Livebetterlife.net
Curr Bladder Dysfunct Rep (2010) 5:212–218DOI 10.1007/s11884-010-0067-2
Benign Prostatic Hyperplasia and Male Lower Urinary TractSymptoms: Epidemiology and Risk Factors
J. Kellogg Parsons
Published online: 7 September 2010
# The Author(s) 2010. This article is published with open access at Springerlink.com
Abstract The epidemiology of benign prostatic hyperpla-
sia (BPH) and male lower urinary tract symptoms (LUTS)has evolved considerably during the past several years. The
The epidemiology of benign prostatic hyperplasia (BPH)
term LUTS describes a distinct phenotype and allows for a
and male lower urinary tract symptoms (LUTS) has
broad epidemiologic description of urinary symptoms at a
evolved considerably during the past several years. The
population level. Although it is becoming the preferred
incidence and prevalence of BPH and LUTS are increasing
term for studying urinary symptoms in populations, LUTS
rapidly as the US population ages. BPH and LUTS are
remains interconnected with BPH in the literature. The
associated with serious medical morbidities, an increased
incidence and prevalence of BPH and LUTS are increasing
risk of falls, depression, diminished health-related quality
rapidly as the US population ages. BPH and LUTS are
of life, and billions of dollars in annual health care costs.
associated with serious medical morbidities, an increased
Although age and genetics play important roles in the
risk of falls, depression, diminished health-related quality
etiology of BPH and LUTS, recent insights at the
of life, and billions of dollars in annual health care costs.
population level have revealed novel risk factors that
Although age and genetics play important roles in the
present new opportunities for treatment and prevention.
etiology of BPH and LUTS, recent insights at the population
Indeed, an exciting notion is that to a large extent, BPH and
level have revealed that modifiable risk factors are likely key
LUTS pathogenesis may be driven by modifiable risk
components as well. Serum dihydrotestosterone, obesity,
factors, including serum dihydrotestosterone (DHT), obesity,
elevated fasting glucose, diabetes, fat and red meat intake,
glucose homeostasis, diet, exercise, and inflammation.
and inflammation increase the risk; vegetables, regularalcohol consumption, exercise, and NSAIDs decrease the
Identification and Definition in Epidemiologic Studies
LUTS represent a cluster of chronic urinary disorders that
Keywords Benign prostatic hyperplasia .
occurs among 15% to 60% of men older than 40 years of
Lower urinary tract symptoms .
age [–]. Specific symptoms associated with the LUTS
American Urological Association Symptom Index . BPH .
complex include frequency, urgency, nocturia, difficulty
LUTS . Epidemiology . Risk factor . Exercise .
initiating urination, sense of incomplete bladder emptying,
Physical activity . Metabolic syndrome . Modifiable .
decreased force of stream, and interruption of stream.
Obesity . Diabetes
The most common etiology of male LUTS is BPH. A
persistent conundrum in designing and interpretingpopulation-based studies of urinary symptoms in older
J. K. Parsons (*)
men is case definition. In the past, chronic urinary
In care of Leslie Parker, Division of Urology,
symptoms in older men were uniformly ascribed to—and
University of California, San Diego,
defined as—BPH. There are at least two reasons why BPH
200 West Arbor Drive #8897,
is a problematic outcome measure for population studies.
San Diego, CA 92103-8897, USAe-mail:
[email protected]
First, BPH has many different definitions, including
Curr Bladder Dysfunct Rep (2010) 5:212–218
histologic analysis of prostate tissue, radiographically
LUTS incidence among older men is also considerable.
determined prostate enlargement, decreased urinary flow
In the Osteoporotic Fractures in Men cohort, a prospective
rates, history of noncancer surgery of the prostate,
study of 6,000 community-dwelling men older than 65 years
physician-diagnosed BPH, and urinary symptoms. Hetero-
of age, 29% of those without LUTS at baseline developed
geneity in defining BPH makes validation across study
clinically significant LUTS within 2 years of follow-up;
populations difficult. Second, BPH excludes other potential
among those 80 years of age or older, this proportion
causes of urinary symptoms in older men, such as
increased to 34% []. In the Olmsted County cohort, 14%
interstitial cystitis and detrusor instability.
