Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Ieeservftp01.unibe.ch

CSTX-13, a highly synergistically acting two-chain
neurotoxic enhancer in the venom of the spider
Cupiennius salei (Ctenidae)
Benno Wullschleger*, Lucia Kuhn-Nentwig*†, Jan Tromp‡, Urs Ka¨mpfer‡, Johann Schaller‡, Stefan Schu¨rch‡,
and Wolfgang Nentwig*
*Zoological Institute, University of Bern, Baltzerstrasse 6, CH-3012 Bern, Switzerland; and ‡Department of Chemistry and Biochemistry, University of Bern,Freiestrasse 3, CH-3012 Bern, Switzerland
Edited by Jerrold Meinwald, Cornell University, Ithaca, NY, and approved June 22, 2004 (received for review April 1, 2004)
The survival of the spider Cupiennius salei depends on its hunting
cysteine in their composition. Some peptides have already been
success, which largely relies on its immediately paralyzing multi-
characterized and named the cupiennin 1 family. These peptides
component venom. Here, we report on the isolation and charac-
exhibit strong bactericidal activities in the submicromolar
terization of CSTX-13, a neurotoxic enhancer in the spider venom.
range, but also cytolytic and insecticidal activities. In addition,
De novo elucidation of the disulfide bridge pattern of CSTX-13 and
cupiennin 1a shows a high synergistic effect with the main
the neurotoxin CSTX-1 by tandem MS revealed an identical ar-
neurotoxin CSTX-1, facilitating a rapid paralysis (12, 13). Pos-
rangement. However, in contrast to CSTX-1, CSTX-13 is a two-chain
itive insecticidal cooperativity between the cytolytically active
peptide with two interchain and two intrachain disulfide bridges.
oxyopinins and neurotoxins is also reported for the spider
Furthermore, the insecticidal activity of CSTX-13 is synergistically
Oxyopes kitabensis (14).
increased in the presence of Kⴙ ions as well as of the cytolytic
The second group involves neurotoxically active peptides with
peptide cupiennin 1a. We demonstrated that the weakly neuro-
molecular masses of ⬇8 kDa, which we named Cupiennius salei
toxic CSTX-13 enhances the paralytic activity of the neurotoxin
toxins (CSTX-1 to -13) (11). There is evidence that CSTX-1
CSTX-1 by 65% when it is administered with the latter at its entirely
inhibits L-type Ca2⫹ channels of GH3 cells (J. S. Cruz, personal
nontoxic physiological concentration, which is 440 times below its
communication). To date, sequence data for the neurotoxins
CSTX-1 and CSTX-9 are available. The four disulfide bridges of
CSTX-9 form linkages between C1–C4; C2–C5; C3–C8; and
Spidersandscorpionsusetheirvenomtoparalyzepreyand兾or C6–C7 (15–17). This arrangement is also found in other spider
to defend against predators. These venoms are complex
neurotoxins belonging to the inhibitor cystine knot (ICK) struc-
mixtures of different components, and the knowledge about
tural motif (18).
their interactions and role in the envenomation process is still
So far, a roughly comparable two-chain peptide structure has
limited. During their evolution, these arthropods have developed
only been reported for the spider Agelenopsis aperta: the -aga-
a large number of neurotoxins that act simultaneously on various
toxins IA and G block presynaptic calcium channels in insect
invertebrate and兾or vertebrate membrane-bound sodium, po-
neuromuscular junction (19, 20). A two-chain structure has also
tassium, and calcium channels. Also, interactions with acid-
been proposed for the Hololena toxin, a presynaptic antagonist
sensing ion channels, glutamate receptors, and as yet unidenti-
of insect neuromuscular transmission (21).
fied targets lead to rapid paralysis or death of the envenomed
Here, we present the amino acid sequence of CSTX-13, an
animals (1, 2).
enhancer peptide from the venom of C. salei, and the de novo
Cupiennius salei (Keyserling, 1877) is a nocturnal hunting
determination of the disulfide bridge pattern of CSTX-1 and
spider living in the Central American rain forest (3). The spider
CSTX-13. Despite its sequence similarity to the neurotoxins
relies on an immediately paralyzing venom activity because, in its
CSTX-1 and CSTX-9, CSTX-13 acts as a neurotoxic enhancer.
arboreal environment, a prey item that escapes is lost. The spider
Moreover, its low neurotoxic activity is also augmented by other
also loses its venom investment and reduces its chance of
venom compounds.
successfully subduing a subsequent prey item, because its venom
storage is limited, regeneration takes ⬇16 days (4), and its
Materials and Methods
production involves high metabolic costs. Behavioral, ecological,
Chemicals. Chemicals were of analytical grade and purchased
and biochemical investigations of the venom economy of C. salei
from Merck unless otherwise specified.
indicate that it alters the amount of venom injected according to
the size, mobility, and defense behavior of its prey (5–8).
Isolation of CSTX-13. Spider maintenance, venom collection, and
This economical venom use is paralleled on the physiological
separation of 425 l of venom by FPLC and HPLC methods were
and biochemical levels by the interactions of different venom
performed as described (ref. 11; see also Supporting Text, which
components (9). In the venom, low molecular mass compounds
is published as supporting information on the PNAS web site).
such as histamine (5.7 mM) and free amino acids, basically
Final purification of CSTX-13 was achieved by RP-HPLC on a
taurine (70 M) and glycine (43.3 M), are present. K⫹, Na⫹,
nucleosil 100–5 C8 column (4 ⫻ 250 mm, Macherey & Nagel)
and Ca2⫹ ions have also been identified. Remarkably, K⫹ ions
using 22% solvent B (0.1% trifluoroacetic acid in acetonitrile) in
are abundant in the venom and rare in the hemolymph (10, 11).
