptoms (National Osteoporosis
The largest class of antiresorptive drugs is the
Foundation, 2002; Cranney et al., 2002).
bisphosphonates (such as alendronate [Fosamax],
etidronate [Didronel], ibandronate [Boniva], pamidronate
The common risk for both therapies is that of deep vein
[Aredia], risedronate [Actonel], zoledronate [Zometa],
thrombosis, especially in conditions of clotting
and tiludronate (Skelid)]. The drugs are distinguished by
abnormalities. This risk is small but nonetheless present
their potency, which is usually positively affected by the
statistically. In other situations the drugs have divergent
presence of a nitrogen atom (e.g., etidronate [low] to
risks. Breast hyperplasia and breast cancer are not found
zoledronate [high]), by their mode of delivery (e.g.,
with the SERM agents as they are with estrogen.
intravenously for pamidronate and zoledronate, orally for
Moreover, the SERM drugs do not cause cervical
alendronate, orally, intravenously, or by injection for
endometrial hyperplasia, menstrual bleeding, or cervical
ibandronate), and by the frequency and size of dosing
(e.g., 5 or 10 mg daily or weekly for alendronate, 2.5 mg
daily for ibandronate, 10-90 mg annually for zoledronate).
The SERM drugs may offer an option for treatment of
An excellent review of these drugs is provided by
prostate cancer. In the presence of decreasing androgens
Reginster (2004).
with aging, estrogen induces prostatic hyperplasia and
neoplasia. Antiestrogens and SERMs suppress prostate
Bisphosphonates are powerful and specific inhibitors of
carcinogenesis. Some preliminary studies suggest that
osteoclasts (Figure 5). They were originally thought to
SERMs may not be useful as a general treatment for male
exert their action via incorporation in the skeleton by
osteoporosis, but there are some male patients, small in
mimicking pyrophosphate and binding to the hydroxy-
number, with the requisite balance of estrogen and
apatite crystals in the bone matrix (Licata, 2005),
testosterone for whom SERMs may be beneficial (Steiner
especially at sites of remodeling, the bone multicellular
and Raghow, 2003; Doran et al., 2001).
units (BMUs) (Russell et al., 1999).
Figure 5: Schematic of bisphosphonate action (Rodan and Fleisch, 1996). Where bisphosphonate (BP in the diagram) has been
incorporated into the bone matrix, osteoclastic resorption of bone cannot occur. Reprinted with permission.
Their actions have since been shown to be complex,
function by "energy starving" the cell. Once incorporated,
however. The amino bisphosphonates inhibit osteoclastic
bisphosphonates remain bound at the bone surface and
cholesterol synthesis and membrane function and increase
exhibit extremely low serum concentrations, thus limiting
cellular apoptosis. The non-amino bisphosphonates
side effects. In general, the third generation (N2
produce ineffective ATP analogs and inhibit osteoclast
containing) bisphosphonates have shown approximately
Gravitational and Space Biology 18(2) June 2005 47
P.R. Cavanagh - Preventing Bone Loss in Space
40-50% reduction in the risk of vertebral and nonvertebral
the upper gastrointestinal (GI) tract, constipation,
fractures compared with placebo in postmenopausal
flatulence, hypocalcemia, and diarrhea), but severe
women (Black et al., 1996; Chesnut et al., 2004; Harris et
esophageal reactions have been reported with alendronate
al., 1999) and have also resulted in increased BMD in the
(Schnitzer et al., 2000). Consequently, its use is not
lumbar spine, total hip, and trochanter in women with and
recommended for patients with a history of upper GI
without osteoporosis (Cooper et al., 2003; Mortensen et
al., 1998; Ravn et al., 1999).
There is some concern that bone formed during the
There are two bed rest studies involving bisphosphonates
administration of bisphosphonates may not have the same
that are relevant to the space program (LeBlanc et al.,
"quality" as normal bone, thus negatively affecting on the
2002; Watanabe et al., 2004). LeBlanc et al. (2002)
mechanical integrity of the skeleton. Animal studies with
administered 10 mg of alendronate daily to eight male
bisphosphonates have shown a delay in fracture healing in
subjects undergoing 17 weeks of horizontal bed rest.
rats and rabbits and an increase in the presence and
Compared with concurrent and historical controls, BMD
persistence of microcracks and reduced remodeling,
loss was significantly attenuated (or eliminated) in the
suggesting a potential change in biomechanical factors (Li
alendronate treatment group in the lumbar spine, femoral
et al., 1999; Li et al., 2001; Mashiba et al., 2001; Lehman
neck, trochanter, and pelvis (but not calcaneus). Most
et al., 2004). In addition, it is notable that Ruggiero et al.
markers of bone collagen breakdown and resorption
(2004) have identified a cluster of patients on chronic
(cross-linked N-teleopeptide of type I collagen [NTX],
bisphosphonate therapy that had an associated risk of
pyridinium [Pyd], and deoxypyridinium [D-Pyd]) in-
osteonecrosis of the jaw. This condition is also seen in
creased in both groups, but significantly less so in the
the myeloma patients treated with i.v. zoledronic acid
treated group than in controls. Markers of bone formation
monthly (Lugassy et al., 2004), which is not the way it is
(alkaline phosphatase, bone-specific alkaline phosphatase,
used for treating osteoporosis. It is possible that such
and osteocalcin) were unchanged in controls, but were
patients may have immune compromise supporting local
decreased in the treated group because of the reduced
dental infection and subsequent bone destruction.
bone turnover. These results demonstrate that the drug
does not ablate the bone loss totally, thus the observed
If sequential combination therapy of different drugs is
clinical effects may require simultaneous mechanical
planned, Gasser et al. (2000) showed in studies of rat
bone that the response to an anabolic drug (see below)
was delayed in animals pretreated with bisphosphonates.
Watanabe et al. (2004) administered 60 mg of pamid-
However, in clinical studies, long-term use of alendronate
ronate to seven male subjects 14 days before 90 days of 6-
and risedronate for 7-10 years shows no similar findings.
degree head-down bed rest. These authors also showed
Both drugs still suppress fractures, which argues against
that alendronates, in addition to their osteoprotective
the adverse effects seen in animal models. Furthermore,
properties, decrease the risk of renal stones. Compared
histomorphometry shows no abnormal characteristics in
with sedentary and resistance training controls, the
patients after 3 or more years of use.
pamidronate-treated subjects not only maintained
significantly more bone in the proximal femur and lumbar
ANABOLIC DRUGS
spine, but also showed no evidence of urolithiasis (stones
in the urinary tract). In the other groups, six subjects
Drugs in this class exert their mode of action by
were found to have radiographic evidence of stone
increasing bone formation rather than by inhibiting
formation during bed rest. All but one of these stone-
resorption. The important role of parathyroid hormone
forming subjects had baseline hypercalciuria (>250 mg
(PTH) in regulating bone and mineral metabolism has
per day). Such patterns of stone formation may be a
been known for more than 70 years (Bisello et al., 2004),
feature of all bed rest studies, and perhaps of long-
but classical teaching identifies PTH as a powerful
duration space flight, that has been previously
mobilizer of skeletal calcium into the serum in the
overlooked. However, it is extremely unusual for healthy
presence of hypocalcemia (i.e., a state of secondary
patients with no prior stone risk to become "at-risk" in
hyperparathyroidism). Evidence from animal exper-
such a short time, and these results, although cautionary,
iments has shown that daily injection of PTH had
need to be replicated.
anabolic effects on bone, and recent work has resolved
these apparently paradoxical effects by showing a
Shapiro et al. (personal communication), in an as yet
dependency on the pattern of exposure. Chronic elevation
unpublished study, showed a reduction of bone loss in the
of PTH (as in primary hyperparathyroidism) leads to
lower extremities of patients with spinal cord injuries who
increased bone resorption, whereas intermittent elevation
had been administered intravenous zoledronate. The
(as in once-daily injections with a short half-life) leads to
paradigm of spinal cord injury has been suggested to be
increased formation. The mechanism of action of PTH
another analog of space flight, although the absence of
appears to be the stimulation of existing osteoblasts via
muscular action may tend to make it even more severe
surface PTH receptors and interaction with RANK-L
from a disuse point of view.