of men without LUTS at baseline subsequently reported
LUTS describes a distinct phenotype of a group of
moderate or severe symptoms within 18 months of follow-
disorders affecting the prostate and bladder that share a
up, and 22% reported moderate or severe symptoms within
common clinical manifestation. The term LUTS allows for
42 months of follow-up [Similarly, 21% of Japanese,
a broad epidemiologic description of urinary symptoms at a
26% of black American, and 20% of Austrian men with no
population level without identification of organ- or disease-
or mild LUTS at baseline reported worsened symptoms
specific etiologies. This approach allows consideration of
after 3, 4, and 5 years of follow-up, respectively [–]
LUTS within a macroscopic context using a uniform
The prevalence and incidence of BPH and LUTS in the
definition unbiased by variable definitions of BPH and
United States increased steadily between 1994 and 2000
other diseases. In recent observational studies, LUTS has
Between 1998 and 2007, the
become the preferred term for studying urinary symptoms in
age-adjusted prevalence of BPH among hospitalized
populations –]. Still, as most men with LUTS have BPH
patients in the United States nearly doubled (Stroup and
and most men with BPH have LUTS, the two terms remain
Parsons, unpublished data). Increases in BPH and LUTS
inextricably interconnected in the contemporary treatment
prevalence and incidence are occurring within the context
and study of urinary disorders in older men. Therefore, this
of an aging population. By 2030, 20% of the US population
review discusses BPH and LUTS as separate outcomes
will be 65 years of age or older, a figure that will include
describing the same spectrum of male urinary disorders.
more than 20 million men. Significantly, the fastest-
The most commonly used measures of LUTS in
growing segment of the older adult population is the oldest
epidemiologic studies, and the primary instruments by
age group: those older than 85 years of age. Current
which BPH is diagnosed in clinical practice are the
estimates indicate that the number of individuals 80 years
American Urological Association Symptom Index (AUA-
of age or older in the United States will rise from 9.3
SI) and its internationally validated counterpart, the
million in 2000 to 19.5 million in 2030, an increase of more
International Prostate Symptom Score (I-PSS). The AUA-
than 100% ]. These trends portend substantial increases
SI and I-PSS are robust and reliable quantitative instru-
in the number of incident and prevalent LUTS and BPH
ments for measuring urinary symptoms in men. As part of
cases within the next few decades.
its clinical guidelines, the American Urological Associationrecommends the routine use of the AUA-SI or I-PSS in the
Public Health Impact of Lower Urinary Tract Symptoms
clinical evaluation of patients with suspected BPH
and Benign Prostatic Hyperplasia
It is important to consider the substantial adverseconsequences of LUTS and BPH for the global health
Epidemiology of Lower Urinary Tract Symptoms
of older men. Although it may be tempting to dismiss
and Benign Prostatic Hyperplasia
LUTS and BPH as relatively harmless disorders repre-senting more of an inexorable, if inconvenient, effect of
Incidence and Prevalence in an Aging Population
aging, this perception belies the substantial medical,psychological, and economic burdens of these conditions.
The prevalence of BPH and LUTS rises markedly with
Despite widespread use of medical therapy (
increased age. BPH affects 70% of US men 60–69 years
), BPH remains associated with a substantial
of age and 80% of those 70 years of age or older In
prevalence of urinary infections, bladder stones, urinary
the Boston Area Community Health survey, LUTS
retention, and acute renal failure (Stroup and Parsons,
prevalence increased from 8% in men 30–39 years of
unpublished data). In addition, the adverse health effects of
age to 35% in men 60–69 years [In the Rancho
LUTS and BPH encompass serious nonurologic conditions.
Bernardo study, 56% of men 50–79 years of age, 70% of
Men with severe LUTS have a 63% increased risk of falling at
men 80–89 years of age, and 90% of men 90 years of age
least twice within 1 year compared with men with no
or older reported LUTS []. Other population-based
symptoms ]. Falls in older adults are associated with
studies have demonstrated similar trends [
debilitating morbidities, including pain and fractures. LUTS
Curr Bladder Dysfunct Rep (2010) 5:212–218
is also associated with a significantly diminished quality of
tigators further estimated that 50% of men undergoing
life, depression, and impairment in instrumental activities of
surgery for BPH who were younger than 60 years of age
daily living [, ].
