Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Pii: s0896-6273(02)00899-
Neuron, Vol. 35, 1147–1156, September 12, 2002, Copyright
2002 by Cell Press
Human Brain Activity during Illusory
Visual Jitter as Revealed by Functional
Magnetic Resonance Imaging
Yuka Sasaki,1,4 Ikuya Murakami,2
demonstrated to reflect retinal slip due to small eye
Patrick Cavanagh,3 and Roger H.B. Tootell1
movements that are normally kept invisible (Murakami
1Athinoula A. Martinos Center for Biomedical
and Cavanagh, 1998).
This unique illusion suggests a specific way that the
Massachusetts General Hospital
brain may normally cancel the motion signals from small
149 13th Street
eye movements. Murakami and Cavanagh (1998, 2001)
Charlestown, Massachusetts 02129
have proposed that retinal motion signals can be used to
2 Human and Information Science Laboratory
compensate for retinal slip due to small eye movements.
NTT Communication Science Laboratories
They postulate two stages of the process. The first is
an adaptable stage that measures local motion signals,
3-1 Morinosato Wakamiya
and the second is a compensation stage that estimates
Atsugi, Kanagawa 243-0198
retinal slip and subtracts it from motion signals nearby.
Suppose that retinal slip is fully represented in early
3 Vision Sciences Laboratory
visual cortex (Galletti et al., 1984; Gur et al., 1997; Gur
Department of Psychology
and Snodderly, 1987, 1997; Ilg and Thier, 1996; Leopold
and Logothetis, 1998). In this early stage, each retino-
topic point is assumed to have a motion vector (direction
33 Kirkland Street
and speed) that is a mixture of eye movements and
Cambridge, Massachusetts 02138
object motion. One of the missions of a subsequent
processing stage is to suppress the component of eye
movements in this velocity field. It is proposed that a
baseline value (i.e., eye velocity) is estimated by finding
the region having the minimum instantaneous velocity.
One central problem in vision is how to compensate
The minimum velocity will usually arise in regions of the
for retinal slip. A novel illusion (visual jitter) suggests
scene where there is no external motion and, so, will
the compensation mechanism is based solely on reti-
represent the eye movement velocity alone. By sub-
nal motion. Adaptation to visual noise attenuates the
tracting this baseline estimate from the velocities of all
motion signals used by the compensation stage, pro-
points, the desired zero velocity for the stationary re-
ducing illusory jitter due to the undercompensation of
gions and the correct velocity for the moving objects
retinal slip. Here, we investigated the neural substrate
could be recovered.
of retinal slip compensation during this illusion using
This model also explains why the visual jitter illusion
high-field fMRI and retinotopic mapping in flattened
occurs. First, adaptation to dynamic noise desensitizes
cortical format. When jitter perception occurred, MR
motion detectors in the adapted region. This means that
signal decreased in lower stages of the visual system
the retinal slip after adaptation is encoded with a smaller
but increased prominently in area MT⫹
. In conclusion,
gain in the adapted region, although there is no change
visual areas as early as V1 are responsible for the
in small eye movements and corresponding retinal slip
adaptation stage, and MT⫹
is involved in the compen-
with and without adaptation. This creates a new baseline
sation stage. The present finding suggests the path-
minimum there. In the unadapted region, the unattenu-
way from V1 to MT⫹
has an important role in stabilizing
ated motion response to eye movements is above this
the visual world.
new, artificially low baseline. Therefore, the retinal slip
occurring in this region is undercompensated and is
perceived as jitter.
Visual jitter clearly differs from the classical motion
Whenever the eyes move, there is concomitant retinal
aftereffect (an illusory motion in the opposite direction
image slip of a stationary outer world at a speed corre-
after adaptation to unidirectional motion [Wohlgemuth,
sponding to the rotation speed of the eyes. Importantly,
1911]). First, jitter occurs in the unadapted region, while
such a retinal slip is usually not noticed. Clearly, the
conventional motion aftereffect is confined within the
brain somehow compensates for retinal slip due to eye
adapted region (Culham et al., 1999; He et al., 1998).
movements in recovering a veridical visual world. How-
Second, the instantaneous speed and direction of jitter
ever, under certain circumstances we can defeat the
are consistent with eye movements during test, whereas
compensation and perceive our own retinal slip. Specifi-
the direction of motion aftereffect is opposite to the
cally, after adaptation to a patch (e.g., an annulus) of
direction of the adapting stimulus. Third, motion afteref-
dynamic random noise, a larger pattern of static random
fect partially transfers across eyes (Ibbotson and Mad-
noise is presented. The static noise in the unadapted
dess, 1994; Murakami, 1995; Wade et al., 1993), but
region then appears to "jitter" coherently in random di-
visual jitter does not (Murakami and Cavanagh, 1998).
rections for several seconds (please access the visual
Therefore, these two types of illusions are distinct from
jitter demonstration [first figure] at http://www.brl.
each other.
ntt.co.jp/people/ikuya/). This jittery motion has been
As described before, the proposed mechanism of the
jitter aftereffect postulates two distinct stages: (1) an
early adaptable stage where local motion signals are
FMRI experiments were carried out in a 3T scanner in
eight normal subjects, using flattened cortical analysis
at a spatial resolution of 3 ⫻ 3 ⫻ 3 mm. Data were shifted
by 4 s to compensate for the known hemodynamic delay.
Retinotopic Representation of Stimulus Regions
First, we tested if retinotopically separate regions were
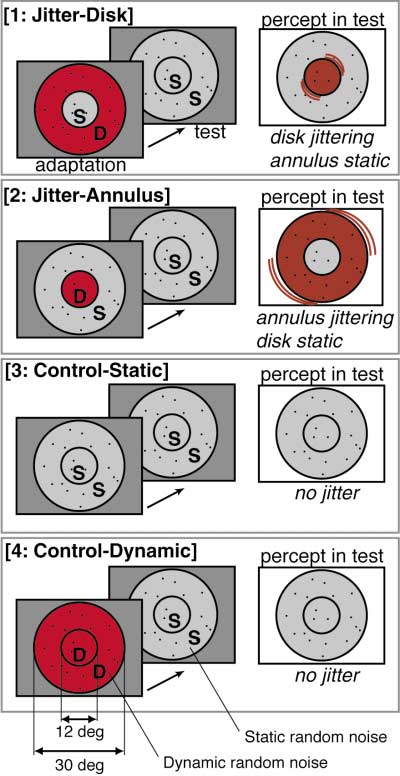
activated by our concentric stimuli for visual jitter. Note
that in jitter-disk and jitter-annulus conditions in Figure
1, jitter perception occurs at different retinotopic re-
gions—although the test stimulus was identical. That is,
jitter occurs in the disk after the annulus was adapted,
whereas jitter occurs in the annulus after adaptation
in the disk. In both cases, jitter is confined within the
retinotopically unadapted region. Figure 2A shows the
retinotopic representation of eccentricity in the right oc-
cipital cortex of one representative subject, with borders
between the visual areas superimposed. Figure 2B
shows the differential BOLD (blood oxygenation level
dependent) activity (p value map) obtained by sub-
tracting the activity in the first 10 s of the test period
for the jitter-annulus condition (in which jitter was per-
ceived in the annulus) from the activity in the first 10 s
of the test period for the jitter-disk condition (in which
jitter was perceived in the disk) within the same flattened
cortex. The differential BOLD activities were positive
Figure 1. Visual Stimulus Configuration
(red-orange) in retinotopically central regions and nega-
There were four conditions, with one condition being presented in
tive (blue-cyan) in more peripheral regions (see Figure
each box: 1, jitter-disk; 2, jitter-annulus; 3, control-static; and 4,
2B). According to the eccentricity map from the same
control-dynamic. In each condition, a trial consisted of an adaptation
subject, these two cortical regions corresponded to the
period (32 s) and a subsequent test period (32 s). The adapting
stimulus varied across conditions. The letter D indicates dynamic
representations of the disk and annulus in the visual
random noise (also emphasized by red in this figure) and the letter
S indicates static random noise. In the test period, the visual stimulus
Thus, two points were clearly revealed. First, in the
was identical throughout the four conditions (i.e., static random
central representations of V1, V2, and other retinotopic
noise occupied both regions). However, perception in the test period
areas, the activity of the disk representation was signifi-
differed across conditions. For illustrative purposes, noise is shown
cantly greater for the jitter-disk condition than for jitter-
as if sparse, but actually it was 50% density.