(NF-κB; see below) (Deal and Gideon, 2003). It is
The side effects of bisphosphonate therapy from the major
known that the amino-terminal region of PTH (the first 34
study series have generally been mild (adverse effects in
amino acids) is necessary and sufficient for full activity,
48 Gravitational and Space Biology 18(2) June 2005
P.R. Cavanagh - Preventing Bone Loss in Space
and the only anabolic agent that is currently Food and
activator of nuclear factor-κB ligand (RANK-L) ratio (see
Drug Administration (FDA) approved for use in the
below) (Locklin et al., 2001).
treatment of osteoporosis is recombinant teriparatide
(rhPTH [1-34] [Forteo]).
RANK-L/OPG
Administration of teriparatide (daily subcutaneous
In 1997, a new pathway regulating bone resorption was
injection 20 µg or 40 µg for 19 months) to women with
identified (serendipitously) by a group looking for novel
low bone mass and a history of prior fracture resulted in
genes in the rat intestine (Simonet et al., 1997). The
an almost 10% increase in vertebral BMD; treatment
transgenic mouse overexpressing one particular gene was
reduced the risk of a second vertebral fracture by
found to have ostopetrosis and a deficiency of osteoclasts
approximately 65% and that of a nonvertebral fracture by
(Khosla, 2001) and the responsible protein was called
approximately 50% compared with placebo. There is
osteoprotegerin for its protective role in maintaining bone
some concern that high doses of teriparatide (up to 60
mass. Simultaneously, Yasuda et al. (1998) found the
times greater than approved human doses) have caused
same protein in a targeted search for the signaling link
osteosarcoma in rats, but not monkeys. No similar
that had been previously hypothesized to exist between
complications have been observed in human studies, but
osteoclasts and ostoblasts (Rodan and Martin, 1982). The
an initially promising trial conducted in men (Orwoll et
pathway that has been identified as a result of these and
al., 2003) was terminated because of concern regarding
subsequent studies is shown in Figure 6. OPG is secreted
the animal results.
as a soluble protein from bone marrow stromal cells and
appears to be a decoy receptor, which binds to RANK-L.
It is also known that PTH-related peptide (PTHrP), a
Since RANK-L is a major factor in osteoclast differ-
protein with some homology to PTH that is produced by
entiation, activation, and apoptosis inhibition, it follows
tumors and leads to hypercalcemia, shares many of the
that the binding of RANK-L to OPG, rather than to its
actions of PTH but has receptors that are much more
target RANK on the osteoclast precursor cell, will prevent
widely distributed (Bisello et al., 2004). The authors have
bone resorption. Because RANK-knockout mice also
initial evidence from human studies that PTHrP has the
exhibited osteopetrosis and absence of osteclasts (Li et al.,
potential to be a powerful anabolic agent, and clinical
2000), the existence of a new OPG/RANK/RANK-L
trials to explore this possibility are ongoing.
pathway in the control of bone resorption was confirmed.
Several genetic mutations of this pathway are associated
It is possible that the mechanism for the differential
with bone diseases such as the family of hyperphos-
effects of intermittent vs. continuous levels of PTH is in
phatasias, Paget's disease, and possible bone loss in
the modulation of the osteoprotegerin (OPG)/receptor
inflammatory arthritis (Boyle et al., 2003; Khosla, 2001).
Figure 6: Schematic of the OPG/RANK-L pathway (Khosla, 2001). Note that OPG acts as a decoy receptor preventing RANK from
attaching to its ligand RANK-L and therefore inhibiting osteoclast differentiation. Copyright 2001, The Endocrine Society. Reprinted with
permission.
Gravitational and Space Biology 18(2) June 2005 49
P.R. Cavanagh - Preventing Bone Loss in Space
OPG was an obvious choice as a clinical therapeutic agent
feature of calcitonin is that it can be administered by
to prevent osteoporosis, and indeed two forms of the
many routes, including nasally in the form of a daily (or
protein were examined by Amgen in clinical trials of
intermittently administered [Tekeoglu et al., 2005]) spray.
osteoporosis (Bekker et al., 2001) and multiple myeloma
and breast carcinoma (Body et al., 2003). A presumed
BONE GENETICS
combination of concerns regarding efficacy, safety,
treatment duration and manufacturing factors has resulted
In the last 5 years, early insights into some of the genetic
in OPG's no longer being examined for clinical use. OPG
determinants of bone mass have been obtained. Ralston
does, however, continue to be explored for the treatment
(2003) and Recker (2004) have recently reviewed the
of bone tumors (Wittrant et al., 2004). A fully human
status of present knowledge in this area. Johnson et al.
monoclonal antibody for RANK-L, AMG 162, is being
(2004) have commented regarding "how little we really
developed as an osteoporosis treatment instead (Bekker et
know about the genes that control bone mass." The
al., 2004; McClung et al., 2004). Phase III clinical trials
genetic basis for diseases caused by a defect in osteoclasts
were initiated in late 2004 for AMG 162.
is discussed by Helfrich (2003).
CALCITONIN
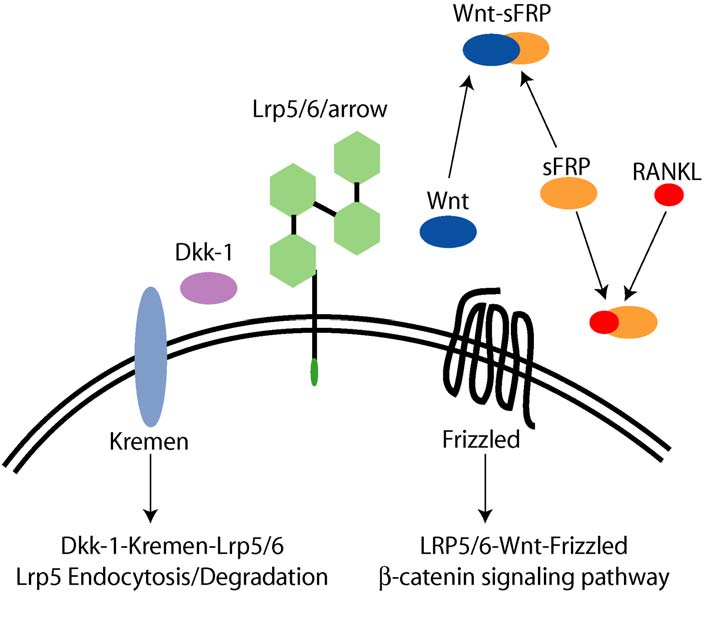
Gong et al. (2001) found that that the LRP5 gene, which
encodes the low-density lipoprotein receptor-related
The peptide calcitonin exerts a complex inhibitory action
protein 5, is important in bone mass accrual. They
on osteoclast function (Kajiya et al., 2003). It has been
reported that loss-of-function mutations in LRP5 caused
used in trials of both men and women with low bone mass
the autosomal recessive disorder osteoporosis-
and has been shown to stabilize (or prevent) bone loss
pseudoglioma syndrome and that Wnt-mediated signaling
(Toth et al., 2005) and, in women, to decrease vertebral
via LRP5 affects bone accrual during growth and peak
fracture rate (Munoz-Torres et al., 2004). One attractive
bone mass (Figure 7).