had a heritable form of disease ]. These findings and
Another important public health issue is the cost
those of others have suggested an autosomal dominant
associated with diagnosis and treatment. In 2000, the most
pattern of inheritance ]. Men with inherited forms of
recent year for which comprehensive data are available,
BPH tend to have larger prostates and younger age at onset
BPH generated 1.1 billion dollars in direct health care
than men with sporadic BPH ].
expenditures and accounted for more than 4.4 million office
Monozygotic twin concordance rates of 63% and 26%
visits, 117,000 emergency department visits, 105,000
have been observed for LUTS and BPH, respectively, with
hospitalizations, and 21 to 38 million hours in lost
one study estimating that genetic factors may contribute as
productivity in the United States. During the same year,
much as 72% to the risk of high moderate or severe LUTS
the direct costs of treatment for overactive bladder in men
among older men ,
totaled 1.8 billion dollars, with another 3.9 billion dollarsdirected to ancillary health care expenditures, including
Sex Steroid Hormones
treatment for urinary tract infections. Estimated annualcosts of BPH treatment currently total 3.9 billion dollars
Prostate tissue is composed of two basic elements: a
glandular element composed of secretory ducts and acini,
The high prevalence of LUTS in the older male
and a stromal element composed primarily of collagen and
population, its detrimental impact on male health, and the
smooth muscle. In BPH, unregulated cellular proliferation
substantial costs associated with diagnosis and treatment
leads to increased prostate volume and increased stromal
underscore the significance of this problem, particularly in
smooth muscle tone; increased prostate volume and stromal
an era of rapidly increasing male longevity.
smooth muscle tone in turn cause physical compression ofthe urethra and mechanical obstruction of the bladderoutlet. In prostatic secretory cells, the hormone 5-α
Risk Factors Associated with Lower Urinary Tract
reductase converts testosterone to DHT, a potent stimulator
Symptoms and Benign Prostatic Hyperplasia
of prostate growth that, in addition to being necessary forprostate development, appears to play a central role in BPH
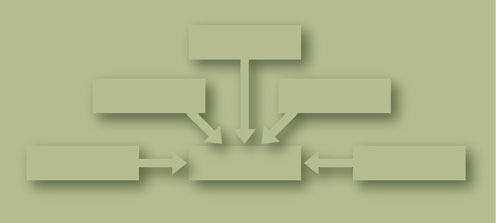
On a population level, there are five broad categories of risk
pathogenesis. Multiple studies have explored associations
factors for BPH and LUTS (Fig. Aside from age, the
of endogenous sex steroid hormones with BPH and LUTS.
other categories are genetics, sex steroid hormones,modifiable lifestyle factors, and inflammation.
At least seven observational studies have reported noassociations and five inverse associations of serum testos-
Evidence suggests that there are strong genetic components
terone (total, bioavailable, or free) with BPH or LUTS [
to both BPH and LUTS. One case-control analysis, in
•, ]. No studies to date have reported an increased risk
which participants were men younger than 64 years of age
of BPH or LUTS with higher serum testosterone levels. A
who underwent surgery for BPH, noted fourfold and sixfold
prominent but theoretical concern of testosterone replace-
increased age-specific risks of BPH surgery among all male
ment therapy is the potential for it to exacerbate BPH and
relatives and brothers, respectively, of cases. These inves-
LUTS [In this sense, these observations imply thathigher serum testosterone concentrations do not promoteBPH.
Several studies have noted an increased risk of BPH withincreased serum concentrations of DHT and its metabolites.
In one recent prospective study of community-dwellingmen, those with the highest midlife levels of DHT hadnearly three times the risk of subsequent BPH comparedwith those with the lowest levels [].
These results are consistent with those of three prior
Fig. 1 Broad categories of epidemiologic risk factors for benignprostatic hyperplasia (BPH) and lower urinary tract symptoms (LUTS)
studies of serum concentrations of two DHT metabolites:
Curr Bladder Dysfunct Rep (2010) 5:212–218
17b-diol-glucuronide and androstanediol glucuronide.
increased prevalence of LUTS compared with those with
These metabolites are surrogate markers for DHT activity,
no components. Other studies have shown that men with
with higher concentrations indicating increased and lower
heart disease are at significantly increased risk of clinical
concentrations indicating decreased levels of DHT. Two
BPH and LUTS [–
cross-sectional studies and one prospective study haveshown direct associations of these DHT metabolites with
BPH or LUTS [, ].