annulus. Second, the opposite pattern of activation was
found in the more peripheral retinotopic representations
of the annulus: activities in these regions were signifi-
encoded and (2) a compensation stage where visual
cantly greater for the jitter-annulus condition. Since the
jitter is represented explicitly. The present study tests
test stimulus was identical (thus cancelling out in the
for the location of each of these two stages (adaptation
subtractive analysis), these activation patterns during
and compensation) by using functional magnetic reso-
test should be due to the effects of adaptation.
nance imaging (fMRI). In addition to testing whether or
Does this result reflect the neural correlate of visual
not the above model is correct, it is of interest to identify
jitter, or is it a result of neural adaptation uncorrelated
the areas in the visual system that exhibit the effects of
with perception? A critical test is to compare the activity
adaptation and jitter. Specifically, we mapped visual
in the test period of the jitter-disk condition with that of
cortical activity while subjects were looking at stimuli
the control-static condition. In the jitter-disk condition,
that generate the visual jitter illusion (e.g., adaptation to
the annulus was adapted to dynamic noise and the disk
dynamic noise and subsequent test in static noise).
appeared to jitter. In the control-static condition, neither
There were four presentation conditions, as depicted
the disk nor the annulus was adapted, and neither ap-
in Figure 1. As a result, two distinct activity patterns
peared to jitter. If the differential activity reflects jitter
emerged at different stages of visual cortical hierarchy:
perception, it should be confined within the disk repre-
an MR signal decrease after adaptation to dynamic
sentation. If, on the other hand, the activity reflects neu-
noise was observed in lower areas, whereas higher ar-
ral adaptation, it should be confined within the annulus
eas showed an increase when the observer perceived
jitter. In conjunction with previous psychophysical find-
We found that the latter was actually the case: the
ings (Murakami and Cavanagh, 1998), these findings
annulus representation (which had been exposed to dy-
lead to tentative brain localization of the two psycho-
namic random noise in the adaptation period of the jitter-
disk condition) gave rise to a significant signal decrease
fMRI during Illusory Visual Jitter
1149
Figure 2. The Visual Cortex in the Right Hemisphere of One Representative Subject Shown in Flattened Format with Borders between Visual
Areas
(A) The representation of retinotopic eccentricity, as revealed by phase-encoded mapping. As shown in the legend, red indicates the foveal
representation, and progressively more peripheral eccentricities are coded blue, and then green. The retinotopic border between disk and
annulus representations in eccentricity are shown as dashed yellow line. The calcarine sulcus is indicated by the letters CS. Asterisks indicate
the foveal representations, which are located near the occipital pole.
(B) Differential BOLD activity between conditions jitter-disk and jitter-annulus for the first 10 s of the test period. Blue-cyan regions indicate
lower BOLD activity (p ⬍ 0.01) in jitter-disk than in jitter-annulus conditions, and red-yellow regions indicate higher activity (p ⬍ 0.01), indicated
by the pseudo color scale.
compared to that of the control-static condition (com-
nal change from their grand mean value. Then they were
pare [1] and [3] of Figure 3B; see below for details). This
averaged across hemispheres and subjects.
signal decrease was observed in V1 and other retino-
topic cortical areas. In contrast, there was no compara-
Activity Reduction after Adaptation
ble change in activation in the disk representation in
to Dynamic Random Noise
early visual areas. Thus, the decrease in activity ob-
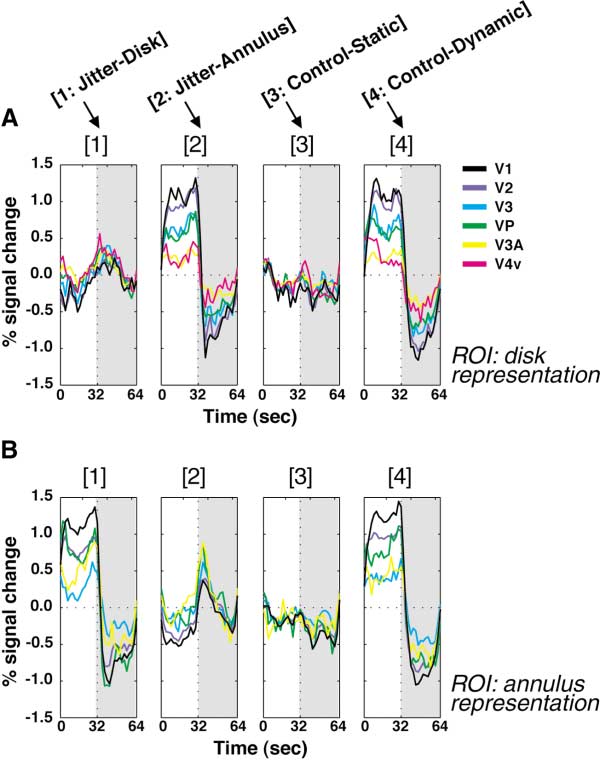
Figures 3A and 3B show the time courses from the reti-
served in lower visual areas seems to result from neural
notopic analysis. The signal changes are plotted as a
adaptation at the regions that were exposed to dynamic
function of time (with 0 and 32 being the beginning of
the adaptation and test periods, respectively). The data
In MT⫹, however, a conspicuous increase in BOLD
from several cortical areas (indicated by colors) are over-
signals was seen in conditions jitter-disk and jitter-
laid. The signal changes in the ROI of the disk represen-
annulus, compared to control-static. Simple neural ad-
tation are plotted in Figure 3A. Figure 3B shows the
aptation cannot explain this increase. Below we con-
analogous results from the annulus representation. The
ducted more detailed analyses to clarify the sources of
data for the four conditions (see Figure 1) are plotted in
this and other activation patterns.