Figure 7: The Wnt signaling pathway that has been discovered through genetic studies of patients with high bone mass (Johnson et al.,
2004). Adapted and reproduced from J Bone Miner Res 2004;19:1749-1757 with permission of the American Society for Bone and
Mineral Research.
Subsequently, mutations in the same gene were also
Johnson et al. (2004). Genes regulating lipoxygenase are
found to be associated with diseases in which there was
also believed to influence bone mass (Klein et al., 2004).
high bone mass (Boyden et al., 2002; Little et al., 2002).
The lack of inhibitory action of the protein Dkk-1 on the
There are indications that the Wnt signaling pathway is
Wnt signaling pathway suggested this protein as a
activated in response to mechanical loading (Johnson,
potential therapeutic target for modulating bone mass. A
2004), and this may be a key element in the elusive
review of LRP5 and Wnt signaling is presented by
mechanotransduction that has long been hypothesized to exist.
50 Gravitational and Space Biology 18(2) June 2005
P.R. Cavanagh - Preventing Bone Loss in Space
An alternative approach to the human linkage and
Finally, the early discovery of the vitamin D receptor
association studies described above is the use of mouse
gene helped introduce the notion that bone mass had a
models in quantitative trait locus (QTL) analysis (Liu et
genetic basis (Eisman, 1995).
al., 2003; Rosen et al., 2001). QTL is basically a
statistical analysis, sometimes of the entire genome, to
The complexity of BMD as a trait and the importance of
identify which regions of the genome contain loci that
gene-environment interactions have been emphasized in a
influence the phenotype of interest.
study of risk factors for low spine and hip BMD involving
12 candidate gene loci and lifestyle factors by Lau et al.
The genes encoding type I collagen (COLIA1 and
COLIA2) are mutated in osteogenesis imperfecta and may
be useful markers of other osteoporotic phenotypes
While these various studies of genetic influence on bone
(Mottes et al., 1998).
mass are in their early stages, there is a high likelihood
that they will eventually identify new therapeutic targets.
The estrogen receptor gene may regulate some aspects of
bone density since the discovery of a male patient with a
gene mutation and osteoporosis (Gennari et al., 2005).
Table 1. The major classes of osteoprotective therapeutic drugs
Drug Manufacturer
Class Action
Alendronate sodium
Merck Bisphosphonate
Novartis Bisphosphonate
Procter & Gamble /
Bisphosphonate Inhibit
Inhibit osteoclast
Inhibit osteoclast
Inhibit osteoclasts
PTHrP Osteotrophin
Inhibit osteoclast
SPECIAL CONSIDERATIONS FOR
astronaut corps comprises primarily younger men (see
THERAPEUTIC DRUG USE IN SPACE
remarks above regarding the number of women astro-
nauts/cosmonauts who have undergone long-duration
The use of therapeutic drugs in space requires both the
space flight), and such individuals are likely to be in good
provider and the patient to accept a different set of
bone health at the time of treatment. Such clinical trials
standards, assumptions, and approvals compared with the
typically take many years to accomplish (for example, the
use of the same drugs on Earth. For example, the primary
WHI study, mentioned above, was scheduled for 8.5
criterion that the FDA uses for approval of drugs designed
years), and the time frame could slow the identification
to treat osteoporosis is a demonstrated reduction of
and application of effective therapies.
fracture risk, usually hip or vertebral fracture. Such
Given current NASA priorities, it is almost certain that
evidence usually comes from a clinical trial of
there will not be a sufficient number of astronauts to
postmenopausal women with evidence of osteoporosis
allow a placebo-controlled dose-ranging on-orbit trial
that is blinded, placebo controlled, and randomized. This
with sufficient statistical power to be mounted in the next
approach may not be appropriate for decisions regarding
decade. It is, therefore, likely that the decision to use a
drugs for use in long-duration space flight, since the
therapeutic drug for astronauts will be based on evidence
Gravitational and Space Biology 18(2) June 2005 51
P.R. Cavanagh - Preventing Bone Loss in Space
from a bed rest study supported by experience in a few
Evaluation) randomized trial. Journal of the American
individual volunteers who will take the drugs prior to
Medical Association 287(7):847-57
and/or during space flight.
Bekker, P.J., Holloway, D., Nakanishi, A., Arrighi, M.,
Among the questions that will need to be answered in
Leese, P.T., and Dunstan, C.R. 2001. The effect of a
these human trials are: (1) What is the bioavailability of
single dose of osteoprotegerin in postmenopausal women.
the various drug therapies in 0-g? (2) Are the dose-
Journal of Bone and Mineral Research 16(2):348-60
response curves similar in 0-g to those established in 1-g?
(3) What are the post-flight consequences for bone health
Bekker, P.J., Holloway, D.L., Rasmussen, A.S., Murphy,
of taking osteoprotective drugs? (4) If drugs need to be
R., Martin, S.W., Leese, P.T., Holmes, G.B., Dunstan,
taken on-orbit, how should they be stored for maximum
C.R., and DePaoli, A.M. 2004. A single-dose placebo-
effectiveness? (5) How will a drug's effectiveness be
controlled study of AMG 162, a fully human monoclonal
determined on-orbit so that doses can be modulated? (6)
antibody to RANKL, in postmenopausal women. Journal
What is the best combination of drug and exercise
of Bone and Mineral Research 19(7):1059-66
countermeasures?
Biriukov, E.N., and Krasnykh, I.G. 1970. Changes in the
SUMMARY AND CONCLUSIONS
Optical Density of Bone Tissue an din teh Calcium
Metabolism of the Astronauts. In: Kosmicheskaia
This review has defined the current status of exercise and
Biologiia i Meditsina. (Nikivaev, A.G., and Sevastianov,
therapeutic drug countermeasures for bone loss during
V.I. Eds) Moscow: pp. 42-45.
long-duration space flight. The available data indicate
that exercise countermeasures to date have not been
Bisello, A., Horwitz, M.J., and Stewart, A.F. 2004.
effective and crew members continue to lose significant
Parathyroid hormone-related protein: an essential
bone mass in the lower extremities and lumbar spine.
physiological regulator of adult bone mass.
Better-designed studies are needed to determine if the
Endocrinology 145(8):3551-3
entire distributed daily dose of exercise that occurs in 1-g
can be successfully replaced by short periods of high-
Black, D.M., Cummings, S.R., Karpf, D.B., Cauley, J.A.,
intensity exercise on-orbit. Exercise dose on-orbit must
Thompson, D.E., Nevitt, M.C., Bauer, D.C., Genant,
also be quantified.
