Masspec.scripps.edu
Organic Anion Transporter 3 Contributes to theRegulation of Blood Pressure
Volker Vallon,*†‡ Satish A. Eraly,* William R. Wikoff,§ Timo Rieg,*‡ Gregory Kaler,*David M. Truong,* Sun-Young Ahn,* Nitish R. Mahapatra,* Sushil K. Mahata,*‡Jon A. Gangoiti,储 Wei Wu,* Bruce A. Barshop,储 Gary Siuzdak,§ and Sanjay K. Nigam*储¶
Departments of *Medicine, †Pharmacology, 储Pediatrics, and ¶Cellular and Molecular Medicine, University ofCalifornia, San Diego, ‡Department of Medicine, San Diego VA Healthcare System, and §Department of MolecularBiology and the Center for Mass Spectrometry, Scripps Research Institute, La Jolla, California
ABSTRACTRenal organic anion transporters (OAT) are known to mediate the excretion of many drugs, but theirfunction in normal physiology is not well understood. In this study, mice lacking organic anion transporter3 (Oat3) had a 10 to 15% lower BP than wild-type mice, raising the possibility that Oat3 transports anendogenous regulator of BP. The aldosterone response to a low-salt diet was blunted in Oat3-null mice,but baseline aldosterone concentration was higher in these mice, suggesting that aldosterone dysregu-lation does not fully explain the lower BP in the basal state; therefore, both targeted and globalmetabolomic analyses of plasma and urine were performed, and several potential endogenous sub-strates of Oat3 were found to accumulate in the plasma of Oat3-null mice. One of these substrates,thymidine, was transported by Oat3 expressed
in vitro.
In vivo, thymidine, as well as two of the mostpotent Oat3 inhibitors that were characterized, reduced BP by 10 to 15%; therefore, Oat3 seems toregulate BP, and Oat3 inhibitors might be therapeutically useful antihypertensive agents. Moreover,polymorphisms in human OAT3 might contribute to the genetic variation in susceptibility tohypertension.
J Am Soc Nephrol 19: 1732–1740, 2008. doi: 10.1681/ASN.2008020180
The proximal tubule of the mammalian kidney ef-
Organic anion transporter 1 (Oat1),9,10 a gene
ficiently secretes a large variety of organic anions
originally identified as novel kidney transporter
(OA) leading to the rapid urinary excretion of those
(NKT) and proposed to function in organic ion
compounds. Basolateral uptake from plasma intothe tubular cell, the first step in this secretory pro-
Received February 13, 2008. Accepted April 11, 2008.
cess, operates
via anion exchange, coupling the en-
Published online ahead of print. Publication date available at
try of OA to the exit of dicarboxylates along their
concentration gradient. Subsequent apical efflux
V.V. and S.A.E. contributed equally to this work.
from tubular cell into urine does not seem to be ratelimiting and may occur
via efflux pumps and/or
N.R.M.'s current affiliation is Department of Biotechnology, IITMadras, Chennai, India.
anion exchange. Substrates for this secretory pro-cess include numerous important drugs (
e.g., most
Correspondence: Dr. Volker Vallon, Division of Nephrology andHypertension, Departments of Medicine and Pharmacology, Univer-
nonsteroidal anti-inflammatory drugs, -lactam
sity of California San Diego and VASDHCS, 3350 La Jolla Village
antibiotics, diuretics, nucleoside analog antivirals).
Drive (9151), San Diego, CA 92161. Phone: 858-552-8585, ext.
Accordingly, renal OA secretion has largely been
5945; Fax: 858-642-1438; E-mail:
[email protected]; or Dr. Sanjay K.
Nigam, Department of Medicine, University of California San Diego,
studied from a pharmacologic perspective; how-
San Diego, 9500 Gilman Drive, La Jolla, CA 92093. Phone: 858-822-
ever, physiologic substrates and functions are not
3482; Fax: 858-822-3483; E-mail:
[email protected]
well understood (for review, see references1–8).