Furthermore, C. salei possesses a complex multicomponent
system consisting of a few proteins with molecular masses ⬎10
This paper was submitted directly (Track II) to the PNAS office.
kDa, among them a highly active hyaluronidase. About 100
Abbreviation: ESI, electrospray ionization
different peptides with molecular masses between 2 and 8 kDa
Data deposition: The sequences reported in this paper have been deposited in the Swiss-
have been detected by electrospray ionization (ESI)-MS. The
Prot and TrEMBL databases [accession nos. P83919 (CSTX-13 chain A) and P83920 (CSTX-13
peptides can be roughly divided into two groups. The first group
contains the smaller peptides with molecular masses of ⬇3–4
†To whom correspondence should be addressed. E-mail: [email protected].
kDa, which are mainly highly cationic ␣-helical peptides without
2004 by The National Academy of Sciences of the USA
PNAS 兩 August 3, 2004 兩 vol. 101 兩 no. 31 兩 11251–11256

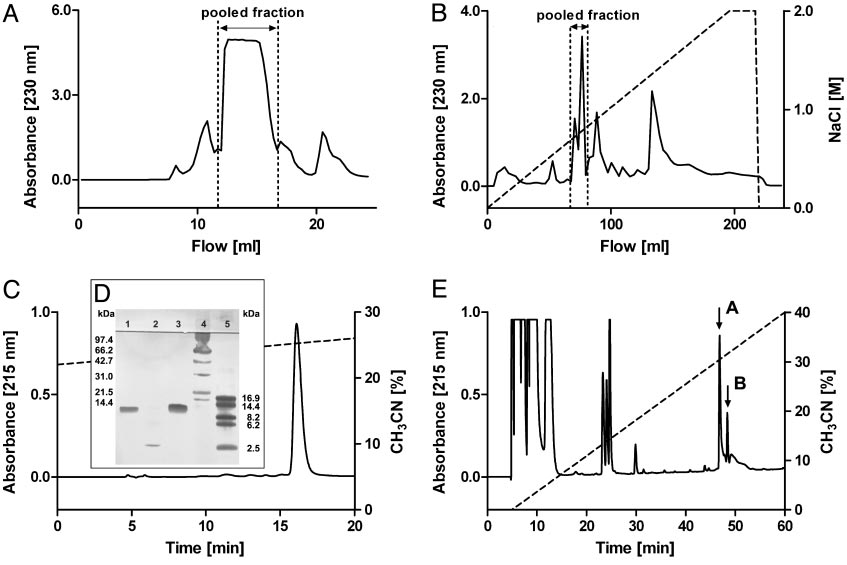
Isolation of CSTX-13 from the venom of C. salei. (A) Crude venom was first separated by gel filtration on a Superdex 75 column. (B) Further separation
of the pooled fraction was achieved by cationic exchange chromatography on a Mono S HR column. (C) When RP-HPLC was used, CSTX-13 was finally isolatedon a nucleosil 100 –5 C8 column, and the purity was controlled by SDS兾PAGE. (D) Lanes: 1, native CSTX-13; 2, reduced CSTX-13; 3, native CSTX-1; 4, molecular massmarkers (14.4 –97.4 kDa; Bio-Rad); and 5, molecular mass markers (2.5–16.9 kDa; Amersham Pharmacia). (E) RP-HPLC of reduced and alkylated CSTX-13 on anucleosil 100 –5 C8 column resulted in the separation of chain A (first arrow) and chain B (second arrow).
solvent A (0.1% trifluoroacetic acid in water) with a flow rate of
supernatant was recovered for separation by RP-HPLC. The
0.5 ml兾min. Directly after injection of the sample, the gradient
digested and purified peptides were subjected to collision-
(22–28% solvent B) was started for 20 min (Fig. 1C). This step
induced dissociation using nitrogen as the collision gas. Collision
was repeated several times to obtain CSTX-13 (variability be-
energies were in the range of 20–80 eV.
tween different preparations: 0.5–1.3 mg).
Amino Acid Analysis and Amino Acid Sequence Analysis. Samples
PAGE. SDS兾PAGE and silver staining of native and reduced
were hydrolyzed in the gas phase with 6 M hydrochloric acid
(2-mercaptoethanol) CSTX-13 were performed with the Phast-
containing 0.1% (by volume) phenol for 24 h at 115°C under N2
System using high density PhastGel (Amersham Pharmacia).
vacuum according to Chang and Knecht (22). N-terminal se-
quence analysis was carried out either in a Procise cLC 492
Reduction and Alkylation. Fifty micrograms of CSTX-13 was
protein sequencer or in a pulsed liquid-phase sequencer 477A,
reduced and alkylated according to the published procedure (ref.
both from Applied Biosystems.
15, see Supporting Text). Chains A and B were further desalted
and separated by RP-HPLC on a nucleosil 100-5 C8 column (4 ⫻
Experiments with Spider Digestive Liquid. Digestive liquid from C.
250 mm; Macherey & Nagel) using 100% solvent A with a flow
salei was obtained by electrical stimulation and collected in glass
rate of 0.5 ml兾min for 0–5 min followed by a 55-min gradient of
capillary tubes, and 8 l of diluted digestive liquid (1:100 with
0.73% solvent B in solvent A per min (Fig. 1E).
water) was mixed with 17 g of CSTX-13 in 8 l of water. The
mixture was kept at 24°C and, after 0.5, 1, and 24 h, aliquots of
MS. Mass spectrometric analyses were performed on a QSTAR
2 l were analyzed by ESI-MS.