In the control-static condition, no discernible MR
Region of Interest
change was observed in either the adaptation or test
In order to reveal the time course of BOLD activity, we
period (Figures 3A and 3B, see [3: control-static]). This
defined the region of interest (ROI) in two ways. The first
is presumably because the visual stimulus was identical
approach was based on retinotopic representations.
throughout the trial. Thus below, all the results in other
The representations of the disk and annulus were imme-
conditions will be described relative to this stable base-
diately obvious as iso-eccentric semicircular shapes,
and were clearly segregated in V1, V2, V3, VP, V3A, and
What happens after prolonged exposure to dynamic
V4v (see Figure 2). Their border could be confirmed
random noise? In the control-dynamic condition, dy-
based on the retinotopic eccentricity map from the same
namic noise presented in both the disk and annulus
subject (cf. Figures 2A and 2B). To analyze the data on
regions changed abruptly to static noise after adapta-
this disk versus annulus basis, we defined the group of
tion without producing a jitter aftereffect in either region
voxels that reached a significant difference between
(Figure 1). As clearly seen, the dynamic noise per se
conditions as the ROI for each of the disk and annulus
produced an MR signal increase (i.e., greater than the
representations and each visual area. In less retinotopic
baseline activity in [3]) during the adaptation period (Fig-
visual areas such as MT⫹, we defined the ROI over the
ures 3A and 3B, see [4: control-dynamic]). Then, how-
entire functionally defined (moving versus stationary)
ever, strong negative activity (i.e., less than the baseline)
visual area.
was observed in both the disk and annulus representa-
The time course data from these ROIs were averaged
tions during the subsequent test period. This tendency
for each hemisphere and normalized as the percent sig-
was most pronounced in V1. Similar responses were
3A), and a relatively steep MR increase was seen in the
annulus representation (Figure 3B). In both conditions,
the MR signals decreased in the representation of the
adapted region after exposure to dynamic random
noise, whereas the unadapted region (where jitter was
perceived) showed an increase of MR signals. We inter-
pret these MR decreases as reflecting the effect of the
dynamic random noise adaptation stimulus. The MR
increases are consistent with the occurrence of jitter in
these regions.
In the retinotopic analysis, both the MR increases
during adaptation and the decreases during the test
period were most prominent in V1 and progressively less
pronounced as the processing stage increased from V1
to V3 or V4v ([2] and [4] of Figure 3A and [1] and [4] of
Figure 3B). In comparison, the positive activity in the
test period was more pronounced as the processing
stage increased ([1] of Figure 3A and [2] of Figure 3B).
Thus, an effect of adaptation was more evident in lower
cortical areas, whereas jitter-consistent activity ap-
peared to increase in higher order motion-selective cor-
tical areas.
Subsequent tests confirmed that the MR signal reduc-
tion during test was larger in V1 than in any other visual
area. MR signals were averaged from adapted regions
(namely, the disk and annulus regions in the control-
dynamic condition, the disk region in the jitter-annulus
condition, and the annulus region in the jitter-disk condi-
Figure 3. Time Course Results of the Retinotopic Analysis
tion) and MR signal intensity was summed during the
MR signal changes are plotted in separate panels for the four condi-
first 10 s of the test period in each subject. A nonpara-
tions. (A) shows the latency-corrected time course of the signal
metric test (p ⬍ 0.01, Friedman test) confirmed that the
change in each visual area (black, V1; purple, V2; cyan, V3; green,
VP; yellow, V3A; magenta, V4v) in the disk representation during
MR signal reduction was largest in V1 in each subject.
adaptation (0–32 s) and subsequent test (32–64 s; indicated by gray).
(B) shows the same analysis for the annulus representation. No
Increased Signals in MT⫹
subject showed activation in the annulus representation in V4v. The
Thus far we have excluded MT⫹ from the analysis be-
ordinate indicates the signal change relative to the average activity
cause the retinotopic analysis is not easily applicable
level across all the conditions; thus its zero level had no functional
to this cortical area (Tootell et al., 1998d). Now we will
meaning. Instead, the virtually flat profiles in the control-static condi-
tion (3) were considered to reflect the baseline activity relative to
compare the nonretinotopic activities of visual areas
which the signal changes were assessed in other conditions.
including MT⫹ by summing up the voxels within each
defined visual area. Figure 4 shows the time course of
the MR signal change in each condition obtained by this
obtained in higher visual areas, albeit with progressively
nonretinotopic analysis. Thus except for area MT⫹, the
data shown here are essentially an average of Figures
Because the test stimulus was identical for all condi-
3A and 3B.
tions, these MR decreases presumably reflect the effect
In both of the control conditions (where no jitter per-
of adaptation to dynamic random noise. Note that the
ception occurred), the MR signal did not increase during
control-dynamic condition never yielded a visual jitter
the test period (Figure 4, [3] and [4]). For example, MR
aftereffect (see Figure 1) after adaptation to dynamic
signals in the control-static condition showed a flat time
noise. Thus, subsequently observed static noise was as
course. Likewise, in the control-dynamic condition, MR
stable as in the adaptation-free visual world. However,
signals in retinotopic visual areas showed the expected
the brain activity during the control-dynamic condition
decrease in the test period following the increase in the
was not the same as during the control-static condition.
adaptation period: similar responses were previously
In the control-dynamic condition, there was a negative
observed in the retinotopic analysis. The behavior of
aftereffect in the MR signals.
MT⫹ was qualitatively similar to other areas in these
What happens in the brain during the test period (in
conditions jitter-disk and jitter-annulus) after dynamic
However, in both conditions jitter-disk and jitter-
random noise was switched to static noise, when jitter
annulus (where jitter perception occurred in the disk and
was actually perceived? In the jitter-disk condition, MR
annulus regions, respectively), the MR signals in MT⫹
decreases were observed in the annulus representation
increased abruptly at the beginning of the test period
during the test period (Figure 3B), whereas the MR signal
(Figure 4, [1] and [2]). V3A (which is also motion selective
increased modestly in the disk representation (Figure
[Tootell et al., 1997]), and V4v showed modest MR signal
3A) during the same period. Similarly, in the jitter-
increases as well. Other cortical areas showed only mi-
annulus condition, negative MR activity was observed
nor positive-negative profiles, as expected from the data
in the disk representation during the test period (Figure
shown in Figure 3.
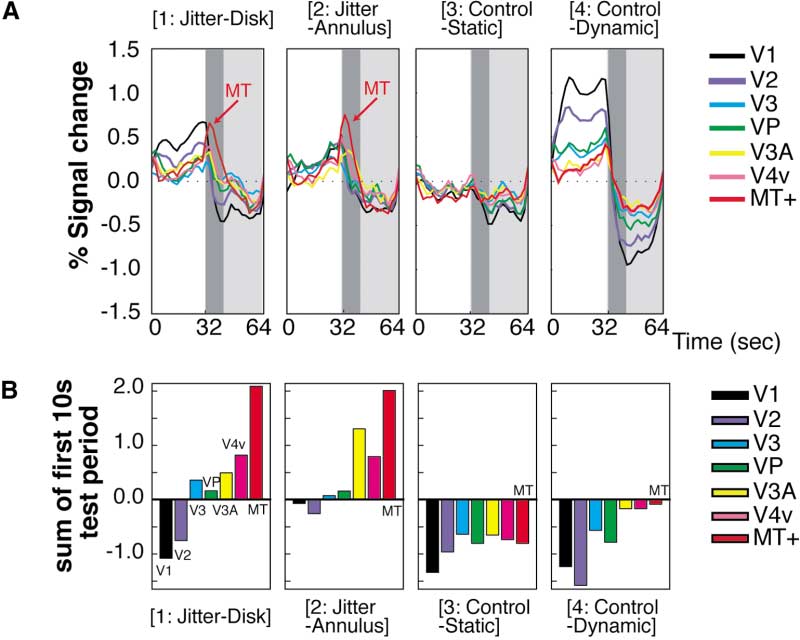
fMRI during Illusory Visual Jitter
1151
Figure 4. Time Course Results of the Nonre-
tinotopic Analysis
(A) The latency-corrected time course of sig-
nal change in each visual area. MR signal
changes in MT⫹ are colored red; otherwise
the conventions are identical to those in Fig-
ure 3.