Copyright 䊚 2008 by the American Society of Nephrology
ISSN : 1046-6673/1909-1732
J Am Soc Nephrol 19: 1732–1740, 2008
transport,11,12 and Oat3, a gene originally identified as Roct
(reduced in oc transporter),13 seem to account largely for the
basolateral uptake step of renal OA secretion: Both transport-
ers have been immunolocalized to the basolateral membrane
of the proximal tubule of human, rat, and mouse.14–21 More-
over, when expressed in
Xenopus oocytes or epithelial cell lines,both Oat1 and Oat3 function as exchangers, coupling substrate
entry to dicarboxylate exit.10,22,23
We, and subsequently others, recently characterized mice
null for Oat1 and Oat3, finding specific defects in OA transport
resulting in reduced renal excretion of diuretics and other
systolic blood pressure (mmHg
drugs.24–28 In the course of performing these experiments, we
discovered, unexpectedly, that the
Oat3⫺/⫺ mice, although
otherwise grossly physiologically normal, manifested lowered
BP. (Renal blood flow [as determined by paraaminohippuricacid clearance] and glomerular filtration [inulin clearance]
were unaffected,26 indicating that altered renal hemodynamics
do not account for the observed OA excretory defects in thesemice.) BP was not affected in
Oat1⫺/⫺ mice or in those null
for the related proximal tubular OAT,
URAT1.29–31 These
findings raised the possibility that Oat3 mediates the specific
transport of an endogenous compound(s) involved in the reg-ulation of BP. Moreover, they suggested the possibility that
inhibition of Oat3 might also lower BP and, therefore, that
Oat3 inhibitors might be useful in the treatment of hyperten-
mean blood pressure (mmHg)
sion. Here we describe experiments addressing these hypothe-
arterial catherization
Figure 1. Low BP in Oat3 knockout mice. Systolic BP was deter-
mined by tail-cuff method in awake mice, or mice were anesthe-tized and mean arterial BP was directly measured
via an intra-arterial catheter. Data are means ⫾ SEM of measurements in six
Oat3 Knockout Mice Manifest Reduced BP
to 20 mice of each genotype. BP measurements under anesthesia
Tail-cuff measurements consistently revealed a 10 to 15% low-
were previously reported for Oat1 knockout (⫺/⫺) mice.28 Mean
ering of both systolic (Figure 1) and diastolic arterial BP (data
arterial pressure was significantly reduced in
Oat3⫺/⫺ compared
not shown) in awake
Oat3⫺/⫺ compared with wild-type
with WT mice (**
P ⬍ 0.01) but not in mice lacking the related
(WT) mice, in the absence of significant differences in heart
proximal tubular OAT Oat1 or mUrat1 (RST), raising the possibility
rate (data not shown). This was confirmed when BP was di-
that an endogenous BP-regulating compound is transported by
rectly measured
via arterial catheterization in anesthetized
mice (Figure 1); again, heart rate was not significantly differentcompared with WT mice (data not shown). Moreover, there
compound. We reasoned that such substrates should be in-
were no differences between
Oat3⫺/⫺ and WT mice in general
creased in the plasma and possibly decreased in the urine of
aspects of renal function (GFR, renal blood flow,26 and frac-
Oat3⫺/⫺ mice, owing to loss of Oat3-mediated tubular secre-
tional excretion of fluid and electrolytes; data not shown). By
tion. We initially performed targeted gas chromatography/
contrast, BP was unaffected in mice null for the other major
mass-spectrometry (GC/MS) as described previously,28 to de-
basolateral proximal tubular OAT,
Oat1 (Figure 1), or in those
termine the concentrations of approximately 30 of the most
null for m
Urat1 (
RST), an apical proximal tubular OAT in-
abundant small OA in the urine and plasma of WT and
volved in the renal reabsorption of urate.29–31 These results
Oat3⫺/⫺ mice, including various Krebs cycle intermediates
suggest the possibility of specific transport of an endogenous
and fatty and amino acid metabolites. No significant differ-
BP-regulating compound by Oat3.
ences between WT and null mice were noted (Figure 2A, left),indicating that this set of compounds, although including sev-
Identification of Potential Endogenous Oat3
eral putative novel endogenous Oat1 substrates (Figure 2A,
right) does not seem to contain any authentic substrates of
We performed analyses of
Oat3⫺/⫺ mouse urine and plasma
Oat3. Accordingly, we performed global (metabolomic) rather
so as to identify endogenous substrates of Oat3, among which
than targeted mass spectrometric analyses of
Oat3⫺/⫺ plasma
might be included the aforementioned putative BP-regulating
and urine, assaying the levels of several thousands of molecules
J Am Soc Nephrol 19: 1732–1740, 2008
Oat3 and BP Control
Figure 2. Detection of potential endogenous Oat3
substrates. (A) GC/MS analysis was performed to de-
termine the levels of approximately 30 of the most
abundant endogenous OA in plasma and urine. For
each compound, the ratio of the concentration in urine
(normalized to creatinine) to the concentration in
plasma was calculated, representing a measure of the
efficiency of renal excretion. Urine/plasma ratios of
these compounds were largely unaffected in
Oat3⫺/⫺
[urine/plasma] Oat3 -/ 10-3
mice (left). In comparison, several compounds mani-
fested markedly lower urine/plasma ratios (
i.e., lower
apparent renal excretion) in
Oat1⫺/⫺ compared with
10-5 10-4 10-3 10-2 10-1 100 101 102 103 104 105
10-5 10-4 10-3 10-2 10-1 100 101 102 103 104 105
WT mice, indicating the involvement of Oat1 in their
[urine/plasma] WT
[urine/plasma] WT
tubular secretion (data taken from reference28). Nota-bly, the urine/plasma ratio for ␣-ketoglutarate, which
serves as a counterion for OA uptake, was greater in
Oat1⫺/⫺ compared with WT mice but unaffected in
Oat3⫺/⫺ mice. Data are means ⫾ SEM (
n ⫽ 4 per
genotype). (B) A global mass-spectrometric (metabolo-
mic) analysis was performed to compare the plasma
and urine metabolite composition of
Oat3⫺/⫺ and WTmice. Data presented are the mean intensity values for
the 100 compounds each in plasma and urine that were
the most significantly different in intensity between
Oat3⫺/⫺ and WT mice. The majority (81%) of the
significantly varying compounds in plasma were in-creased in
Oat3⫺/⫺ relative to WT, whereas the majority (64%) of those in urine were decreased in
Oat3⫺/⫺ relative to WT, consistentin each case with diminished renal excretion of the corresponding compounds.