The 5-α reductase inhibitors (finasteride and dutasteride)
Prior studies have consistently observed that increased
decrease serum concentrations of DHT [and prevent
adiposity is positively associated with prostate volume—
progression of clinical BPH [However, it is not known
that is, the greater the amount of adiposity, the greater the
whether finasteride or dutasteride will prevent incident
prostate volume. Body weight, body mass index (BMI),
and waist circumference all have been positively associatedwith prostate volume in multiple different study populations
–]. In the Baltimore Longitudinal Study of Agingcohort, for example, each 1-kg/m2 increase in BMI
No clear patterns of estrogen, LUTS, and BPH have yet
corresponded to a 0.41-mL increase in prostate volume.
emerged. Prior studies have reported positive, negative, and
Moreover, obese (BMI ≥35 kg/m2) participants had a 3.5-fold
null associations of endogenous estrogens with BPH and
increased risk of prostate enlargement compared with non-
obese (BMI <25 kg/m2) participants ]. A preponderanceof published epidemiologic evidence also demonstrates that
obesity increases the risks of BPH surgery, urinary symptomprogression, initiation of BPH medical therapy, and LUTS
Other sex steroid hormones also have not demonstrated
consistent links with LUTS or BPH. One study observed apositive association of DHEAS (dehydroepiandrosterone
Diabetes and Disruptions in Glucose Homeostasis
sulfate, a primary adrenal androgen with little intrinsicandrogenic activity) with BPH and two inverse associations
Disruptions in glucose homeostasis at multiple different
with LUTS [].
levels—from alterations in serum insulin growth factorconcentrations to diagnosis of clinical diabetes—are asso-
ciated with higher likelihoods of prostate enlargement,BPH, and LUTS. Higher serum concentrations of insulin-
A notable development in the epidemiology of BPH and
like growth factor-1 and insulin-like growth factor binding
LUTS is the recognition that modifiable lifestyle factors
protein-3 have been associated with increased risk of
substantially influence the natural history of these
clinical BPH and BPH surgery ]. Physician-diagnosed
conditions. Accumulating data intimate that many of the
diabetes, increased serum insulin, and elevated fasting
same metabolic disturbances associated with cardiovas-
plasma glucose have been associated with increased
cular disease—and the lifestyle factors that modulate
prostate size and increased risks of prostate enlargement,
these disturbances—influence the risk of BPH and
clinical BPH, BPH surgery, and LUTS in multiple different
LUTS. These observations are important because they
cohorts cumulatively incorporating tens of thousands of
suggest the existence of modifiable pathways for BPH
and LUTS that may present novel targets for preventionand treatment.
The Metabolic Syndrome and Cardiovascular Disease
Relatively few data are available on potential associationsof lipids (high-density lipoprotein, low-density lipoprotein,
The metabolic syndrome is a clinical constellation of
and triglycerides) with BPH and LUTS. At least five
metabolic abnormalities—obesity, glucose intolerance,
studies have been conducted, with three showing positive
dyslipidemia, and hypertension—that increases the risk of
and two showing null associations [, ].
cardiovascular disease and results primarily from dietaryand other lifestyle practices endemic to Westernized
societies ].
In one cohort, men diagnosed with at least three
There are some indications that macronutrients and
components of the metabolic syndrome had an 80%
micronutrients may affect the risk of BPH and LUTS,
Curr Bladder Dysfunct Rep (2010) 5:212–218
although the patterns are somewhat inconsistent. For
BPH represents a nonmalignant pathway of unregulated
macronutrients, increased total energy intake, energy-
prostate growth promoted by oxidative stress, inflamma-
adjusted total protein intake, red meat, fat, milk and dairy
tory mediators, and insulin growth pathways.
products, cereals, bread, poultry, and starch potentially
There are strong links between BPH and histologic
increase the risks of clinical BPH and BPH surgery;
inflammation in surgical specimens, with the extent and
vegetables, fruits, polyunsaturated fatty acids, linoleic
severity of the inflammation corresponding to the magni-
acid, and vitamin D potentially decrease the risks of BPH
tude of prostate enlargement and BPH area –Men
and LUTS [, ]. With respect to micronutrients,
with LUTS are more likely to have higher serum C-reactive
higher circulating concentrations of vitamin E, lycopene,
protein, a robust marker of systemic inflammation [
selenium, and carotene have been inversely associated
while prior gonorrheal infection or prostatitis increase the
with BPH and LUTS [•, ]; zinc has been
likelihood of BPH surgery and LUTS [A history of
associated with increased and decreased risk ,
infection with gonorrhea, chlamydia, or trichomonosisincreases the risk of elevated prostate-specific antigen
Physical Activity
]; high serum IgG antibody titers to cytomegalovirus,herpes virus, human papilloma virus, and hepatitis are
Increased physical activity and exercise have been
associated with LUTS ].