(B) MR signal integration over the first 10 s
of the test period (dark gray in [A]).
Figure 4 shows that MT⫹ exhibited a prominent MR
(64 s in total). Again, we found that the MR signal in
signal increase when visual jitter occurred. The MR sig-
both the left and right MT⫹ showed increased MR sig-
nal increased very rapidly at the transition between the
nals during the test period when jitter perception oc-
adaptation and test periods and rapidly decayed within
curred in either the left or the right half of the visual
roughly one-third of the test period. This time course is
consistent with our perception of jitter: it appears most
strongly at the beginning of the test period and typically
lasts for 10–15 s (Murakami and Cavanagh, 1998, 2001).
Following visual stimulation, the BOLD signals in area
Furthermore, a statistical test showed that the MR
V1 show a transient decrease across many experiments
increase associated with jitter perception in the test
and many stimuli. This so-called poststimulus under-
period was larger in MT⫹ than in any other visual area.
shoot (e.g., Kwong et al., 1992) is thought due to a
MR signals were averaged in conditions jitter-disk and
temporal mismatch between the cessation of CBF (cere-
jitter-annulus, and signal intensity was summed during
bral blood flow) increase, coupled with a slower recovery
the first 10 s of the test period in each subject. The
of CBV (cerebral blood volume) equilibrium associated
signal increase in MT⫹ was significantly larger than in
with brain activity (Buxton et al., 1998; Hoge et al., 1999;
other visual areas (Friedman test, p ⬍ 0.01). Figure 4B
Kruger et al., 1999; Mandeville et al., 1999a, 1999b,
shows this result more intuitively. The MR signal inten-
1998). Here, we conducted a control experiment to see
sity was summed over the first 10 s of the test period
if the MR signal decrease after adaptation to dynamic
for each visual area and plotted as a bar chart. The value
random noise (see Figure 3) was simply another example
of MT⫹ was greatest of all areas in conditions jitter-disk
of this poststimulus undershoot or whether an additional
(Figure 4B, [1]) and jitter-annulus (Figure 4B, [2])—the
effect (as postulated here) was included.
two conditions in which illusory jitter was perceived.
The trial started with a blank period for 32 s. During
To approximate our retinotopic analysis (e.g., Figure
such blank periods, the stimulus was a spatially uniform
2) in the less-retinotopic area MT⫹, we compared the
gray, of luminance equal to the mean luminance of the
levels of MT⫹ activation when jitter was confined within
random noise; the central fixation point was always pre-
a single hemifield. Dynamic random noise was pre-
sented. In the subsequent 32 s period, the standard
sented only in the left (or right) half of the visual field
adapting stimulus was presented (as in the main experi-
in the 32 s adaptation period, while static noise was
ment, e.g., Figure 1, [1] and [2]). In the following 64 s,
presented in the opposite half of the visual field. In the
subjects were presented with either of two conditions.
subsequent 32 s test period, static noise was presented
In one condition (the jitter condition) we presented the
in both hemifields. In this configuration, jitter perception
same standard test stimulus used in the main experi-
occurred in the right (or left) half of the visual field (i.e.,
ment: static random noise in both the disk and annulus
in the unadapted region) when dynamic random noise
regions. Illusory jitter occurred in this stimulus. For the
ceased, as expected (Murakami and Cavanagh, 1998,
alternative undershoot condition, all visual noise was
2001). Note that this approach is comparable to condi-
removed; thus the screen was spatially uniform except
tions jitter-disk and jitter-annulus in the main (retino-
for the fixation point. Since the visual jitter illusion re-
topic) analysis. To define a baseline activity level, we
quires the presence of static noise, nothing appeared
also included the control-static condition, in which static
to move in this uniform screen. In this condition, the MR
noise was presented in both hemifields in both periods
signal undershoot was expected to occur without the
were almost equivalent in the adaptation period
(32–62 s).
Most importantly, the MR signal differed during the
test period. This difference was most significant during
the first half of the test period (64–94 s) when the jitter
percept occurred. A nonparametric paired-comparison
test revealed a statistically significant difference (p ⬍
0.0001, two-tailed sign test) in this time period (64–94
s), as indicated by the shaded region (Figure 5C). Statis-
tically significant differences were not found in any of
the remaining time periods (e.g., 0–30 s, 32–62 s, etc.).
In Figure 5D, these third-quarter data are magnified.
The difference reached near-significance even during
the first 10 s (64–72 s) where most of the jitter perception
occurs (p ⫽ 0.0625, two-tailed sign test), and the signifi-
cance increased monotonically with longer sample
times (for example, p ⬍ 0.05 for the first 12 s, p ⬍ 0.002
for the remaining 76–94s period). Thus, the MR signal
reduction in the jitter condition was larger in amplitude,
Figure 5. Results of Undershoot Control
of longer duration, and was less erratic, compared to
(A and B) The time courses of MR signal decreases in V1 were
the undershoot condition. If the MR reduction in V1 (elic-
averaged and plotted separately for the jitter (A) and undershoot
ited by changing dynamic noise to static noise) was due
(B) conditions. The signal change was defined relative to the baseline
activity during the initial blank period. The green curve represents
to just the BOLD undershoot, the residual curve would
the averaged time course from the brain regions that were adapted
be the same as in the undershoot condition. Therefore,
to dynamic random noise, and the orange curve represents the
even if there is a contribution from the undershoot effect
averaged time course from the brain regions that were exposed to
to the MR signal reduction in V1, there is also a signifi-
static random noise. The thick curves represent the residual brain
cant further decrease due to the adaptation.
activity between those two profiles.
(C) The overlay of the residual signals in the jitter and undershoot
conditions is shown. The curves are colored consistently across
panels. In the gray-coded interval, the MR signal reduction in the
jitter condition remained low longer than the undershoot.
Our fMRI experiments demonstrated that the BOLD ac-
(D) Differences in the residual values in the third quarter are empha-
tivity of early cortical areas (e.g., V1) decreased after
sized, with the time axis enlarged 400%; the ordinate is also enlarged
adapted to dynamic random noise. Control tests con-
firmed that this decrease in V1 was distinguishable from
perception of illusory jitter. Our interest here was to see
the commonly described poststimulus undershoot,
whether the signal decrease we had observed in the
which is thought due to vascular phenomena. In con-
jitter condition was different from the signal decrease
trast, in motion-selective/higher-tier cortical areas such
in the undershoot condition.