Pulsar hybrid quadrupole time-of-flight mass spectrometer (Ap-
For further experiments, 50 g of CSTX-13 was dissolved in
plied Biosystems) equipped with a nanoelectrospray ion source.
31.8 l of water, mixed with 31.8 l of diluted digestive liquid
The instrument was tuned for a mass resolving power of 12,000
(1:100 with water), and incubated for 24 h at 24°C, and the
(m兾⌬m, full width at half maximum) and calibrated with caesium
fragment was isolated by RP-HPLC on a nucleosil 120–5 C18
iodide and reserpine (Sigma). Samples were dissolved in meth-
column (2 ⫻ 125 mm; Macherey & Nagel) using a gradient of
anol兾water (1:1 vol兾vol) containing 1% formic acid. The final
0.2% B in A兾min for 200 min and a flow rate of 0.5 ml兾min. For
peptide concentration was 5 pmol兾l. All analyses were per-
ESI-MS analysis, the CSTX-13 fragment was again dissolved in
formed in the positive ion mode. Numbers represent monoiso-
100 l of buffer [100 mM Tris䡠HCl, pH 8.0, containing 135 M
topic masses.
TLCK (N␣-p-tosyl-L-lysine chloromethyl ketone, Sigma) and 220
For elucidation of the disulfide bridge pattern, 50 g of native
M TPCK (N-tosyl-L-phenylalanine chloromethyl ketone,
CSTX-13 was cleaved with immobilized trypsin (23 l wet gel
Sigma)], reduced, alkylated, and separated as described above.
containing 0.5 units of trypsin (Sigma) in 50 l of 0.1 M Tris䡠HCl
buffer, pH 8.1, and 1.0 mM iodoacetamide, Fluka) under gentle
Bioassays and Calculations. Bioassays were performed according
shaking for 17 h at 24°C. The suspension was centrifuged, and the
to Escoubas et al. (23) using 1- to 3-day-old Drosophila melano-
Wullschleger et al.

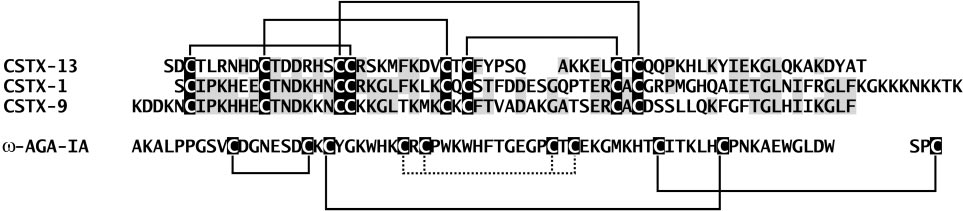
Sequence comparison and disulfide bridge arrangement of CSTX-13, CSTX-1, CSTX-9 (C. salei), and -agatoxin IA (A. aperta). Identical amino acids are
shaded gray, the disulfide bridges are represented by lines, and the corresponding cysteine residues are shaded black. The disulfide bridge patterns of CSTX-13and CSTX-1 were determined by nanoelectrospray tandem MS of the corresponding disulfide-linked tryptic fragment.
gaster female flies. The injected volume was 0.05 l of 0.1 M
successive RP-HPLC (Fig. 1C). The retention profile of
ammonium acetate, pH 6.1 (control), and all further injections
CSTX-13 revealed no impurities. CSTX-13 was characterized by
with different components were carried out in this solution,
ESI-MS and amino acid composition. The yield of CSTX-13
except nifedipine (Calbiochem), which was injected in ⱖ0.06 M
obtained by purification of crude venom was 1.2–3.0 g兾l
ammonium acetate, pH 6.1, and ⱕ5.6 M dimethyl sulfoxide. To
depending on the separation protocol.
estimate the LD50 (24 h after injection), 20 flies were used as
N-terminal sequence analysis of native CSTX-13 provided
control, and 20 flies were used for each concentration.
evidence for a two-chain molecule: Ser兾Ala–Asp兾Lys–Xaa兾Lys–
To investigate synergistic effects between CSTX-13 and fur-
ther venom components such as cupiennin 1a (9.6 M) (in
Xaa兾Gln. Therefore, CSTX-13 was reduced and alkylated, and
nontoxic concentration), histamine (5.7 mM, Sigma), taurine
chains A and B were separated by RP-HPLC. Both chains were
(0.07 mM, Sigma), and KCl (215 mM) (all in physiological venom
sequenced by Edman degradation from the N to the C termini
concentrations), bioassays were performed with 12.6 pmol of
without any ambiguity. Chain A is composed of 34 residues
CSTX-13 per mg of fly. We tested CSTX-13 alone and in
(measured, 4,342.73 Da; calculated, 4,342.76 Da), and chain B is
combination with each of the above mentioned venom compo-
composed of 29 residues. ESI-MS of chain B gave a monoiso-
nents [n ⫽ 2 ⫻ (15 ⫻ 5) for each assay]. Venom components in
topic mass of 3,475.80 Da, which is one mass unit less than the
above-mentioned concentrations were injected alone as control
expected theoretical mass of 3,476.83 Da, thus indicating C-
(n ⫽ 20). The paralytic activity of physiological KCl concentra-
terminal amidation of chain B. The determined amino acid
tion was measured by comparing the awake time of a control
sequences of both chains agree well with the amino acid com-
group (n ⫽ 20) and a treated group (n ⫽ 20) (Mann–Whitney U
position of native CSTX-13 as well as with the individual chains.
test, SPSS 10 software).