H.K., Haskell, W.L., Marcus, R., Ott, S.M., Torner, J.C.,
Quandt, S.A., Reiss, T.F., and Ensrud, K.E. 1996.
Drug therapeutics for bone have not yet been used in
Randomised trial of effect of alendronate on risk of
space, and, given the considerable experience using
fracture in women with existing vertebral fractures.
several classes of osteoprotective drugs on Earth (mostly
Fracture Intervention Trial Research Group. Lancet
in postmenopausal women with low bone mass), it seems
348(9041):1535-41
wise to explore such interventions for use during space
flight. However, the many differences between the 1-g
Body, J.J., Greipp, P., Coleman, R.E., Facon, T., Geurs,
clinical studies and the 0-g individual prescription must
F., Fermand, J.P., Harousseau, J.L., Lipton, A., Mariette,
be carefully considered. Many new therapies can be
X., Williams, C.D., Nakanishi, A., Holloway, D., Martin,
expected in the future as investigators achieve a better
S.W., Dunstan, C.R., and Bekker, P.J. 2003. A phase I
understanding of the genetic regulation of bone mass, and
study of AMGN-0007, a recombinant osteoprotegerin
genetic screening may offer a means of selecting crew
construct, in patients with multiple myeloma or breast
members with a low susceptibility to bone loss.
carcinoma related bone metastases. Cancer 97(3
Boyden, L.M., Mao, J., Belsky, J., Mitzner, L., Farhi, A.,
Supported by National Space Biomedical Research
Mitnick, M.A., Wu, D., Insogna, K., and Lifton, R.P.
Institute grants BL00401 and BL00402 through NASA
2002. High bone density due to a mutation in LDL-
NCC 9-58. The assistance of Ted Bateman, Ph.D., was
receptor-related protein 5. New England Journal of
Medicine 346(20):1513-21
REFERENCES
Boyle, W.J., Simonet, W.S., and Lacey, D.L. 2003.
Osteoclast differentiation and activation. Nature
Ammann, P., and Rizzoli, R. 2003. Bone strength and its
423(6937):337-42
determinants. Osteoporosis International 14 Suppl 3:S13-
Brodzinski, R.L., Rancitelli, L.A., Haller, W.A., and
Barrett-Connor, E., Grady, D., Sashegyi, A., Anderson,
Dewey, L.S. 1971. Calcium, potassium, and iron loss by
P.W. , Cox, D.A., Hoszowski, K., Rautaharju, P., and
Apollo VII, VIII, IX, X and XI astronauts. Aerospace
Harper, K.D. 2002. Raloxifene and cardiovascular events
Medicine 42(6):621-26
in osteoporotic postmenopausal women: four-year results
from the MORE (Multiple Outcomes of Raloxifene
Carani, C., Qin, K., Simoni, M., Faustini-Fustini, M., Serpente, S., Boyd, J., Korach, K.S., and Simpson, E.R.
52 Gravitational and Space Biology 18(2) June 2005
P.R. Cavanagh - Preventing Bone Loss in Space
1997. Effect of testosterone and estradiol in a man with
Eisman, J.A. 1995. Vitamin D receptor gene alleles and
aromatase deficiency. New England Journal of Medicine
osteoporosis: An affirmative view. Journal of Bone and
Mineral Research 10(9):1289-93
Chapuy, M.C., Arlot, M.E., Duboeuf, F., Brun, J.,
Ettinger, B. , Black, D.M., Mitlak, B.H., Knickerbocker,
Crouzet, B., Arnaud, S., Delmas, P.D., and Meuntier, P.J.
R.K., Nickelsen, T., Genant, H.K., Christiansen, C.,
1992. Vitamin D3 and Calcium to prevent hip fractures in
Delmas, P.D., Zanchetta, J.R., Stakkestad, J., Gluer, C.C.,
elderly women. New England Journal of Medicine
Krueger, K., Cohen, F.J., Eckert, S., Ensrud, K.E., Avioli,
L.V., Lips, P., and Cummings, S.R. 1999. Reduction of
vertebral fracture risk in postmenopausal women with
Chesnut, C.H., III, Skag, A., Christiansen, C., Recker, R.,
osteoporosis treated with raloxifene: results from a 3-year
Stakkestad, J.A., Hoiseth, A., Felsenberg, D., Huss, H.,
randomized clinical trial. Multiple Outcomes of
Gilbride, J., Schimmer, R.C., and Delmas, P.D. 2004.
Raloxifene Evaluation (MORE) Investigators. Journal of
Effects of oral ibandronate administered daily or
the American Medical Association 282(7):637-45
intermittently on fracture risk in postmenopausal
osteoporosis. Journal of Bone and Mineral Research
Gasser, J.A., Kneissel, M., Thomsen, J.S., and Mosekilde,
L. 2000. PTH and interactions with bisphosphonates.
Journal of Musculoskeletal and Neuronal Interactions
Cooper, C., Emkey, R.D., McDonald, R.H., Hawker, G.,
Bianchi, G., Wilson, K., and Schimmer, R.C. 2003.
Efficacy and safety of oral weekly ibandronate in the
Gazenko, O.G., Gurovsky, N.N., Genin, A.M., Bryanov,
treatment of postmenopausal osteoporosis. Journal of
I.I., Eryomin, A.V., and Egorov, A.D. 1976. Results of
Clinical Endocrinology and Metabolism 88(10):4609-15
medical investigations carried out on board the Salyut
orbital stations. Life Sciences and Space Research 14:145-
Cranney, A., Tugwell, P., Zytaruk, N., Robinson, V.,
Weaver, B., Adachi, J., Wells, G., Shea, B., and Guyatt,
G. 2002. Meta-analyses of therapies for postmenopausal
Gennari, L., Merlotti, D., De Paola, V., Calabro, A.,
osteoporosis. IV. Meta-analysis of raloxifene for the
Becherini, L., Martini, G., and Nuti, R. 2005. Estrogen
prevention and treatment of postmenopausal osteoporosis.
receptor gene polymorphisms and the genetics of
Endocrine Reviews 23(4):524-8
osteoporosis: a HuGE review. American Journal of
Epidemiology 161(4):307-20
Cullen, D.M. , Smith, R.T., and Akhter, M.P. 2001. Bone-
loading response varies with strain magnitude and cycle
Gong, Y., Slee, R.B., Fukai, N., Rawadi, G., Roman-
number. Journal of Applied Physiology 91(5):1971-6
Roman, S., Reginato, A.M., Wang, H., Cundy, T.,
Glorieux, F.H., Lev, D., Zacharin, M., Oexle, K.,
Daly, E., Vessey, M.P., Hawkins, M.M., Carson, J.L.,
Marcelino, J., Suwairi, W., Heeger, S., Sabatakos, G.,
Gough, P., and Marsh, S. 1996. Risk of venous
Apte, S., Adkins, W.N., Allgrove, J., Arslan-Kirchner,
thromboembolism in users of hormone replacement
M., Batch, J.A., Beighton, P., Black, G.C., Boles, R.G.,
therapy. Lancet 348(9033):977-80
Boon, L.M., Borrone, C., Brunner, H.G., Carle, G.F.,
Dallapiccola, B., De Paepe, A., Floege, B., Halfhide,
Deal, C., and Gideon, J. 2003. Recombinant human PTH
M.L., Hall, B., Hennekam, R.C., Hirose, T., Jans, A.,
1-34 (Forteo): an anabolic drug for osteoporosis.