as MT⫹ (and to a lesser extent V3A, and V4v), BOLD
Figure 5 compares the time courses in the jitter condi-
activity increased when illusory jitter perception oc-
tion (Figure 5A) and the undershoot condition (Figure
5B) in V1. In Figures 5A and 5B, the green and orange
These results suggest a locus of adaptation in area
curves represent the time courses from the brain regions
V1 followed by a compensation mechanism for retinal
that were exposed to dynamic noise and static noise,
slip located in the V1→MT⫹ pathway. This is consistent
respectively, during the adaptation period. The thick
with the proposed model for visual jitter (Murakami and
curve in each panel shows the difference (denoted as
Cavanagh, 1998). This model proposed that a retinal-
residual) between the green and orange. Thus, the blue
slip compensation mechanism explained the visual jitter
curve in (A) corresponds to the effect of removal of
illusion and also how retinal slip caused by small eye
flicker from the noise pattern, and the negative values
movements normally remains invisible. A unique and
during 64–96 s are considered to comprise the adapta-
important point of this model is that it postulates two
tional jitter effect. In (B), the green and orange curves
stages for this retinal slip correction mechanism: an
showed the typical temporal profile of the BOLD under-
adaptable motion measurement stage and a compensa-
shoot, and the red curve in (B) corresponds to the resid-
tion stage (see Introduction). Consistent with this predic-
ual difference between both undershoots. If the adapta-
tion, the MR signal decreased in early visual areas, such
tional jitter effect was just another form of undershoot
as V1, where dynamic random noise had been presented
(with the background of static noise instead of the blank
retinotopically (see Figures 2 and 3). This signal de-
background) the residual signals in (A) and (B) would be
crease may be interpreted to correspond to the adapt-
equal because the background effect had been sub-
able motion measurement stage. In contrast, the MR
tracted out. Figure 5C compares the residual curves
signal was found to increase mainly in MT⫹ (and to some
from (A) (blue) and (B) (red). The residuals during the
extent in V3A and V4v) when illusory jitter perception
initial uniform period (0–30 s) and the last 32 s (96–126 s)
occurred, and this may correspond to the compensation
were almost identical between the jitter and undershoot
conditions, and they were nearly zero. Moreover, they
Previous psychophysical experiments have revealed
fMRI during Illusory Visual Jitter
1153
that this jitter adaptation is monocular and selective for
directionally selective cells as well, eventually desensi-
direction and spatial frequency (Murakami and Cava-
tizing them. As a result, these cells should become less
nagh, 1998, 2001). This suggests the involvement of
sensitive to incessant retinal slip during the test period.
early cortical areas such as V1. Consistent with this
Such a transient loss of sensitivity should lead to de-
aspect of the model, we observed that the effect of
creased BOLD signals.
adaptation on BOLD signals was most pronounced in
In contrast, according to the model (Murakami and
V1 for our human subjects. The effect decreased pro-
Cavanagh, 2001), MT⫹ and some other extrastriate ar-
gressively at higher-tier cortical levels.
eas monitor the activity map of earlier stages such as
On the other hand, two psychophysical facts suggest
V1 in order to calibrate the velocity field with respect to
that the second (compensation) stage should occur at
the minimum baseline. A biologically plausible way to
higher cortical levels. First, the jitter percept transfers
do this would be to use center-surround antagonism
between the left and right hemifields, even if the adapted
with respect to motion, and in fact, such cells have been
and unadapted regions are separated up to 5⬚–6⬚ (Mura-
reported in areas MT and MST (Allman et al., 1985; Born
kami and Cavanagh, 2001). Second, jitter is greatest
et al., 2000; Born and Tootell, 1992; Tanaka et al., 1986).
when the disk size matches the average receptive field
Such cells would fire when they detected objects mov-
size in the macaque MT at a given eccentricity (Mura-
ing faster than the background. Similarly, in jitter percep-
kami and Cavanagh, 2001). In agreement with these
tion, they would also fire when they detected a faster
psychophysical findings, we have found that the BOLD
motion velocity in a region (the unadapted region, in
signal in MT⫹ (which is bilaterally driven to some extent
this case) compared to the transiently lowered baseline
in humans [Tootell et al., 1998d, 1995b]) increased when
minimum in motion velocity in a region resulting from
jitter perception was present. The same pattern of MR
adaptation to dynamic random noise (i.e., the adapted
signal increase was obtained both when adapted and
region). Therefore, the positive BOLD signals in MT⫹
unadapted regions were located concentrically and also
observed in this study are both theoretically feasible
when they were separated across the left and right hemi-
and consistent with the electrophysiological literature.
fields. This is again consistent with size of the receptive
Our current fMRI technique could not segregate MT
field and bilaterality in primate MT/MST.
from MST, but previous psychophysical measurements
In fMRI studies, human MT⫹ is activated when a mo-
of the optimal stimulus size (Murakami and Cavanagh,
tion aftereffect is perceived in a physically stationary
2001) suggest that MT may be more responsible for jitter
stimulus (Culham et al., 1999; He et al., 1998; Huk et al.,
perception than MST.
2001; Tootell et al., 1995a). Putting this fact together
It has been asked why the V1 decrease is more slug-
with the present finding, there is thus some superficial
gish (Figure 5D), compared to the apparently faster on-
similarity in the MT⫹ responses to motion aftereffect
set in the MT increase (Figure 4). First, this comparison
and illusory visual jitter. In both cases, MT⫹ is active
is somewhat misleading because the V1 response is
when one sees illusory motion in stationary stimuli. Addi-
corrected for the undershoot, whereas the MT response
tional evidence suggests that the activity of MT⫹ can
is not. Secondly, differences as large as 0.25%–0.5%
be tightly related to one's perception of motion rather
occur throughout the 64–96 s period (note that the blue
than actual motion information (Kourtzi and Kanwisher,
curve is always lower than the red curve in Figure 5D),
2000; Zeki et al., 1993).
although those in the earlier half tend to be obscured
Recently, Huk et al. (2001) pointed out that fMRI acti-
by the steep decline of each curve. Third, it is certainly
vation in human MT⫹ correlated with the perception of
possible that the neural interaction between V1 and MT
motion aftereffect can be confounded with the effect of
would not produce a simple linear MR inversion. Com-
attention. While attention could surely contribute to the
pared to the adapted population, many potential factors
present MR signal changes (e.g., in MT⫹), the following
could reshape the MR signal in MT or V1, such as (1)
facts suggest that our effect is not entirely (nor even
threshold nonlinearities, (2) differences in the time
predominantly) due to attention. First, the BOLD signal
course of gain control, and (3) fewer cells being involved
showed both increases and decreases in different visual
in jitter perception. MR signals during visual illusion can
areas (e.g., MT⫹ and V1) simultaneously. Second, even
have either a sharp onset (Tootell et al., 1995a) or a
within a visual area such as V1, the BOLD signal showed
much slower onset (Hadjikhani et al., 1998)—even with
both an increase and decrease at the same time in reti-
equally abrupt onsets in percept. Hence, the minor dif-
notopic subdivisions consistent with the percept (e.g.,
ference in the shape of the time courses between V1 and
Figure 2). Finally, in current experiments we have found
MT remains compatible with our overall interpretation.
the effect of attention modulates, but does not fully
The BOLD signal increase associated with jitter per-
account, for conventional fMRI-based motion afteref-
ception also occurred to a lesser extent in visual areas
fects (Sasaki, Y., Murakami, I., Watanabe, T., Tootell,
such as V3A and V4v. Human V3A is known to be motion
R.B.H., and Nishida, N. (2002). Neuroimaging of direc-
sensitive (Tootell et al., 1997) and thus may be directly
tion-selective mechanisms for first-order and second-
involved in the perception of visual jitter. The degree
order motion stimuli. Paper presented at: Vis. Sci. Soc.
of processing independence between cortical areas is
Annual Meeting (Sarasota, FL).
unclear, but feedback signals may exist from human
After adaptation, why does V1 activity decrease and
MT⫹ to V3A (Hupe et al., 1998). In the macaque, connec-
MT⫹ activity increase while we see jitter? Dynamic ran-
tions between MT and V3A have been reported, as well
dom noise has a flat spatiotemporal-frequency power
as between MT and V4 (Maunsell and Van Essen, 1983).