Taking into account the four disulfide bridges of both peptide
To highlight synergistic effects between the neurotoxins
chains and the amidation, the calculated monoisotopic mass of
CSTX-1 and CSTX-13, corresponding to their molar ratio in the
CSTX-13 is in agreement with the measured mass of native
crude venom (9:1), bioassays were performed with 0.315 pmol of
CSTX-13 (measured, 7,354.51 Da; calculated, 7,354.37 Da) (Fig.
CSTX-1 per mg of fly alone and in combination with 0.035 pmol
2 and Fig. 5, which is published as supporting information on the
of CSTX-13 per mg of fly, and three further concentrations down
PNAS web site).
to 0.63 fmol of CSTX-13 per mg of fly [n ⫽ 2 ⫻ (12 ⫻ 5) for each
SDS兾PAGE analysis of purified native CSTX-13 revealed a
assay]. CSTX-13 alone was used in the above mentioned con-
single band at 12 kDa, whereas reduced CSTX-13 revealed a
centrations as a control (n ⫽ 2 ⫻ 20).
single band at ⬇3 kDa, obviously containing the peptide chains
The influence of CSTX-13 on two different Ca2⫹ channel
A and B. This supports the two-chain structure of the native
blockers was further investigated. NiCl2 was administered in a
CSTX-13 (Fig. 1 C and D).
concentration of 5.26 nmol兾mg of fly alone and in combination
To exclude the possibility that the two-chain structure of
with 0.035 pmol of CSTX-13 per mg of fly [n ⫽ 2 ⫻ (12 ⫻ 5) for
CSTX-13 is a proteolytic artifact because of contamination of the
each assay]. Nifedipine was tested in a concentration of 0.105
venom with digestive liquid (24), CSTX-13 was incubated with
nmol兾mg of fly alone and in combination with 0.035 pmol of
fresh digestive liquid. After 0.5, 1, and 24 h of incubation, the
CSTX-13 per mg of fly [2 ⫻ (6 ⫻ 5) for each assay], and three
obtained mass indicates a proteolytic degradation of the 14
further concentrations up to 5.5 pmol of CSTX-13 per mg of fly
C-terminal amino acid residues of chain B (measured, 5,746.51
[n ⫽ 6 ⫻ 5 for each assay].
Da; calculated, 5,746.51 Da). The CSTX-13 fragment was puri-
The relative mortality of D. melanogaster was arcsin square
fied by RP-HPLC, and reduced and alkylated in the presence of
root-transformed and treated as the dependent variable,
two protease inhibitors. ESI-MS analysis of the purified com-
whereas the venom components or CSTX-13 were treated as
pounds revealed an intact chain A (measured, 4,342.80 Da;
nominal independent variables. The experiment was analyzed by
calculated, 4,342.76 Da) and a truncated chain B (measured,
generalized linear models. The means of the nominal indepen-
1,868.03 Da; calculated, 1,867.98 Da). These findings are in
dent variables venom components or CSTX-13, respectively,
accordance with the result described above and support the
were compared pairwise by the Bonferroni method. Fulfillment
assumption of a native two-chain structure of CSTX-13 in the
of the model assumptions was checked by visual inspection of the
venom. CSTX-13 seems to be present in the venom as a
residuals distribution for every statistical test conducted. Statis-
two-chain molecule and to the best of our knowledge does not
tics were performed with S-PLUS 6.0 PROFESSIONAL software.
represent a purification artifact.
Because of the unique amino acid sequences of CSTX-1 and
CSTX-13 with cysteine residues arranged in close proximity,
Purification and Sequence Analysis of CSTX-13. The crude venom
classical approaches to determine the disulfide bridge pattern,
(425 l) was separated in a four-step protocol using gel filtration
based on specific enzymatic or chemical cleavages, failed. Con-
(Fig. 1 A), cationic exchange chromatography (Fig. 1B), and
sequently, the disulfide bridge patterns of CSTX-1 and CSTX-13
Wullschleger et al.
PNAS 兩 August 3, 2004 兩 vol. 101 兩 no. 31 兩 11253

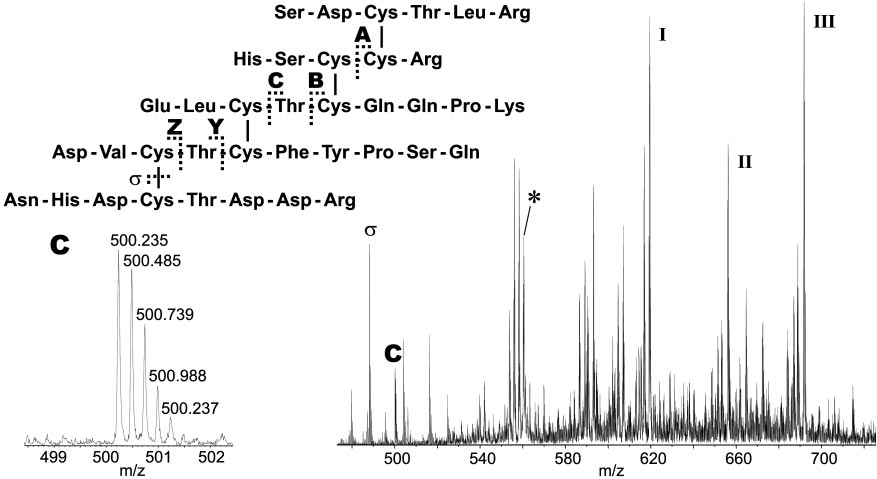
Sequence of the cystine-containing fragment obtained from tryptic digest of native CSTX-13 (4,473.73 Da). The asterisk (*) indicates the [M ⫹ 8H]8⫹ ion
with m兾z 560.23, which was selected as the precursor for CID. Typical fragmentation pathways include loss of terminal amino acids in conjunction with loss ofwater (ions I, I, and III), disulfide cleavage (ion ), and cleavage of peptide bonds between cystines (disulfide bridge-defining ions A–Z). The enlargement showsthe isotopic pattern of fragment ion C with m兾z 500.22, which defines, in combination with fragment ion A, the Cys 1–Cys 4 and Cys 2–Cys 5 bridges.