robustly and consistently linked with decreased risks of
Conversely, inhibition of inflammatory pathways poten-
BPH surgery, clinical BPH, histologic BPH, and LUTS
tially attenuates BPH risk. In one community cohort, men
. A meta-analysis of 11 published studies (n =
who reported daily NSAID use experienced significantly
43,083 men) indicated that moderate to vigorous physical
decreased risks of LUTS, low urinary flow rate, increased
activity reduced the risk of BPH or LUTS by as much as
prostate volume, and elevated prostate-specific antigen
25% relative to a sedentary lifestyle, with the magnitude
of the protective effect increasing with higher levels ofactivity [].
In summary, BPH and LUTS are of significant impor-
Like exercise, moderate alcohol intake also appears to be
tance to public health, affecting millions of older men
protective against multiple outcomes related to BPH [].
and contributing to billions of dollars in health care cost
However, unlike exercise, the same protective effect does
each year. Current disease trends in the United States
not appear to apply to LUTS. A meta-analysis of 19
suggest that the number of men suffering from these
published studies (n = 120,091 men) observed up to a 35%
conditions will swell markedly in the very near future.
decreased likelihood of BPH among men who drank daily,
The rapid aging of the US population, coupled with the
but an increased risk of LUTS [].
obesity and diabetes epidemics is poised to substantiallyincrease the prevalence of BPH and LUTS within the
general population and place even greater burdens onfinite health care resources. Although the relatively
Although several studies support the existence of an
immutable consequences of age and genetics factor
inverse protective effect of smoking on the risk of BPH
substantially in the development of BPH and LUTS,
and LUTS, several others have reported no risk or
many modifiable variables contribute as well—factors
increased risk [Thus, no definitive conclusions can
that may be manipulated to delay onset, prevent
be drawn at this time.
progression, or attenuate symptoms. Potential strategiesinclude inhibition of DHT synthesis with 5-α reductase
inhibitors, modulation of metabolic risk factors withcomprehensive lifestyle interventions incorporating diet
Most observational studies suggest that inflammation is
change and physical activity, and suppression of inflam-
intimately linked to the development of BPH and LUTS.
matory pathways with NSAIDs.
The mechanisms underpinning this relationship areunclear. One potential explanation is that the metabolic
Dr. Parsons has received grant funding from the
syndrome, which promotes systemic inflammation and
National Institutes of Health.
oxidative stress, mediates the connection between them
Dr. Parsons has served as a consultant for American Medical
[Inflammation has been implicated as a primary
Systems and the University of California Physician Enhancement
stimulus for prostate carcinogenesis, and it is possible that
Curr Bladder Dysfunct Rep (2010) 5:212–218
This article is distributed under the terms of the
18. Rohrmann S, Fallin MD, Page WF, et al.: Concordance rates and
Creative Commons Attribution Noncommercial License which per-
modifiable risk factors for lower urinary tract symptoms in twins.
mits any noncommercial use, distribution, and reproduction in any
Epidemiology 2006, 17:419–427.
medium, provided the original author(s) and source are credited.
19. Partin AW, Page WF, Lee BR, et al.: Concordance rates for benign
prostatic disease among twins suggest hereditary influence.
Urology 1994, 44:646–650.
20. Trifiro MD, Parsons JK, Palazzi-Churas K, et al.: Serum sex
hormones and the 20-year risk of lower urinary tract symptoms incommunity-dwelling older men. BJU Int 2010, 105:1554–1559.
Papers of particular interest, published recently, have been
21. • Parsons JK, Palazzi-Churas K, Bergstrom J, Barrett-Connor E: A
prospective study of serum dihydrotesterone and subsequent risk
of benign prostatic hyperplasia in community dwelling men: the
• Of importance
Rancho Bernardo study. J Urol 2010 Jul 17 (Epub ahead of print).