spectra, containing a lot of motion energy in all direc-
V4v is one of the subdivisions of V4 (Boussaoud et al.,
tions at all speeds. This stimulus should strongly drive
1991; Felleman and Van Essen, 1991; Gattass et al.,
subcortical cells such as magnocellular LGN and V1
1988). Currently, how these cortical areas mutually inter-
stimuli were presented. In the jitter condition, the same static noise
act remains unsolved in humans (Tootell and Hadjikhani,
used in the main experiment was presented; in the alternative under-
shoot condition, a blank (spatially uniform gray) screen with the
One of the fundamental tasks of visual processing
fixation point was presented instead of static random noise during
from V1 to MT⫹ is considered to include visual motion
this period.
processing. However, the present study suggests that
For each condition, the time courses from the brain regions that
these areas are also involved in stabilizing the visual
were adapted to dynamic random noise were averaged across ROIs,
hemispheres, and subjects. These data are plotted as green curves
world, processing visual motion inputs even during fixa-
in Figures 5A and 5B (denoted as dynamic); these included the
tion of static scenes. V1, as the adaptation stage, regis-
annulus region in the jitter-disk condition and the disk region in the
ters retinal slip (although with lower gain after adapta-
jitter-annulus condition. Similarly, the time courses from the brain
tion), providing inputs for subsequent compensation.
regions that were exposed to static random noise were averaged
MT⫹, as the compensation stage, uses these inputs
and plotted by orange curves (denoted as static).
and subtracts the minimum baseline motion from all
other motions to counteract retinal slip. If these stages
General Imaging Procedures
malfunction, then a static visual scene should not be
Experimental details were similar to those described elsewhere
perceived as static. That may be why some dyslexia
(Hadjikhani et al., 1998; Mendola et al., 1999; Somers et al., 1999;
patients who have reading difficulties and often com-
Tootell et al., 1997). Scans were acquired using either a 3T General
Electric MR scanner retrofitted with ANMR echo-planar imaging or
plain that letters appear blurred and jittering (Stein and
(in later experiments) a 3T Siemens Allegra. A custom-built, quadra-
Walsh, 1997) reportedly show dysfunction in MT⫹
ture-based, semicylindrical surface coil was used to acquire high-
(Demb et al., 1997, 1998; Eden et al., 1996). In this re-
sensitivity MR images including occipital, parietal, and posterior
spect, the underlying mechanisms of dyslexia patients'
temporal lobes bilaterally. Voxels were 3.1 mm2 in-plane and 3 mm
reading deficits and our illusory visual jitter may be simi-
thick. Functional MR images were acquired using gradient echo
lar to each other (G. Hebb, personal communication).
sequences (TE ⫽ 30 ms) with 128 images in 16 contiguous slices,
oriented approximately orthogonal to the calcarine sulcus. TR was
This issue is currently open to investigation.
2 s for the main fMRI experiments, and each fMRI scan took 256 s.
Subjects were run for 8–15 scans for the purpose of signal averaging.
A total of 75 functional scans (153,600 images) were obtained.
The statistical maps were generated using linear regression analy-
sis. The fMRI signal was modeled as a linear convolution of a hemo-
Eight healthy subjects with normal or corrected-to-normal visual
dynamic impulse function (Dale and Buckner, 1997). The activation
acuity passively viewed visual stimuli in the magnetic resonance
amplitude for each condition was estimated from the fMRI time
(MR) scanner. All subjects gave informed written consent. This study
course on a voxel-by-voxel basis. T-tests were performed to com-
was approved by Massachusetts General Hospital Human Studies
pare activation amplitudes between conditions. Their p values were
Protocol 96-7464 and 2000p-001155.
projected onto the flattened activity maps.
Stimuli
Dynamic random noise was used as the adapting stimulus. 50% of
the dots (each dot: 0.3⬚ ⫻ 0.3⬚) were black (0.4 cd/m2 ), and the
In a separate session, retinotopic visual areas and borders were
remaining ones were white (62.7 cd/m2 ); the background was gray
mapped using phase-encoded stimuli and field sign analysis (Dale
(31.3 cd/m2 ). The pattern was updated to a totally new pattern on
and Buckner, 1997; Hadjikhani et al., 1998; Sereno et al., 1995;
each frame at 60 Hz. There were two concentric regions centered
Somers et al., 1999; Tootell et al., 1998a, 1997, 1998b, 1998c, 1998d).
around the fixation point: a central disk (12⬚ in diameter) and a
This analysis identified visual areas V1, V2, V3/VP, V3A, V4v, V7,
surrounding annulus (30⬚ in diameter) except for the interhemi-
and V8 (DeYoe et al., 1996, 1994; Engel et al., 1997; Schneider et
spheric tests, as described in the text.
al., 1993; Sereno et al., 1995; Tootell et al., 1998a, 1997, 1998b,
1998c, 1998d). Low-contrast, moving and stationary concentric
rings were also presented to localize area MT⫹ (Beauchamp et al.,
A trial consisted of an adaptation period (32 s) and a subsequent
1997; Dupont et al., 1994; Lueck et al., 1989; McCarthy et al., 1995;
test period (32 s) (Figure 1). In the adaptation period, each of the
Tootell et al., 1995a, 1995b; Watson et al., 1993; Zeki et al., 1991).
disk and annulus regions was filled with either dynamic random
Regions of interests (ROIs) were defined based on these functional
noise or static random noise. Hence, there were four varieties of
borders. The TR was 4 s for retinotopic scans (8 min, 32 s duration)
adapting stimuli. In the jitter-disk condition, the annulus was dy-
and 2 s for the MT⫹ localization (4 min, 16 s duration).
namic and the disk was static. In the jitter-annulus condition, the
annulus was static and the disk was dynamic. In the control-static
Flattening the Visual Cortex
condition, both annulus and disk regions were static. In the control-
In a separate session, structural images of the whole brain were
dynamic condition, both were dynamic. In the subsequent test pe-
obtained with high resolution (1.0 ⫻ 1.0 ⫻ 1.3 mm3, 1.5T) to provide
riod, the visual stimuli were always equivalent, namely, both disk
data for three-dimensional reconstruction (Dale et al., 1999; Fischl
and annulus regions were filled with static random noise. In the test
et al., 1999), which allowed us to generate an unfolded and flattened
period, jitter was perceived in the disk and annulus in conditions
cortical surface for each subject (FreeSurfer, http://www.nmr.
jitter-disk and jitter-annulus, respectively. In conditions control-
static and control-dynamic, no illusory motion was perceived.
In each MRI scan (256 s), these four conditions (64 s each) were
repeated in counterbalanced order across scans.
This study was supported by a grant from the National Eye Institute
We conducted another experiment to test the MR signal decrease
(EY07980) to R.B.H.T. Y.S. was supported by a fellowship from the
observed after adaptation to dynamic noise, compared with an MR
Japan Society for the Promotion of Science. We thank Nouchine
signal decrease expected from BOLD undershoot (Figure 5). We
Hadjikhani for her technical assistance; Ken Kwong for his com-
presented a blank screen with the fixation point (32 s) and then
ments; Terrance Campbell, Larry Wald, Mary Foley, Larry White,
presented the same adapting stimulus (32 s) used in conditions
and Bruce Rosen for MRI support; Tommy Vaughan for building
jitter-disk (see Figure 1, [1]) or jitter-annulus (see Figure 1, [2]) in the
the customized coil; and the Rowland Institute for machining MR-
main experiment. In the following test period (64 s), two possible
fMRI during Illusory Visual Jitter
1155
Received: August 10, 2001
rons in primary visual cortex (V1) move in space with the eye move-
Revised: June 17, 2002
ments of fixation. Vision Res. 37, 257–265.