were identified de novo by nanoelectrospray tandem MS. Diges-
calculated masses. Fig. 3 also shows a section of the product ion
tion of the native toxin with immobilized trypsin yielded main
spectrum obtained by CID of the [M ⫹ 8H]8⫹ precursor ion of
fragments consisting of five short peptide chains cross-linked by
the tryptic CSTX-13 fragment. The high mass accuracy and
four disulfide bridges.
resolving power of the tandem mass spectrometer allow unam-
Multiply charged [M ⫹ nH]n⫹ ions (n ⫽ 3–9) of the cystine
biguous peak assignment, as demonstrated for the quadruply
containing tryptic fragment (measured, 4,473.75 Da; calculated,
charged fragment ion C. Additional information was obtained by
4,473.76 Da) of CSTX-13 were selected as precursor ions for
assigning peaks generated by disulfide bridge cleavage. The same
subsequent collision-induced dissociation (CID). The resulting
strategy was applied for the elucidation of the disulfide bridge
product ion spectra are characterized by abundant peaks of
pattern of CSTX-1, which exhibits identical disulfide bridges
fragment ions generated by cleavage of the disulfide bridges.
These ions define the order of peptide chains. Further abundant
peaks indicate repetitive loss of amino acids from the termini of
Synergistic Insecticidal Effects. To evaluate the biological impor-
the peptide chains, often occurring in conjunction with the loss
tance of CSTX-13, comparative bioassays with D. melanogaster
of water. Detailed information on the disulfide bridge pattern
were performed. The LD50 of 16.3 pmol兾mg of fly (14.5–27.1;
was obtained by detection of the less abundant fragment ions of
95% confidence limits) indicates a lower toxicity than other
mass 968.43 Da (A), 1,895.80 Da (B), 1,995.85 Da (C), 1,289.45
neurotoxins of C. salei. CSTX-13 is ⬇49 times less toxic than the
Da (Y), and 1,390.49 Da (Z), generated by cleavage of the
neurotoxin CSTX-1, and 2.8 times less toxic than the cytolytically
peptide backbone between adjacent cystines. The corresponding
active peptide cupiennin 1a, both key components identified in
cleavage sites are indicated in Fig. 3 (see Fig. 6, which is
the venom of C. salei (24, 12) (Table 1).
published as supporting information on the PNAS web site).
Synergistic interactions of different venom components (taurine,
Measurements exhibit a maximum deviation of 0.02 Da from the
histamine, KCl) with the paralytic activity of CSTX-13 were ana-
Table 1. Insecticidal activity of spider venom components
Physiological venom
Synergistically tested
concentration, mM
Estimation of the lethal doses (LD50) in a Drosophila bioassay, where 50% of the test flies died of intoxication
24 h after injection. Different amounts of peptides, histamine, taurine, and KCl were dissolved in 0.1 M ammoniumacetate, at a pH of 6.1, and 0.05 l was injected into the flies. The physiological concentrations of CSTX-1 (13),taurine (13), histamine (13), KCl (13), CSTX-9 (15), and cupiennin 1a (12) in the venom were reported.
Wullschleger et al.

only in high concentrations (LD50 309 M) when applied alone,
but synergistically enhances the paralytic activity of the main
neurotoxin CSTX-1 at low concentrations (0.7 M).
In the venom, CSTX-13 is constitutively present at a 7–8 times
lower concentration than the main neurotoxin CSTX-1 and in an
up to 2.8 times lower concentration than a further neurotoxin
CSTX-9. Similarly, its insecticidal activity, expressed as a LD50
value, is 49 times lower than the activity of CSTX-1 and 1.5 times
lower than that of CSTX-9 (Table 1) (24). Protein database
search using BLASTP 2.2.8 (29) resulted in a high sequence identity
of 56% (70% similarity) between CSTX-1 and CSTX-9, but in
lower sequence identities of 35% between CSTX-13 and CSTX-1
(51% similarity), and of 31% between CSTX-9 and CSTX-13
Synergistic effects between CSTX-13 and venom components. (A)
(49% similarity). Nevertheless, all three peptides exhibit iden-
Synergistic effects between CSTX-13 and low molecular venom components.
tical disulfide bridge patterns (15–17) (Fig. 2). Sequence com-
In a Drosophila bioassay, the lethal effect of CSTX-13 injected alone (239.4 M)
parison implies that, upon processing, a short peptide is excised
was compared with the lethal effect of coinjected CSTX-13 (239.4 M) with
in the loop forming the disulfide bridge C6–C7, thus leading to
taurine (0.07 mM; not significant), histamine (5.7 mM; not significant), KCl
the two-chain structure of CSTX-13.