This cohort study demonstrated that higher concentrations of
1. Wei JT, Calhoun E, Jacobsen SJ: Urologic diseases in America
serum DHT increase the risk of BPH. Serum total and
project: benign prostatic hyperplasia. J Urol 2005, 173:1256–1261.
bioavailable testosterone are not associated with BPH risk.
2. Kupelian V, Wei JT, O'Leary MP, et al.: Prevalence of lower
22. Kristal AR, Schenk JM, Song Y, et al.: Serum steroid and sex
urinary tract symptoms and effect on quality of life in a racially
hormone-binding globulin concentrations and the risk of incident
and ethnically diverse random sample: the Boston Area Community
benign prostatic hyperplasia: results from the Prostate Cancer
Health (BACH) survey. Arch Intern Med 2006, 166:2381–2387.
Prevention Trial. Am J Epidemiol 2008, 168:1416–1424.
3. Taylor BC, Wilt TJ, Fink HA, et al.: Prevalence, severity, and
23. Bhasin S, Singh AB, Mac RP, et al.: Managing the risks of
health correlates of lower urinary tract symptoms among older
prostate disease during testosterone replacement therapy in older
men: the MrOS study. Urology 2006, 68:804–809.
men: recommendations for a standardized monitoring plan. J
4. Parsons JK, Bergstrom J, Silberstein J, Barrett-Connor E:
Androl 2003, 24:299–311.
Prevalence and characteristics of lower urinary tract symptoms
24. Amory JK, Wang C, Swerdloff RS, et al.: The effect of 5alpha-
in men aged > or = 80 years. Urology 2008, 72:318–321.
reductase inhibition with dutasteride and finasteride on semen
5. Parsons JK, Wilt TJ, Wang PY, et al.; Osteoporotic Fractures in
parameters and serum hormones in healthy men. J Clin Endocrinol
Men Research Group: Progression of lower urinary tract symp-
Metab 2007, 92:1659–1665.
toms in older men: a community based study. J Urol 2010,
25. McConnell JD, Roehrborn CG, Bautista OM, et al.: The long-term
effect of doxazosin, finasteride, and combination therapy on the
6. Jacobsen SJ, Girman CJ, Guess HA, et al.: Natural history of
clinical progression of benign prostatic hyperplasia. N Engl J Med
prostatism: longitudinal changes in voiding symptoms in community
dwelling men. J Urol 1996, 155:595–600.
26. Haffner S, Taegtmeyer H: Epidemic obesity and the metabolic
7. Masumori N, Tsukamoto T, Rhodes T, Girman CJ: Natural history
syndrome. Circulation 2003, 108:1541–1545.
of lower urinary tract symptoms in men—result of a longitudinal
27. Meigs JB, Mohr B, Barry MJ, et al.: Risk factors for clinical
community-based study in Japan. Urology 2003, 61:956–960.
benign prostatic hyperplasia in a community-based population of
8. Sarma AV, McLaughlin JC, Jacobsen SJ, et al.: Longitudinal
healthy aging men. J Clin Epidemiol 2001, 54:935–944.
changes in lower urinary tract symptoms among a cohort of black
28. Rohrmann S, Smit E, Giovannucci E, Platz EA: Association between
American men: the Flint Men's Health study. Urology 2004,
markers of the metabolic syndrome and lower urinary tract symptoms
in the Third National Health and Nutrition Examination Survey
9. Temml C, Brossner C, Schatzl G, et al.: The natural history of
(NHANES III). Int J Obes (Lond) 2005, 29:310–316.
lower urinary tract symptoms over five years. Eur Urol 2003,
29. Joseph MA, Harlow SD, Wei JT, et al.: Risk factors for lower
urinary tract symptoms in a population-based sample of African-
10. Centers for Disease Control and Prevention: Trends in aging—
American men. Am J Epidemiol 2003, 157:906–914.
United States and worldwide. MMWR Morb Mortal Wkly Rep
30. Parsons JK, Carter HB, Partin AW, et al.: Metabolic factors
2003, 52:101–104, 106.
associated with benign prostatic hyperplasia. J Clin Endocrinol
11. Parsons JK, Mougey J, Lambert L, et al.: Lower urinary tract
Metab 2006, 91:2562–2568.
symptoms increase the risk of falls in older men. BJU Int 2009,
31. Parsons JK: Modifiable risk factors for benign prostatic hyperplasia
and lower urinary tract symptoms: new approaches to old problems. J
12. Engstrom G, Henningsohn L, Walker-Engstrom ML, Leppert J:
Urol 2007, 178:395–401.