Gur, M., Beylin, A., and Snodderly, D.M. (1997). Response variability
of neurons in primary visual cortex (V1) of alert monkeys. J. Neurosci.
17, 2914–2920.
Allman, J., Miezin, F., and McGuinness, E. (1985). Direction- and
Hadjikhani, N., Liu, A.K., Dale, A.M., Cavanagh, P., and Tootell, R.B.
velocity-specific responses from beyond the classical receptive field
(1998). Retinotopy and color sensitivity in human visual cortical area
in the middle temporal visual area (MT). Perception 14, 105–126.
V8. Nat. Neurosci. 1, 235–241.
Beauchamp, M.S., Cox, R.W., and DeYoe, E.A. (1997). Graded ef-
He, S., Cohen, E.R., and Hu, X. (1998). Close correlation between
fects of spatial and featural attention on human area MT and associ-
activity in brain area MT/V5 and the perception of a visual motion
ated motion processing areas. J. Neurophysiol. 78, 516–520.
aftereffect. Curr. Biol. 8, 1215–1218.
Born, R.T., and Tootell, R.B. (1992). Segregation of global and local
Hoge, R.D., Atkinson, J., Gill, B., Crelier, G.R., Marrett, S., and Pike,
motion processing in primate middle temporal visual area. Nature
G.B. (1999). Stimulus-dependent BOLD and perfusion dynamics in
human V1. Neuroimage 9, 573–585.
Born, R.T., Groh, J.M., Zhao, R., and Lukasewycz, S.J. (2000). Segre-
Huk, A.C., Ress, D., and Heeger, D.J. (2001). Neuronal basis of the
gation of object and background motion in visual area MT: effects
motion aftereffect reconsidered. Neuron 32, 161–172.
of microstimulation on eye movements. Neuron 26, 725–734.
Hupe, J.M., James, A.C., Payne, B.R., Lomber, S.G., Girard, P.,
Boussaoud, D., Desimone, R., and Ungerleider, L.G. (1991). Visual
and Bullier, J. (1998). Cortical feedback improves discrimination
topography of area TEO in the macaque. J. Comp. Neurol. 306,
between figure and background by V1, V2 and V3 neurons. Nature
Buxton, R.B., Wong, E.C., and Frank, L.R. (1998). Dynamics of blood
Ibbotson, M.R., and Maddess, T. (1994). The effects of adaptation
flow and oxygenation changes during brain activation: the balloon
to visual stimuli on the velocity of subsequent ocular following re-
model. Magn. Reson. Med. 39, 855–864.
sponses. Exp. Brain Res. 99, 148–154.
Culham, J.C., Dukelow, S.P., Vilis, T., Hassard, F.A., Gati, J.S.,
Ilg, U.J., and Thier, P. (1996). Inability of rhesus monkey area V1 to
Menon, R.S., and Goodale, M.A. (1999). Recovery of fMRI activation
discriminate between self-induced and externally induced retinal
in motion area MT following storage of the motion aftereffect. J.
image slip. Eur. J. Neurosci. 8, 1156–1166.
Kourtzi, Z., and Kanwisher, N. (2000). Activation in human MT/MST
Dale, A., and Buckner, R. (1997). Selective averaging of rapidly pre-
by static images with implied motion. J. Cogn. Neurosci. 12, 48–55.
sented individual trials using fMRI. Hum. Brain Mapp. 5, 326–340.
Kruger, G., Kastrup, A., Takahashi, A., and Glover, G.H. (1999). Simul-
Dale, A.M., Fischl, B., and Sereno, M.I. (1999). Cortical surface-
taneous monitoring of dynamic changes in cerebral blood flow and
based analysis. I. Segmentation and surface reconstruction. Neuro-
oxygenation during sustained activation of the human visual cortex.
image 9, 179–194.
Demb, J.B., Boynton, G.M., and Heeger, D.J. (1997). Brain activity in
Kwong, K.K., Belliveau, J.W., Chesler, D.A., Goldberg, I.E., Weiss-
visual cortex predicts individual differences in reading performance.
koff, R.M., Poncelet, B.P., Kennedy, D.N., Hoppel, B.E., Cohen, M.S.,
Proc. Natl. Acad. Sci. USA 94, 13363–13366.
Turner, R., et al. (1992). Dynamic magnetic resonance imaging of
Demb, J.B., Boynton, G.M., and Heeger, D.J. (1998). Functional mag-
human brain activity during primary sensory stimulation. Proc. Natl.
netic resonance imaging of early visual pathways in dyslexia. J.
Acad. Sci. USA 89, 5675–5679.
Leopold, D.A., and Logothetis, N.K. (1998). Microsaccades differen-
DeYoe, E., Carman, G., Bandettini, P., Glickman, S., Wieser, J., Cox,
tially modulate neural activity in the striate and extrastriate visual
R., Miller, D., and Neitz, J. (1996). Mapping striate and extrastriate
cortex. Exp. Brain Res. 123, 341–345.
visual areas in human cerebral cortex. Proc. Natl. Acad. Sci. USA
Lueck, C.J., Zeki, S., Friston, K.J., Deiber, M.P., Cope, P., Cunning-
ham, V.J., Lammertsma, A.A., Kennard, C., and Frackowiak, R.S.
DeYoe, E.A., Bandettini, P., Neitz, J., Miller, D., and Winans, P. (1994).
(1989). The colour centre in the cerebral cortex of man. Nature 340,
Functional magnetic resonance imaging (FMRI) of the human brain.
J. Neurosci. Methods 54, 171–187.
Mandeville, J.B., Marota, J.J., Kosofsky, B.E., Keltner, J.R., Weiss-
Dupont, P., Orban, G.A., De Bruyn, B., Verbruggen, A., and Mortel-
leder, R., Rosen, B.R., and Weisskoff, R.M. (1998). Dynamic func-
mans, L. (1994). Many areas in the human brain respond to visual
tional imaging of relative cerebral blood volume during rat forepaw
motion. J. Neurophysiol. 72, 1420–1424.
stimulation. Magn. Reson. Med. 39, 615–624.
Eden, G.F., VanMeter, J.W., Rumsey, J.M., Maisog, J.M., Woods,
Mandeville, J.B., Marota, J.J., Ayata, C., Moskowitz, M.A., Weisskoff,
R.P., and Zeffiro, T.A. (1996). Abnormal processing of visual motion
R.M., and Rosen, B.R. (1999a). MRI measurement of the temporal
in dyslexia revealed by functional brain imaging. Nature 382, 66–69.
evolution of relative CMRO(2) during rat forepaw stimulation. Magn.
Engel, S.A., Glover, G.H., and Wandell, B.A. (1997). Retinotopic orga-
Reson. Med. 42, 944–951.
nization in human visual cortex and the spatial precision of functional
Mandeville, J.B., Marota, J.J., Ayata, C., Zaharchuk, G., Moskowitz,
MRI. Cereb. Cortex 7, 181–192.
M.A., Rosen, B.R., and Weisskoff, R.M. (1999b). Evidence of a cereb-
Felleman, D.J., and Van Essen, D.C. (1991). Distributed hierarchical
rovascular postarteriole windkessel with delayed compliance. J.
processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47.