Juppner, H., Kim, C.A., Keppler-Noreuil, K.,
Cleveland Clinic Journal of Medicine 70(7):585-6, 589-
Kohlschuetter, A., LaCombe, D., Lambert, M., Lemyre,
90, 592-4 passim
E., Letteboer, T., Peltonen, L., Ramesar, R.S.,
Romanengo, M., Somer, H., Steichen-Gersdorf, E.,
Dietlein, L.F. 1965. Experiment M-3, inflight exerciser on
Steinmann, B., Sullivan, B., Superti-Furga, A., Swoboda,
Gemini IV. In: Manned Space Flight Experiments
W., van den Boogaard, M.J., Van Hul, W., Vikkula, M.,
Symposium. Gemini missions III and IV. Washington DC:
Votruba, M., Zabel, B., Garcia, T., Baron, R., Olsen,
National Aeronautics and Space Administration,
B.R., and Warman, M.L. 2001. LDL receptor-related
protein 5 (LRP5) affects bone accrual and eye
Dietlein, L.F., and Rapp, R.M. 1966. Experiment M-3,
development. Cell 107(4):513-23
inflight exercise work tolerance. In: Gemini Midprogram
Conference Including Experiment Results.NASA Special
Hanzlik, R.P., Fowler, S.C., and Eells, J.T. 2005.
Publication, NASA-SP-121., pp. 393-96.
Absorption and elimination of formate following oral
administration of calcium formate in female human
Doran, P.M., Riggs, B.L., Atkinson, E.J., and Khosla, S.
subjects. Drug Metabolism and Disposition 33(2):282-86
2001. Effects of raloxifene, a selective estrogen receptor
Harris, S.T. , Watts, N.B., Genant, H.K., McKeever, C.D.,
modulator, on bone turnover markers and serum sex
Hangartner, T., Keller, M., Chesnut, C.H. 3rd, Brown, J.,
steroid and lipid levels in elderly men. Journal of Bone
Eriksen, E.F., Hoseyni, M.S., Axelrod, D.W., and Miller,
and Mineral Research 16(11):2118-25
P.D. 1999. Effects of risedronate treatment on vertebral
and nonvertebral fractures in women with
Gravitational and Space Biology 18(2) June 2005 53
P.R. Cavanagh - Preventing Bone Loss in Space
postmenopausal osteoporosis: a randomized controlled
Yu, A. 2004. Cortical and trabecular bone mineral loss
trial. Vertebral Efficacy With Risedronate Therapy
from the spine and hip in long-duration spaceflight.
(VERT) Study Group. Journal of the American Medical
Journal of Bone and Mineral Research 19(6):1006-12
Association 282(14):1344-52
Langton, C.M., Haire, T.J., Ganney, P.S., Dobson, C.A.,
Helfrich, M.H. 2003. Osteoclast diseases. Microscopy
Fagan, M.J., Sisias, G., and Phillips, R. 2000.
Research and Technique 61(6):514-32
Stochastically simulated assessment of anabolic treatment
following varying degrees of cancellous bone resorption.
Iki, M., Kajita, E., Dohi, Y., Nishino, H., Kusaka, Y.,
Bone 27(1):111-8
Tsuchida, C., Yamamoto, K., and Ishii, Y. 1996. Age,
menopause, bone turnover markers and lumbar bone loss
Lanyon, L.E. 1996. Using functional loading to influence
in healthy Japanese women. Maturitas 25(1):59-67
bone mass and architecture: objectives, mechanisms, and
relationship with estrogen of the mechanically adaptive
Johnson, M.L. 2004. The high bone mass family--the role
process in bone. Bone 18(1 Suppl 1):37S-43S
of Wnt/Lrp5 signaling in the regulation of bone mass.
Journal of Musculoskeletal and Neuronal Interactions
Lau, H.H., Ng, M.Y., Ho, A.Y., Luk, K.D., and Kung,
A.W. 2005. Genetic and environmental determinants of
bone mineral density in Chinese women. Bone [Available
Johnson, M.L., Harnish, K., Nusse, R., and Van Hul, W.
online via doi:10.1016/j.bone.2005.01.014.]
2004. LRP5 and Wnt signaling: a union made for bone.
Journal of Bone and Mineral Research 19(11):1749-57
LeBlanc, A., Schneider, V., Shackelford, L., West, S.,
Oganov, V., Bakulin, A., and Voronin, L. 2000. Bone
Kajiya, H., Okamoto, F., Fukushima, H., and Okabe, K.
mineral and lean tissue loss after long duration space
2003. Calcitonin inhibits proton extrusion in resorbing rat
flight. Journal of Musculoskeletal and Neuronal
osteoclasts via protein kinase A. Pflugers Archiv
Interactions 1(2):157-60
LeBlanc, A., Shackelford, L., and Schneider, V. 1998.
Kanis, J.A. 2002. Diagnosis of osteoporosis and
Future human bone research in space. Bone 22(5
assessment of fracture risk. Lancet 359(9321):1929-36
Kessel, B. 2004. Hip fracture prevention in
LeBlanc, A.D., Driscol, T.B., Shackelford, L.C., Evans,
postmenopausal women. Obstetrical and Gynecological
H.J., Rianon, N.J., Smith, S.M., Feeback, D.L., and Lai,
Survey 59(6):446-55; quiz 485
D. 2002. Alendronate as an effective countermeasure to
disuse induced bone loss. Journal of Musculoskeletal and
Khosla, S. 2001. Minireview: the OPG/RANKL/RANK
Neuronal Interactions 2(4):335-43
system. Endocrinology 142(12):5050-5
LeBlanc, A.D., Schneider, V., Shackelford, L., West, S.,
Khosla, S., Melton, L.J. 3rd, and Riggs, B.L. 2002.
Oganov, V., Bakulin, A., and Veronin, L. 1996. Bone
Clinical review 144: Estrogen and the male skeleton.
mineral and lean tissue loss after long duration space
Journal of Clinical Endocrinology and Metabolism
flight (Abstract). Journal of Bone and Mineral Research
Khosla, S., Riggs, B.L., Atkinson, E.J., Oberg, A.L.,
Lehman, R.A. Jr, Kuklo, T.R., Freedman, B.A., Cowart,
Mavilia, C., Del Monte, F., Melton, L.J. 3rd, and Brandi,
J.R., Mense, M.G., and Riew, K.D. 2004. The effect of
M.L. 2004. Relationship of estrogen receptor genotypes to
alendronate sodium on spinal fusion: a rabbit model.
bone mineral density and to rates of bone loss in men.
Spine Journal 4(1):36-43
Journal of Clinical Endocrinology and Metabolism
Li, J., Mashiba, T., and Burr, D.B. 2001. Bisphosphonate
Treatment Suppresses Not Only Stochastic Remodeling
Klein, R.F., Allard, J., Avnur, Z., Nikolcheva, T.,
but Also the Targeted Repair of Microdamage. Calcified
Rotstein, D., Carlos, A.S., Shea, M., Waters, R.V.,
Tissue International 69(5):281-6
Belknap, J.K., Peltz, G., and Orwoll, E.S. 2004.
Regulation of bone mass in mice by the lipoxygenase
Li, J., Mori, S., Kaji, Y., Mashiba, T., Kawanishi, J., and
gene Alox15. Science 303(5655):229-32
Norimatsu, H. 1999. Effect of bisphosphonate
(incadronate) on fracture healing of long bones in rats.