(215 mM; *, P ⬍ 0.05), or cupiennin 1a (9.6 M; ***, P ⬍ 0.001). As controls,
No sequence similarities were detected with further neuro-
taurine, histamine, KCl, and cupiennin 1a showed no toxic effect when
toxins and the two other sequenced two-chain calcium channel
administered alone. (B) Synergistic effects of CSTX-13 on the toxicity ofCSTX-1. The lethal effect of CSTX-1 (5.99
M) was compared with the lethal
-agatoxin IA (66 and 3 residue chains) and -agatoxin
effect of coinjected CSTX-1 (5.99 M) with CSTX-13 (0.67 M兾mg of fly;
G (62 and 3 residue chains) (20) from the spider Agelenopsis
P ⬍ 0.001) (molar ratio of 9:1) corresponding to their concentrations in the
aperta. In contrast to CSTX-13, which contains two interchain
venom. CSTX-13 was also injected alone as control and showed no toxic effect
and two intrachain disulfide linkages, these two neurotoxins
on the flies. Statistical analysis was done by using the Bonferroni method.
possess four intrachain and one interchain disulfide linkage. -
Standard error bars are shown for every treatment.
agatoxin IA is formed from its precursor by excision of an
internal heptapeptide leading to a major peptide chain that is
lyzed. Taurine itself was not toxic to D. melanogaster up to 8.9
connected to the minor peptide chain (three residues) by one
nmol兾mg of fly. The LD
disulfide bridge (19) (Fig. 2).
50 of histamine was 51.0 nmol兾mg of fly
(44.2–61.2; 95% confidence limits) and KCl showed a LD
Unlike the above mentioned -agatoxins, CSTX-13 is neuro-
nmol兾mg of fly (91.7–118.4; 95% confidence limits) (Table 1).
toxic by itself only at a high micromolar concentration. This
When tested alone at physiological concentrations, taurine
circumstance raised the question of whether the two-chain
(3.88 pmol兾mg of fly) and histamine (316.67 pmol兾mg of fly)
structure of CSTX-13 might be the result of a purification artifact
showed no effects in a Drosophila bioassay. Injection of KCl
caused by contamination with proteases. The purification
(11.94 nmol兾mg of fly) showed a short significant paralytic effect
protocols of CSTX-13 over the last 5 years have always resulted
(564.7 s ⫾ SD 288.9 s; P ⬍ 0.001) when compared with the
in a pure peptide with identical molecular masses. Experiments
control group (234.5 s ⫾ SD 116.5 s). An injection of 12.6 pmol
with spider digestive liquid, which could be the major source of
of CSTX-13兾mg of fly resulted in a mortality of 39%. No
protease contaminations, resulted only in a C-terminal trunca-
statistically significant differences were observed by coinjection
tion of 14 residues of chain B. As shown previously, C-terminal
of CSTX-13 with taurine (mortality of 34%) or with histamine
proteolytic degradation of CSTX-1 by spider digestive liquid
(mortality of 42%). In contrast, coinjection of CSTX-13 with
stopped at position 49 (Gly) (24). Therefore, we conclude that
KCl significantly increased the mortality to 59% (P ⬍ 0.05) (Fig.
the two-chain structure of CSTX-13 is not a purification artifact
4A). When injected alone, the cytolytic cupiennin 1a is not toxic
or protease degradation product, but a valid constitutive
to D. melanogaster in a concentration of 0.53 pmol兾mg of fly, but
component of the C. salei venom. Whether the two-chain
it increases the mortality of CSTX-13 from 39% to 97% (P ⬍
structure is posttranslationally generated, however, remains
0.001) (Fig. 4A).
to be investigated.
In addition, we investigated the synergistic effect of CSTX-13
on the toxicity of CSTX-1. At physiological concentrations in the
Biological Function of CSTX-13 in the Venom. To analyze the bio-
venom, the molar ratio of CSTX-1 and CSTX-13 is 9:1. With
logical function of CSTX-13, we investigated possible interac-
administration of one peptide alone, 0.315 pmol of CSTX-1 per
tions between CSTX-13 and different venom components in a
mg of fly caused a mortality of 31%, and injection of 0.035 pmol
Drosophila bioassay. Previously, we have shown that the neuro-
of CSTX-13 per mg of fly had no effect. Surprisingly, coinjection
toxicity of the main neurotoxin CSTX-1 to blow flies (Proto-
of CSTX-1 and CSTX-13 in the above mentioned molar ratio of
phormia sp.) could be increased when coinjected with taurine
9:1 significantly increased the mortality to 96% (P ⬍ 0.001) (Fig.
and histamine (9). However, coinjection of CSTX-13 with
4B), and, even in a molar ratio of 500:1, the enhancing effect of
taurine or histamine in its physiological venom concentration did
CSTX-13 was observed (45%, not significant).
not increase its insecticidal activity. Nevertheless, histamine as a
In view of the fact that CSTX-1 inhibits L-type Ca2⫹ channels
neurotransmitter and taurine as a neuromodulator play an
and that, in Drosophila muscle, a 1,4-dihydropyridine-sensitive
important role in the insect nerve system (30–33).
(25) homolog of the mammalian L-type兾␣1D (Dmca1D) subunit
Remarkably, the venom of C. salei exhibits a very high K⫹ ion
gene is expressed (26), the influence of CSTX-13 on the activity
concentration that is 32-fold higher than in the hemolymph, and
of the L-type calcium channel blocker nifedipine (27) as well as
even 2.7-fold higher than in the prevenom of the scorpion
NiCl2, a general inhibitor of calcium channels (28), was inves-
Parabuthus transvaalicus (11, 34). Hammock and coworkers (34)
tigated. No synergistic effects between NiCl2 or nifedipine and
suggest an economically motivated strategy in venom utilization
CSTX-13 were detected.
for this scorpion. P. transvaalicus first secretes a prevenom
containing a high K⫹ ion concentration at a low protein content,
whereas the subsequently secreted venom is characterized by a
The Structure of CSTX-13. In CSTX-13, we have characterized a
high protein content and a 15-fold lower K⫹ ion concentration.
two-chain peptide from the venom of C. salei. It paralyzes flies
The synergistic activity in the prevenom between the ‘‘inexpen-
Wullschleger et al.