Cereb. Blood Flow Metab. 19, 679–689.
Fischl, B., Sereno, M.I., and Dale, A.M. (1999). Cortical surface-
Maunsell, J.H., and van Essen, D.C. (1983). The connections of the
based analysis. II: Inflation, flattening, and a surface-based coordi-
middle temporal visual area (MT) and their relationship to a cortical
nate system. Neuroimage 9, 195–207.
hierarchy in the macaque monkey. J. Neurosci. 3, 2563–2586.
Galletti, C., Squatrito, S., Battaglini, P.P., and Grazia Maioli, M.
McCarthy, G., Spicer, M., Adrignolo, A., Luby, M., Gore, J., and
(1984). ‘Real-motion' cells in the primary visual cortex of macaque
Allison, T. (1995). Brain activation associated with visual motion
monkeys. Brain Res. 301, 95–110.
studied by functional magnetic resonance imaging in humans. Hum.
Gattass, R., Sousa, A.P., and Gross, C.G. (1988). Visuotopic organi-
Brain Mapp. 2, 234–243.
zation and extent of V3 and V4 of the macaque. J. Neurosci. 8,
Mendola, J.D., Dale, A.M., Fischl, B., Liu, A.K., and Tootell, R.B.
(1999). The representation of illusory and real contours in human
Gur, M., and Snodderly, D.M. (1987). Studying striate cortex neurons
cortical visual areas revealed by functional magnetic resonance
in behaving monkeys: benefits of image stabilization. Vision Res.
imaging. J. Neurosci. 19, 8560–8572.
Murakami, I. (1995). Motion aftereffect after monocular adaptation
Gur, M., and Snodderly, D.M. (1997). Visual receptive fields of neu-
to filled-in motion at the blind spot. Vision Res. 35, 1041–1045.
Murakami, I., and Cavanagh, P. (1998). A jitter after-effect reveals
motion-based stabilization of vision. Nature 395, 798–801.
Murakami, I., and Cavanagh, P. (2001). Visual jitter: evidence for
visual-motion-based compensation of retinal slip due to small eye
movements. Vision Res. 41, 173–186.
Schneider, W., Noll, D., and Cohen, J. (1993). Functional topographic
mapping of the cortical ribbon in human vision with conventional
MRI scanners. Nature 365, 150–153.
Sereno, M.I., Dale, A.M., Reppas, J.B., Kwong, K.K., Belliveau, J.W.,
Brady, T.J., Rosen, B.R., and Tootell, R.B. (1995). Borders of multiple
visual areas in humans revealed by functional magnetic resonance
imaging. Science 268, 889–893.
Somers, D.C., Dale, A.M., Seiffert, A.E., and Tootell, R.B. (1999).
Functional MRI reveals spatially specific attentional modulation in
human primary visual cortex. Proc. Natl. Acad. Sci. USA 96, 1663–
1668.
Stein, J., and Walsh, V. (1997). To see but not to read; the magnocel-
lular theory of dyslexia. Trends Neurosci. 20, 147–152.
Tanaka, K., Hikosaka, K., Saito, H., Yukie, M., Fukada, Y., and Iwai,
E. (1986). Analysis of local and wide-field movements in the superior
temporal visual areas of the macaque monkey. J. Neurosci. 6,
134–144.
Tootell, R.B., and Hadjikhani, N. (2001). Where is ‘dorsal v4' in human
visual cortex? retinotopic, topographic and functional evidence.
Cereb. Cortex 11, 298–311.
Tootell, R.B., Reppas, J.B., Dale, A.M., Look, R.B., Sereno, M.I.,
Malach, R., Brady, T.J., and Rosen, B.R. (1995a). Visual motion
aftereffect in human cortical area MT revealed by functional mag-
netic resonance imaging. Nature 375, 139–141.
Tootell, R.B., Reppas, J.B., Kwong, K.K., Malach, R., Born, R.T.,
Brady, T.J., Rosen, B.R., and Belliveau, J.W. (1995b). Functional
analysis of human MT and related visual cortical areas using mag-
netic resonance imaging. J. Neurosci. 15, 3215–3230.
Tootell, R.B., Mendola, J., Hadjikhani, N., Ledden, P., Liu, A., Rep-
pas, J., Sereno, M., and Dale, A. (1997). Functional analysis of V3A
and related areas in human visual cortex. J. Neurosci. 17, 7060–7078.
Tootell, R.B., Hadjikhani, N., Mendola, J., Marrett, S., and Dale, A.
(1998a). From retinotopy to recognition: fMRI in human visual cortex.
Trends Cogn. Sci. 2, 174–183.
Tootell, R.B., Hadjikhani, N., Hall, E.K., Marrett, S., Vanduffel, W.,
Vaughan, J.T., and Dale, A.M. (1998b). The retinotopy of visual spa-
tial attention. Neuron 21, 1409–1422.
Tootell, R.B., Hadjikhani, N.K., Vanduffel, W., Liu, A.K., Mendola,
J.D., Sereno, M.I., and Dale, A.M. (1998c). Functional analysis of
primary visual cortex (V1) in humans. Proc. Natl. Acad. Sci. USA 95,
811–817.
Tootell, R.B., Mendola, J.D., Hadjikhani, N.K., Liu, A.K., and Dale,
A.M. (1998d). The representation of the ipsilateral visual field in
human cerebral cortex. Proc. Natl. Acad. Sci. USA 95, 818–824.
Wade, N.J., Swanston, M.T., and de Weert, C.M. (1993). On interocu-
lar transfer of motion aftereffects. Perception 22, 1365–1380.
Watson, J.D., Myers, R., Frackowiak, R.S., Hajnal, J.V., Woods, R.P.,
Mazziotta, J.C., Shipp, S., and Zeki, S. (1993). Area V5 of the human
brain: evidence from a combined study using positron emission
tomography and magnetic resonance imaging. Cereb. Cortex 3,
79–94.
Wohlgemuth, A. (1911). On the after-effect of seen movement. British
Journal of Psychology Monograph Supplement 1, 1–117.
Zeki, S., Watson, J.D., Lueck, C.J., Friston, K.J., Kennard, C., and
Frackowiak, R.S. (1991). A direct demonstration of functional spe-
cialization in human visual cortex. J. Neurosci. 11, 641–649.
Zeki, S., Watson, J.D., and Frackowiak, R.S. (1993). Going beyond
the information given: the relation of illusory visual motion to brain
activity. Proc. R. Soc. Lond. B Biol. Sci. 252, 215–222.
Source: http://lpp.psycho.univ-paris5.fr/pdf/PapersPC/2002/Sasaki-35-2002-1147-56.pdf
Nature's Aide® Vitamins Nature's Aide® Vitamins Takes the Ache Out of Flu Season Every year it seems the common cold and the current year's flu bring a little more misery than the year before—racking coughs, drips, sniffles, sinuses that feel as if they've been hit with a blowtorch, aches in places most of us didn't know could ache—you've been there. We all have.
Spezial-Tees zum Genießen und Kurieren An g u rat é -Tee - Magentee aus Peru Traditionell angewendet unterstützt Anguraté wirksam die Magen-Darm-Funktion. Anguraté (lat. Mentzelis cordifolia) stammt aus Peru und wird dort in den peruanischen Anden in Höhenlagen 70 g 09100 von 1500-2300 m unter beschwerlichen Bedingungen aus Wild wuchs geerntet. Anguraté hat