Journal of Bone and Mineral Research 14(6):969-79
Krasnykh, I.G. 1969. Mineral saturation of bone tissue
Li, J., Sarosi, I., Yan, X.Q., Morony, S., Capparelli, C.,
under conditions of prolonged hypodynamia. NASA TT F-
Tan, H.L., McCabe, S., Elliott, R., Scully, S., Van, G.,
Kaufman, S., Juan, S.C., Sun, Y., Tarpley, J., Martin, L.,
Christensen, K., McCabe, J., Kostenuik, P., Hsu, H.,
Lang, T., LeBlanc, A., Evans, H., Lu, Y., Genant, H., and
Fletcher, F., Dunstan, C.R., Lacey, D.L., and Boyle, W.J.
54 Gravitational and Space Biology 18(2) June 2005
P.R. Cavanagh - Preventing Bone Loss in Space
2000. RANK is the intrinsic hematopoietic cell surface
Mashiba, T., Turner, C.H., Hirano, T., Forwood, M.R.,
receptor that controls osteoclastogenesis and regulation of
Johnston, C.C., and Burr, D.B. 2001. Effects of
bone mass and calcium metabolism. Proceedings of the
suppressed bone turnover by bisphosphonates on
National Academy of Sciences of the United States of
microdamage accumulation and biomechanical properties
America 97(4):1566-71
in clinically relevant skeletal sites in beagles. Bone
Licata, A.A. 2005. Discovery, clinical development, and
therapeutic uses of bisphosphonates. Annals of Pharma-
McCarthy, I., Goodship, A., Herzog, R., Oganov, V.,
cotherapy 39(4):668-77
Stussi, E., and Vahlensieck, M. 2000. Investigation of
bone changes in microgravity during long and short
Linde, F., Norgaard, P., Hvid, I., Odgaard, A., and
duration space flight: comparison of techniques.
Soballe, K. 1991. Mechanical properties of trabecular
European Journal of Clinical Investigation 30(12):1044-
bone: dependency on strain rate. Journal of Biomechanics
McClung, M.R., Lewiecki, E.M., Bolognese, M.A.,
Little, R.D. , Carulli, J.P., Del Mastro, R.G., Dupuis, J.,
Woodson, G., Moffell, A., Peacock, M., Miller, P.D.,
Osborne, M., Folz, C., Manning, S.P., Swain, P.M., Zhao,
Lederman, S., Chesnut, C.H., Murphy, R., Holloway,
S., Eustace, B., Lappe, M.M., Spitzer, L., Zweier, S.,
D.L., and Bekker, P.J. 2004. AMG 162 increases bone
Braunschweigner, K., Benchekroun, Y., Hu, X., Adair,
mineral density (BMD) within 1 month in
R., Chee, L., Fitzgerald, G., McGuiere, S., Nogues, X.,
postmenopausal women with low BMD (Abstract).
Gong, G., Allen, K.M., Tulig, C., Caruso, A., Tzellas, N.,
Journal of Bone and Mineral Research 19(Suppl 1):S20
Bawa, A., Franklin, B., Anisowicz, A., Morales, A.J.,
Lomedico, P.T., Recker, S.M., Van Eedewegh, P.,
McCrory, J.L., Lemmon, D.R., Sommer, H.J., Prout, B.,
Recker, R.R., and Johnson, M.L. 2002. A mutation in the
Smith, D., Korth, D.W., Lucero, J., Greenisen, M.,
LDL receptor-related protein 5 gene results in the
Moore, J., Kozlovskaya, I., Pestov, I., Stapansov, V.,
autosomal dominant high-bone-mass trait. American
Miyakinchenko, Y., Cavanagh, P.R., and (The TVIS
Journal of Human Genetics 70(1):11-19
Study Group). 1999. Evaluation of a treadmill with
vibration isolation and stabilization (TVIS) for use on the
Liu, Y.Z., Liu, Y.J., Recker, R.R., and Deng, H.W. 2003.
International Space Station. Journal of Applied
Molecular studies of identification of genes for
Biomechanics 15:292-302
osteoporosis: the 2002 update. Journal of Endocrinology
Mortensen, L., Charles, P., Bekker, P.J., Digennaro, J.,
and Johnston, C.C. Jr. 1998. Risedronate increases bone
Locklin, R.M., Khosla, S., and Riggs, B.L. 2001.
mass in an early postmenopausal population: two years of
Mechanisms of biphasic anabolic and catabolic effects of
treatment plus one year of follow-up. Journal of Clinical
parathyroid hormone (PTH) on bone cells. Bone
Endocrinology and Metabolism 83(2):396-402
Mosley, J.R., and Lanyon, L.E. 1998. Strain rate as a
Lugassy, G., Shaham, R., Nemets, A., Ben-Dor, D., and
controlling influence on adaptive modeling in response to
Nahlieli, O. 2004. Severe osteomyelitis of the jaw in long-
dynamic loading of the ulna in growing male rats. Bone
term survivors of multiple myeloma: a new clinical entity.
American Journal of Medicine 117(6):440-441
Mottes, M., Gomez Lira, M., Zolezzi, F., Valli, M., Lisi,
Mack, P.B., LaChance, P.A., Vose, G.P., and Vogt, F.B.
V., and Freising, P. 1998. Four new cases of lethal
1967. Bone demineralization of foot and hand of Gemini-
osteogenesis imperfecta due to glycine substitutions in
Titan IV, V and VII astronautis during orbital flight.
COL1A1 and genes. Mutations in brief no. 152. Online.
American Journal of Roentgenology, Radium Therapy
Human Mutation 12(1):71-72
and Nuclear Medicine 100(3):503-11
Munoz-Torres, M., Alonso, G., and Raya, M.P. 2004.
Mack, P.B., and LaChance, P.L. 1967. Effects of
Calcitonin therapy in osteoporosis. Treatments in
recumbency and space flight on bone density. American
Endocrinology 3(2):117-32
Journal of Clinical Nutrition 20(11):1194-205
National Osteoporosis Foundation. 2002. Physician's
Mack, P.B., and Vogt, F.B. 1971. Roentgenographic bone
Guide 2002 (online version; text can be accessed at
density changes in astronauts during representative
Apollo space flight. American Journal of Roentgenology,
Radium Therapy and Nuclear Medicine 113(4):621-33
Nicogossian, A.E., Sawin, C.F., and Crigoriev, A.I. 1994.
Countermeasures to space deconditioning. In: Space
Marx, J. 2004. Coming to grips with bone loss. Science
Physiology and Medicine. (Nicogossian, A.E., Huntoon,
305(5689):1420-2
C.L., and Pool, S.L. Eds) Philadelphia: Lea & Febiger,
Gravitational and Space Biology 18(2) June 2005 55
P.R. Cavanagh - Preventing Bone Loss in Space
Nuttall, M.E., Stroup, G.B., Fisher, P.W., Nadeau, D.P.,
Riggs, B.L., and Hartmann, L.C. 2003. Selective
Gowen, M., and Suva, L.J. 2000. Distinct mechanisms of
estrogen-receptor modulators -- mechanisms of action and
action of selective estrogen receptor modulators in breast
application to clinical practice. New England Journal of
and osteoblastic cells. American Journal of Physiology:
Medicine 348(7):618-29
Cell Physiology 279(5):C1550-7
Rodan, G.A., and Fleisch, H.A. 1996. Bisphosphonates:
Oganov, V.S. , Cann, C., Rakhmanov, A.S., and
mechanisms of action. Journal of Clinical Investigation
Ternovoi, S.K. 1990. [Study of the Musculoskeletal
System of the Spine in Humans After Long-Term Space
Flights by the Method of Computerized Tomography].