Impact on quality of life of different lower urinary tract symptoms
32. Parsons JK, Sarma AV, McVary K, Wei JT: Obesity and benign
in men measured by means of the SF 36 questionnaire. Scand J
prostatic hyperplasia: clinical connections, emerging etiological
Urol Nephrol 2006, 40:485–494.
paradigms and future directions. J Urol 2009, 182(6 Suppl):S27–
13. Hu TW, Wagner TH, Bentkover JD, et al.: Estimated economic
costs of overactive bladder in the United States. Urology 2003,
33. Kristal AR, Arnold KB, Schenk JM, et al.: Race/ethnicity, obesity,
health related behaviors and the risk of symptomatic benign
14. Saigal CS, Joyce G: Economic costs of benign prostatic
prostatic hyperplasia: results from the Prostate Cancer Prevention
hyperplasia in the private sector. J Urol 2005, 173:1309–1313.
Trial. J Urol 2007, 177:1395–1400; quiz 591.
15. Sanda MG, Beaty TH, Stutzman RE, et al.: Genetic susceptibility
34. Sarma AV, Parsons JK, McVary K, Wei JT: Diabetes and benign
of benign prostatic hyperplasia. J Urol 1994, 152:115–119.
prostatic hyperplasia/lower urinary tract symptoms—what do we
16. Pearson JD, Lei HH, Beaty TH, et al.: Familial aggregation of
know? J Urol 2009, 182(6 Suppl):S32–S37.
bothersome benign prostatic hyperplasia symptoms. Urology
35. Parsons JK, Bergstrom J, Barrett-Connor E: Lipids, lipoproteins
2003, 61:781–785.
and the risk of benign prostatic hyperplasia in community-
17. Sanda MG, Doehring CB, Binkowitz B, et al.: Clinical and
dwelling men. BJU Int 2008, 101:313–318.
biological characteristics of familial benign prostatic hyperplasia.
36. Gupta A, Gupta S, Pavuk M, Roehrborn CG: Anthropometric and
J Urol 1997, 157:876–879.
metabolic factors and risk of benign prostatic hyperplasia: a
Curr Bladder Dysfunct Rep (2010) 5:212–218
prospective cohort study of Air Force veterans. Urology 2006,
44. Nickel JC, Downey J, Young I, Boag S: Asymptomatic
inflammation and/or infection in benign prostatic hyperplasia.
37. Nandeesha H, Koner BC, Dorairajan LN, Sen SK: Hyper-
BJU Int 1999, 84:976–981.
insulinemia and dyslipidemia in non-diabetic benign prostatic
45. Theyer G, Kramer G, Assmann I, et al.: Phenotypic characterization
hyperplasia. Clin Chim Acta 2006, 370:89–93.
of infiltrating leukocytes in benign prostatic hyperplasia. Lab Invest
38. • Kristal AR, Arnold KB, Schenk JM, et al.: Dietary patterns,
1992, 66:96–107.
supplement use, and the risk of symptomatic benign prostatic
46. Anim JT, Udo C, John B: Characterisation of inflammatory cells in
hyperplasia: results from the Prostate Cancer Prevention Trial.
benign prostatic hyperplasia. Acta Histochem 1998, 100:439–449.
Am J Epidemiol 2008, 167:925–934. This analysis of the
47. Di Silverio F, Gentile V, De Matteis A, et al.: Distribution of
placebo arm of the Prostate Cancer Prevention Trial included
inflammation, pre-malignant lesions, incidental carcinoma in
several important observations with respect to diet and BPH,
histologically confirmed benign prostatic hyperplasia: a retrospective
namely higher fat and red meat intake increase, while higher
analysis. Eur Urol 2003, 43:164–175.
vegetable intake and regular alcohol consumption decrease BPH
48. Rohrmann S, De Marzo AM, Smit E, et al.: Serum C-reactive
protein concentration and lower urinary tract symptoms in older
39. Tavani A, Longoni E, Bosetti C, et al.: Intake of selected
men in the Third National Health and Nutrition Examination
micronutrients and the risk of surgically treated benign prostatic
Survey (NHANES III). Prostate 2005, 62:27–33.