PNAS 兩 August 3, 2004 兩 vol. 101 兩 no. 31 兩 11255
sive'' K⫹ ion and the assumed inhibitors of rectifier K⫹ channels
neurotoxic enhancer CSTX-13 show that it enhances the efficacy
is proposed as a means of conserving metabolically expensive
of the neurotoxin CSTX-1 at a concentration of 440 times below
neuropeptides in the venom (34). In part, C. salei also uses this
its LD50. Tests with different concentrations of CSTX-13 re-
strategy to enhance its venom efficacy. Coinjection of CSTX-13
vealed a positive correlation between the amount of CSTX-13
with K⫹ ions increases the mortality of the flies by 20%. The
and the efficacy of CSTX-1. The cooperation between CSTX-1
synergistic cooperation of K⫹ ions is also detectable when
and CSTX-13 seems to be highly specific, because no synergistic
applied together with CSTX-1, a suggested L-type Ca2⫹ channel
interactions between CSTX-13 and other Ca2⫹ channel blockers,
blocker (B.W. and L.K.-N., unpublished data). The high K⫹
such as nifedipine and NiCl2, were found.
concentration in the venom alone caused an immediate short
paralysis, and there seems to be a general cooperation between
K⫹ ions and various ion channel blockers described here for a
In summary, the structural and biological characterization of
labidognath spider.
CSTX-13 provide further insight into the complexity of C. salei
Enhancement of insecticidal efficacy through the cooperative
venom as more multiple interactions between different venom
interaction of different venom peptide neurotoxins in spiders
components become apparent. After venom injection into a
(35) and scorpions (36, 37) has been well investigated. Addi-
prey animal, the hyaluronidase seems to act as a spreading
tionally, synergistic interactions between acylpolyamines and
factor, followed by the dual cytolytic activity of the cupiennins.
cysteine-rich peptide neurotoxins (38) as well as between cyto-
They facilitate the activity of the neurotoxins and at the same
lytic peptides and neurotoxins have been described (12–14).
time protect the venom duct and glands against bacterial
These positive interactions were principally demonstrated by
invasion by membrane disturbance and pore building. Addi-
applying both components in toxic concentrations.
tionally, antimicrobial peptides may also modulate intracellu-
In contrast, a nontoxic concentration of the cytolytically active
lar signaling by increasing intracellular Ca2⫹, as reported for
cupiennin 1a (20 times lower than its LD
parabutoporin and opistoporin from scorpion venoms (39).
50) dramatically en-
Simultaneously, the inhibition of ion channels by the neuro-
hances the efficacy of CSTX-1 (12, 13). The same effect was
toxins is further enhanced by the high K⫹ ion concentration in
observed when testing CSTX-13 and cupiennin 1a. It is assumed
the venom, shifting the K⫹ equilibrium potential (34). Finally,
that, in both cases, mainly through the nonspecific cytolytic
the neurotoxins act on different ion channels with a concom-
activity of cupiennin 1a, CSTX-1 and CSTX-13 have better
itant enhancement by CSTX-13.
access to their targets.
Surprisingly, when CSTX-1 and CSTX-13 were administered
We thank Dr. Patrik Kehrli and Dr. Sven Bacher for statistical advice,
together at their venom concentrations, a strong positive coop-
Dr. Heather Murray for critical comments on the manuscript, and the
eration was found. The data presented here on the two-chain
Swiss National Science Foundation for funding.
1. Loret, E. & Hammock, B. (2001) in Scorpion Biology and Research, eds.
22. Chang, J.-Y. & Knecht, R. (1991) Anal. Biochem. 197, 52–58.
Brownell, P. & Polis, G. (Oxford Univ. Press, New York), pp. 204–233.
23. Escoubas, P., Palma, M. F. & Nakajima, T. (1995) Toxicon 33, 1549–
2. Escoubas, P., Diochot, S. & Corzo, G. (2000) Biochimie 82, 893–907.
3. Barth, F. G. (2002) A Spider's World: Senses and Behavior (Springer, New York).
24. Kuhn-Nentwig, L., Schaller, J., Ka¨mpfer, U., Imboden, H., Malli, H. &
4. Boeve´, J.-L., Kuhn-Nentwig, L., Keller, S. & Nentwig, W. (1995) Toxicon 33,
Nentwig, W. (2000) Arch. Insect Biochem. Physiol. 44, 101–111.
25. Gielow, M. L., Gu, G.-G. & Singh, S. (1995) J. Neurosci. 15, 6085–6093.
5. Malli, H., Imboden, H. & Kuhn-Nentwig, L. (1998) Toxicon 36, 1959–1969.
26. Ren, D., Xu, H., Eberl, D. F., Chopra, M. & Hall, L. M. (1998) J. Neurosci. 18,
6. Malli, H., Kuhn-Nentwig, L., Imboden, H. & Nentwig, W. (1999) J. Exp. Biol.
27. Cohen, C. J., Ertel, E. A., Smith, M. M., Venema, V. J., Adams, M. E. &
7. Wigger, E., Kuhn-Nentwig, L. & Nentwig, W. (2002) Toxicon 40, 749–752.
Leibowitz, M. D. (1992) Mol. Pharmacol. 42, 947–951.
8. Wullschleger, B. & Nentwig, W. (2002) Funct. Ecol. 16, 802–807.
28. Wakamori, M., Strobeck, M., Niidome, T., Teramoto, T., Imoto, K. & Mori, Y.
9. Kuhn-Nentwig, L., Bu¨cheler, A., Studer, A. & Nentwig, W. (1998) Naturwis-
(1998) J. Neurophysiol. 79, 622–634.
senschaften 85, 136–138.