Rodan, G.A., and Martin, T.J. 1982 . Role of osteoblasts
[Russian]. Kosmicheskaia Biologiia i Aviakosmicheskaia
in hormonal control of bone resorption - a hypothesis.
Meditsina 24(4):20-1
Calcified Tissue International 34(3):311
Orwoll, E.S. , Scheele, W.H., Paul, S., Adami, S.,
Rosen, C.J., Beamer, W.G., and Donahue, L.R. 2001.
Syversen, U., Diez-Perez, A., Kaufman, J.M., Clancy,
Defining the genetics of osteoporosis: using the mouse to
A.D., and Gaich, G.A. 2003. The effect of teriparatide
understand man. Osteoporosis International 12(10):803-
human parathyroid hormone (1-34). Journal of Bone and
Mineral Research 18(1):9-17
Rossouw, J.E., Anderson, G.L., Prentice, R.L., LaCroix,
Phan, T.C., Xu, J., and Zheng, M.H. 2004. Interaction
A.Z., Kooperberg, C., Stefanick, M.L., Jackson, R.D.,
between osteoblast and osteoclast: impact in bone disease.
Beresford, S.A., Howard, B.V., Johnson, K.C., Kotchen,
Histology and Histopathology 19(4):1325-44
J.M., and Ockene, J. 2002. Risks and benefits of estrogen
plus progestin in healthy postmenopausal women:
Ralston, S.H. 2003. Genetic determinants of susceptibility
principal results From the Women's Health Initiative
to osteoporosis. Current Opinion in Pharmacology
randomized controlled trial. Journal of the American
Medical Association 288(3):321-33
Rambaut, P.C., and Johnston, R.S. 1979. Prolonged
Rubin, C., Turner, A.S., Mallinckrodt, C., Jerome, C.,
weightlessness and calcium loss in man. Acta
McLeod, K., and Bain, S. 2002a. Mechanical Strain,
Astronautica 6:1113-22
Induced Noninvasively in the High-Frequency Domain, Is
Anabolic to Cancellous Bone, but Not Cortical Bone.
Rambaut, P.C., Leach, C.S., and Whedon, G.D. 1979. A
Bone 30(3):445-52
study of metabolic balance in crew members of Skylab
IV. Acta Astronautica 6:1313-22
Rubin, C., Turner, A.S., Muller, R., Mittra, E., Mcleod,
K., Lin, W., and Qin, Y. 2002b. Quantity and quality of
Rambaut, P.C., Smith, M.C., Mack, P.B., and Vogel, J.M.
trabecular bone in the femur are enhanced by a strongly
1975. Skeletal response. In: Biomedical Results of Apollo.
anabolic, noninvasive mechanical intervention. Journal of
(Johnson, R.S., Dietlein, L.F., and Berry, C.A. Eds) pp.
Bone and Mineral Research 17( 2):349-57
Ruggiero, S.L., Mehrotra, B., Rosenberg, T.J., and
Ravn, P., Bidstrup, M., Wasnich, R.D., Davis, J.W.,
Engroff, S.L. 2004. Osteonecrosis of the Jaws Associated
McClung, M.R., Balske, A., Coupland, C., Sahota, O.,
With the Use of Bisphosphonates: a Review of 63 Cases.
Kaur, A., Daley, M., and Cizza, G. 1999. Alendronate and
Journal of Oral and Maxillofacial Surgery 62(5):527-34
estrogen-progestin in the long-term prevention of bone
loss: four-year results from the early postmenopausal
Russell, R.G., Croucher, P.I., and Rogers, M.J. 1999.
intervention cohort study. A randomized, controlled trial.
Bisphosphonates: pharmacology, mechanisms of action
Annals of Internal Medicine 131(12):935-42
and clinical uses. Osteoporosis International 9 Suppl
Recker, R.R. 2004. Genetic research in osteoporosis:
Where are we? Where should we go next? Journal of
Schneider, S.M., Amonette, W.E., Blazine, K., Bentley,
Musculoskeletal and Neuronal Interactions 4(1):86-90
J., Lee, S.M., Loehr, J.A., Moore, A.D. Jr. , Rapley, M.,
Mulder, E.R., and Smith, S.M. 2003. Training with the
Reginster, J.Y. 2004. Prevention of postmenopausal
International Space Station interim resistive exercise
osteoporosis with pharmacological therapy: practice and
device. Medicine and Science in Sports and Exercise
possibilities. Journal of Internal Medicine 255(6):615-28
Rice, A.J., Maender, C.C., Genc, K.O., Ochia, R.S.,
Schnitzer, T., Bone, H.G., Crepaldi, G., Adami, S.,
Snedeker, J.G., and Cavanagh, P.R. 2004. Bone Loss and
McClung, M., Kiel, D., Felsenberg, D., Recker, R.R.,
Lower Extremity Loading During Long-Duration Space
Tonino, R.P., Roux, C., Pinchera, A., Foldes, A.J.,
Flight (Abstract). Journal of Bone and Mineral Research
Greenspan, S.L., Levine, M.A., Emkey, R., Santora, A.C.
2nd, Kaur, A., Thompson, D.E., Yates, J., and Orloff, J.J.
56 Gravitational and Space Biology 18(2) June 2005
P.R. Cavanagh - Preventing Bone Loss in Space
2000. Therapeutic equivalence of alendronate 70 mg
Management, Scientific and Technical Information
once-weekly and alendronate 10 mg daily in the treatment
Division, pp. 23-30.
of osteoporosis. Alendronate Once-Weekly Study Group.
Aging (Milano) 12(1):1-12
Thornton, W. 1989b. Work, exercise and space flight. II.
Modification of adaptation by exercise (Exercise
Shackelford, L.C., LeBlanc, A.D., Driscoll, T.B., Evans,
Prescription). In: NASA CP 3051 Workshop on Exercise
H.J., Rianon, N.J., Smith, S.M., Spector, E., Feeback,
Prescription for Long-Duration Space Flight. (Harris,
D.L., and Lai, D. 2004. Resistance exercise as a
B.A., Jr., and Stewart, D.F., Eds.) NASA Office of
countermeasure to disuse-induced bone loss. Journal of
Management, Scientific and Technical Information
Applied Physiology 97(1):119-29
Division, pp. 107-15.
Simonet, W.S., Lacey, D.L., Dunstan, C.R., Kelley, M.,
Thornton, W. 1989c. Work, exercise and space flight. III.