hyperplasia: a case-control study from Italy. Eur Urol 2006,
49. Sutcliffe S, Giovannucci E, De Marzo AM, et al.: Sexually transmitted
infections, prostatitis, ejaculation frequency, and the odds of lower
40. Dal Maso L, Zucchetto A, Tavani A, et al.: Lifetime occupational
urinary tract symptoms. Am J Epidemiol 2005, 162:898–906.
and recreational physical activity and risk of benign prostatic
50. Sutcliffe S, Zenilman JM, Ghanem KG, et al.: Sexually
hyperplasia. Int J Cancer 2006, 118:2632–2635.
transmitted infections and prostatic inflammation/cell damage as
41. Parsons JK, Kashefi C: Physical activity, benign prostatic
measured by serum prostate specific antigen concentration. J Urol
hyperplasia, and lower urinary tract symptoms. Eur Urol 2008,
51. Sutcliffe S, Rohrmann S, Giovannucci E, et al.: Viral infections
42. Parsons JK, Im R: Alcohol consumption is associated with a
and lower urinary tract symptoms in the Third National Health
decreased risk of benign prostatic hyperplasia. J Urol 2009,
and Nutrition Examination Survey. J Urol 2007, 178:2181–2185.
52. St Sauver JL, Jacobson DJ, McGree ME, et al.: Protective
43. Furukawa S, Fujita T, Shimabukuro M, et al.: Increased oxidative
association between nonsteroidal antiinflammatory drug use and
stress in obesity and its impact on metabolic syndrome. J Clin
measures of benign prostatic hyperplasia. Am J Epidemiol 2006,
Invest 2004, 114:1752–1761.
Source: http://livebetterlife.net/wp-content/uploads/2013/09/11884_2010_Article_67.pdf
The Right to Parenthood An Argument for a Narrow Interpretation1 ABSTRACTThe paper argues for two kinds of limitations on the right to parenthood. First, it claims that the right to parenthooddoes not entail a right to have as many children as one desires. This conclusion follows from the standard justificationsfor the right to parenthood, none of which establishes the need to grant special protection to having as many childrenas one desires. Second, with respect to the right to receive assistance from the state in IVF, it is suggested that the stateshould also be allowed to take non-medical considerations into account in determining whether or not an applicant isentitled to this service, particularly in cases where the applicant seems to lack mothering ability.
Signal Transduction Minocycline Targets the NF-kB Nexus through Suppressionof TGF-b1-TAK1-IkB Signaling in Ovarian Cancer Parvin Ataie-Kachoie1, Samina Badar1,2, David L. Morris1,2, and Mohammad H. Pourgholami2 Substantial evidence supports the critical role of NF-kB in ovarian cancer. Minocycline, a tetracycline, has been shown to exhibit beneficial effects in this malignancy through regulation of a cohort of genes thatoverlap significantly with the NF-kB transcriptome. Here, it was examined whether or not the molecularmechanism could be attributed to modulation of NF-kB signaling using a combination of in vitro and in vivomodels. Minocycline suppressed constitutive NF-kB activation in OVCAR-3 and SKOV-3 ovarian carci-noma cells and was correlated with attenuation of IkBa kinase (IKK) activation, IkBa phosphorylation anddegradation, and p65 phosphorylation and nuclear translocation. The inhibition of IKK was found to beassociated with suppression of TGF-b-activated-kinase-1 (TAK1) activation and its dissociation from TAK1-binding-protein-1 (TAB1), an indispensable functional mediator between TGF-b and TAK1. Further studiesdemonstrated that minocycline downregulated TGF-b1 expression. Enforced TGF-b1 expression inducedNF-kB activity, and minocycline rescued this effect. Consistent with this finding, TGF-b1 knockdownsuppressed NF-kB activation and abrogated the inhibitory effect of minocycline on this transcription factor.These results suggest that the minocycline-induced suppression of NF-kB activity is mediated, in part,through inhibition of TGF-b1. Furthermore, the influence of minocycline on NF-kB pathway activation wasexamined in female nude mice harboring intraperitoneal OVCAR-3 tumors. Both acute and chronicadministration of minocycline led to suppression of p65 phosphorylation and nuclear translocationaccompanied by downregulation of NF-kB activity and endogenous protein levels of its target gene products.These data reveal the therapeutic potential of minocycline as an agent targeting the pro-oncogenic TGF-b–NF-kB axis in ovarian cancer.