29. Altschul, S. F., Madden, T. L., Scha¨ffer, A. A., Zhang, J., Zhang, Z., Miller, W.
10. Loewe, R., Linzen, B. & von Stackelberg, W. (1970) Z. Vergl. Physiol. 66, 27–34.
& Lipman, D. J. (1997) Nucleic Acids Res. 25, 3389–3402.
11. Kuhn-Nentwig, L., Schaller, J. & Nentwig, W. (1994) Toxicon 32, 287–302.
30. Zheng, Y., Hirschberg, B., Yuan, J., Wang, A. P., Hunt, D. C., Ludmerer, S. W.,
12. Kuhn-Nentwig, L., Mu¨ller, J., Schaller, J., Walz, A., Dathe, M. & Nentwig, W.
Schmatz, D. M. & Cully, D. F. (2002) J. Biol. Chem. 277, 2000–2005.
(2002) J. Biol. Chem. 277, 11208–11216.
31. Witte, I., Kreienkamp, H.-J., Gewecke, M. & Roeder, T. (2002) J. Neurochem.
13. Kuhn-Nentwig, L., Schaller, J. & Nentwig, W. (2004) Toxicon 43, 543–553.
14. Corzo, G., Villegas, E., Go´mez-Lagunas, F., Possani, L. D., Belokoneva, O. S.
32. Buchner, E., Buchner, S., Burg, M. G., Hofbauer, A., Pak, W. L. & Pollack, I.
& Nakajima, T. (2002) J. Biol. Chem. 277, 23627–23637.
(1993) Cell Tissue Res. 273, 119–125.
15. Schaller, J., Ka¨mpfer, U., Schu¨rch, S., Kuhn-Nentwig, L., Haeberli, S. &
33. Bicker, G. (1991) Brain Res. 560, 201–206.
Nentwig, W. (2001) Cell. Mol. Life Sci. 58, 1538–1545.
34. Inceoglu, B., Lango, J., Jing, J., Chen, L., Doymaz, F., Pessah, I. N. &
16. Schaller, J., Kuhn-Nentwig, L., Schu¨rch, S., Ka¨mpfer, U., Mu¨ller, J. & Nentwig,
Hammock, B. D. (2003) Proc. Natl. Acad. Sci. USA 100, 922–927.
W. (2001) Chimia 55, 1058–1062.
35. Bindokas, V. P., Venema, V. J. & Adams, M. E. (1991) J. Neurophysiol. 66,
17. Schu¨rch, S., Schaller, J., Ka¨mpfer, U., Kuhn-Nentwig, L. & Nentwig, W. (2001)
Chimia 55, 1063–1066.
36. Herrmann, R., Moskowitz, H., Zlotkin, E. & Hammock, B. D. (1995) Toxicon
18. Norton, R. S. & Pallaghy, P. K. (1998) Toxicon 36, 1573–1583.
19. Santos, A. D., Imperial, J. S., Chaudhary, T., Beavis, R. C., Chait, B. T.,
37. Regev, A., Rivkin, H., Inceoglu, B., Gershburg, E., Hammock, B. D., Gurevitz,
Hunsperger, J. P., Olivera, B. M., Adams, M. E. & Hillyard, D. R. (1992) J. Biol.
M. & Chejanovsky, N. (2003) FEBS Lett. 537, 106–110.
Chem. 267, 20701–20705.
38. Adams, M. E., Herold, E. E. & Venema, V. J. (1989) J. Comp. Physiol. A 164,
20. Saccomano, N. A. & Ahlijanian, M. K. (1994) Drug Dev. Res. 33, 319–343.
21. Bowers, C. W., Phillips, H. S., Lee, P., Jan, Y. N. & Jan, L. Y. (1987) Proc. Natl.
39. Moerman, L., Verdonck, F., Willems, J., Tytgat, J. & Bosteels, S. (2003)
Acad. Sci. USA 84, 3506–3510.
Biochem. Biophys. Res. Commun. 311, 90–97.
Wullschleger et al.
Source: ftp://ieeservftp01.unibe.ch/pub/iee/groups/syn/publications/pdfs/2004/wullschleger2004.pdf
Corso di Perfezionamento Tecnologie per l'autonomia e l'integrazione sociale delle persone disabili Anno Accademico 1999/2000 Storia di ordinaria sclerosi multipla CANDIDATO: Bianca Tovo Abstract. Il caso di studio riguarda Roberta, 61 anni, che ha presentato i primi disturbi motori e sensitivi a 31anni e che dall'età di 43 anni si sposta esclusivamente in carrozzina. Ha conservato un discreto utilizzofunzionale dell'arto superiore sinistro.Ha uno spirito vivace e sensibile e ha affrontato la sua disabilità con coraggio e buon senso.Negli ultimi anni, anche in seguito ad una malattia del marito oltre che al proprio aggravamento motorio, haperso entusiasmo e ha rinunciato poco per volta alle attività che comportano fatica per lei e per chi l'assiste.Il progetto si propone di:• migliorare la stazione seduta in carrozzina
clinical therapeutics Metformin for the Treatment of the Polycystic Ovary Syndrome John E. Nestler, M.D. This Journal feature begins with a case vignette that includes a therapeutic recommendation. A discussion of the clinical problem and the mechanism of benefit of this form of therapy follows. Major clinical studies, the clinical use of this therapy, and potential adverse effects are reviewed. Relevant formal guidelines,