Chang, M.S., Luthy, R., Nguyen, H.Q., Wooden, S.,
Exercise devices and protocols. In: Proceedings of the
Bennett, L., Boone, T., Shimamoto, G., DeRose, M.,
1986 Workshop on Exercise Prescription for Long-
Elliott, R., Colombero, A., Tan, H.L., Trail, G., Sullivan,
Duration Space Flight. (Harris, B.A., Jr., and Stewart,
J., Davy, E., Bucay, N., Renshaw-Gegg, L., Hughes,
D.F., Eds.) NASA Office of Management, Scientific and
T.M., Hill, D., Pattison, W., Campbell, P., Boyle, W.J.,
Technical Information Division, pp. 31-42.
and et, a.l. 1997. Osteoprotegerin: a novel secreted protein
involved in the regulation of bone density. Cell
Thornton, W.E., Cavanagh, P.R., Buczek, F.L., Milliron,
M.J., and Davis, B.L. 1998. The kinematics of treadmill
locomotion in space. In: Three-Dimensional Analysis of
Sirola, J., Kroger, H., Honkanen, R., Jurvelin, J.S.,
Human Locomotion. (Allard, P., Cappozzo, A., Lundberg,
Sandini, L., Tuppurainen, M.T., and Saarikoski, S. 2003.
A., and Vaughan, C.L., Eds.) Chichester: John Wiley &
Factors affecting bone loss around menopause in women
Sons, pp. 375-88.
without HRT: a prospective study. Maturitas 45(3):159-
Thornton, W.E., and Rummel, J.A. 1977. Muscular
deconditioning and its prevention in space flight. In:
Smith, M.C. , Rambaut, P.C., Vogel, J.M., and Whittle,
Biomedical Results From Skylab. (Johnson, R.S., and
M.W. 1977. Bone mineral measurement - experiment
Dietlein, L.F., Eds.) Houston: National Aeronautics and
M078. In: Biomedical Results from Skylab. (Johnston,
Space Administration, pp. 191-97.
R.S., and Dietlein, L.F. Eds) Washington, DC: NASA, pp.
Tilton, F.E., Degioanni, J.J.C., and Schneider, V.S. 1980.
Long-term follow-up of Skylab bone demineralization.
Smith, S.M. , Nillen, J.L., Leblanc, A., Lipton, A.,
Aviation, Space, and Environmental Medicine
Demers, L.M., Lane, H.W., and Leach, C.S. 1998.
Collagen cross-link excretion during space flight and bed
rest. Journal of Clinical Endocrinology and Metabolism
Torgerson, D.J., and Bell-Syer, S.E. 2001. Hormone
replacement therapy and prevention of nonvertebral
fractures: a meta-analysis of randomized trials. Journal of
Smith, S.M. , Wastney, M.E., Morukov, B.V., Larina,
the American Medical Association 285(22):2891-7
I.M., Nyquist, L.E., Abrams, S.A., Taran, E.N., Shih,
C.Y., Nillen, J.L., Davis-Street, J.E., Rice, B.L., and
Toth, E., Csupor, E., Meszaros, S., Ferencz, V., Nemeth,
Lane, H.W. 1999. Calcium metabolism before, during,
L., McCloskey, E.V., and Horvath, C. 2005. The effect of
and after a 3-mo spaceflight: kinetic and biochemical
intranasal salmon calcitonin therapy on bone mineral
changes. American Journal of Physiology 277(1 Pt 2):R1-
density in idiopathic male osteoporosis without vertebral
fractures--an open label study. Bone 36(1):47-51
Steiner, M.S., and Raghow, S. 2003. Antiestrogens and
Turner, C.H. 1998. Three rules for bone adaptation to
selective estrogen receptor modulators reduce prostate
mechanical stimuli. Bone 23(5):399-407
cancer risk. World Journal of Urology 21(1):31-36
Turner, C.H. 2002. Biomechanics of bone: determinants
Tekeoglu, I., Adak, B., Budancamanak, M., Demirel, A. ,
of skeletal fragility and bone quality. Osteoporosis
and Ediz, L. 2005. Comparison of cyclic and continuous
International 13(2):97-104
calcitonin regimens in the treatment of postmenopausal
osteoporosis. Rheumatology International
Turner, C.H., and Pavalko, F.M. 1998.
Mechanotransduction and functional response of the
Thornton, W. 1989a. Work, exercise and space flight. I.
skeleton to physical stress: the mechanisms and
Operations, environment, and effects of spaceflight. In:
mechanics of bone adaptation. Journal of Orthopaedic
Proceedings of the 1986 Workshop on Exercise
Science 3(6):346-55
Prescription for Long-Duration Space Flight. (Harris,
B.A., Jr., and Stewart, D.F. Eds.) NASA Office of
Watanabe, Y., Ohshima, H., Mizuno, K., Sekiguchi, C.,
Gravitational and Space Biology 18(2) June 2005 57
P.R. Cavanagh - Preventing Bone Loss in Space
Fukunaga, M., Kohri, K., Rittweger, J., Felsenberg, D.,
Blanchard, F., Heymann, D., and Redini, F. 2004.
Matsumoto, T., and Nakamura, T. 2004. Intravenous
RANKL/RANK/OPG: New Therapeutic Targets in Bone
pamidronate prevents femoral bone loss and renal stone
Tumours and Associated Osteolysis. Biochimica Et
formation during 90-day bed rest. Journal of Bone and
Biophysica Acta 1704(2):49-57
Mineral Research 19(11):1771-78
Yasuda, H., Shima, N., Nakagawa, N., Mochizuki, S.I.,
Whedon, G.D., Lutwak, L., Rambaut, P., Whittle, M.,
Yano, K., Fujise, N., Sato, Y., Goto, M., Yamaguchi, K.,
Leach, C., Reid, J., and Smith, M. 1977. Effect of
Kuriyama, M., Kanno, T., Murakami, A., Tsuda, E.,
weightlessness on mineral metabolism; metabolic studies
Morinaga, T., and Higashio, K. 1998. Identity of
on skylab orbital space flights. Calcified Tissue Research
osteoclastogenesis inhibitory factor (OCIF) and
21(Suppl):423-30
osteoprotegerin (OPG): a mechanism by which
OPG/OCIF inhibits osteoclastogenesis in vitro.
Whitson, P.A., Pietrzyk, R.A., and Sams, C.F. 1999.
Endocrinology 139(3):1329-37
Space flight and the risk of renal stones. Journal of
Gravitational Physiology 6(1):P87-P88
Yegorov, A.D., Kakurin, L.I., and Nefyodov, Y.G. 1972.
Effects of an 18-day flight on the human body. Life
Wittrant, Y., Theoleyre, S., Chipoy, C., Padrines, M. ,
Sciences and Space Research 10:57-60
58 Gravitational and Space Biology 18(2) June 2005
Source: http://gravitationalandspacebiology.org/index.php/journal/article/download/345/346
The Newsletter of Gray Academy of Jewish Education September 2009 Tevet-Shevet 5769 Jan - Mar 2013 Tevet - Nissan 5773 By SJaiofui JKdsuvu tdsijvs sijf svoijabiqibefahbipu boieoibje ibjodisjbodfibjdab odiabofdob dfiubdbfiu hbaiudbiudfhurtoibjhoidsi ubndiu gadiu biu-biduanbiuds hbiudnbirunb ueiusnbieu unriuhnipaeungaiunrg uriangierun peiunbiae nieurngpiaeunrgpiuan gunaepiurngaipungi-
Nutritional Supplementation in Pregnancy It goes without saying that Nutrition in Pregnancy is vitally important for the developing fetus. Dietary requirements are increased substantially in a pregnant woman, and if she does not have enough in the way of proteins, vitamins and minerals in her own body stores, then her baby could be compromised. It has been clearly established that Pregnant women in Australia are not meeting their daily