simultaneously. These global analyses revealed that the metab-
thymidine handling by Oat1 both
in vitro and
in vivo. We de-
olite composition of plasma and urine of
Oat3⫺/⫺ mice is
termined that thymidine was not appreciably transported by
indeed significantly different from that of WT mice, with 2% of
Oat1
in vitro (Supplemental Figure 1A) and that plasma levels
the features in plasma and 14% of the features in urine signif-
of thymidine were not elevated in the Oat1 knockout (Supple-
icantly different in concentration between the genotypes (two-
mental Figure 1B), indicating that thymidine does not undergo
tailed
t test,
P ⬍ 0.01). Importantly, the observed differences
significant Oat1-mediated uptake/secretion
in vivo.
were markedly skewed in the direction expected for endoge-nous substrates (
i.e., increased in concentration in plasma and
Effect on BP of Administration of Endogenous Oat3
decreased in urine in
Oat3⫺/⫺ mice [Figure 2B]).
The most significantly varying features were analyzed fur-
Thymidine or FMN could be directly involved in BP regulation,
ther; however, although the masses of the corresponding com-
and/or as apparent Oat3 substrates, they could competitively in-
pounds were determined to an accuracy of 8 ppm, only a few
hibit the transport of other substrates, including that of the puta-
could be matched to known metabolites (
e.g., using the Metlin
tive BP-regulating substrate. Accordingly, exogenous administra-
metabolite database). Plasma and urine intensity values in WT
tion of these compounds might reproduce the phenotype of
and
Oat3⫺/⫺ mice for two of the identified compounds, fla-
decreased BP observed in the
Oat3⫺/⫺ mice. FMN, thymidine,
vin mononucleotide (FMN) and thymidine, are presented in
or vehicle (30 l of water) was given as an intravenous bolus in
Figure 3A, along with data for an unknown compound of m/z
anesthetized WT mice while BP was continuously monitored. We
323.03. We subsequently tested the identified compounds for
observed that whereas FMN at dosages up to 100 mg/kg intrave-
interaction with Oat3
in vitro and found that both FMN and
nously and vehicle application were ineffective (data not shown),
thymidine inhibited tracer uptake in Oat3-expressing oocytes
thymidine induced a dosage-dependent reduction in BP (Figure
in a concentration-dependent manner (Figure 3B), as would
3D) without lowering heart rate (data not shown). The BP re-
be expected in the case of genuine Oat3 substrates. Because
sponse peaked within approximately 30 s and lasted for 3 to 5 min.
further experiments (see next paragraph) pointed to a partic-
The lower potency of thymidine
versus FMN for the inhibition of
ular role of thymidine, we directly assessed Oat3-mediated
Oat3-mediated uptake (Figure 3B) suggested that lowering of BP
transport of this compound. We found that labeled thymidine
by this compound might not have been primarily due to Oat3
was taken up into Oat3-expressing oocytes to a much greater
inhibition. In accordance with this inference, a very similar BP
degree than into control oocytes (Figure 3C), indicating that
response to thymidine was observed in
Oat3⫺/⫺ mice (Figure
Oat3 can mediate the transport of thymidine. We also assessed
3D). Thus, the Oat3 substrate thymidine might accumulate in the
Journal of the American Society of Nephrology
J Am Soc Nephrol 19: 1732–1740, 2008
Identification of potential endogenous
Oat3 substrates. (A) Box plots of intensity values fortwo of the identified compounds from the metabolo-mic analysis, FMN and thymidine, and of an unknowncompound of m/z of 323.03. **
P ⬍ 0.01. (B) Traceruptake into Oat3-expressing oocytes was determinedin the presence of the indicated concentrations of theputative substrates, thymidine and FMN, and of thewell-characterized Oat3 substrate estrone-3-sulfateand in their absence (control). Data are means ⫾ SEMof measurements in four groups of four to five oocyteseach, and the IC50 values were calculated by curve-fitting the points using nonlinear regression. (C) Uptakeof labeled thymidine (0.097 M) and ES (0.017 M)was determined in oocytes microinjected with Oat3and in control (noninjected) oocytes. Data are means ⫾SEM of measurements in five groups of four to eightoocytes each. Oat3-expressing oocytes manifestedsignificantly greater uptake of both ES and thymidinethan did control oocytes. #
P ⬍ 0.001. (D) Intravenousapplication of thymidine induced a dosage-dependentreduction in BP in anesthetized WT and
Oat3⫺/⫺mice. Data are means ⫾ SEM in four mice. *
P ⬍ 0.05,**
P ⬍ 0.01, #
P ⬍ 0.001
versus vehicle.
plasma of mice lacking Oat3 and contribute to the lower BP in
mately 2 to 5 mM in the case of the fluorescent compounds),
followed by recording of any changes in BP. We found thatadministration of the two highest potency compounds (of the
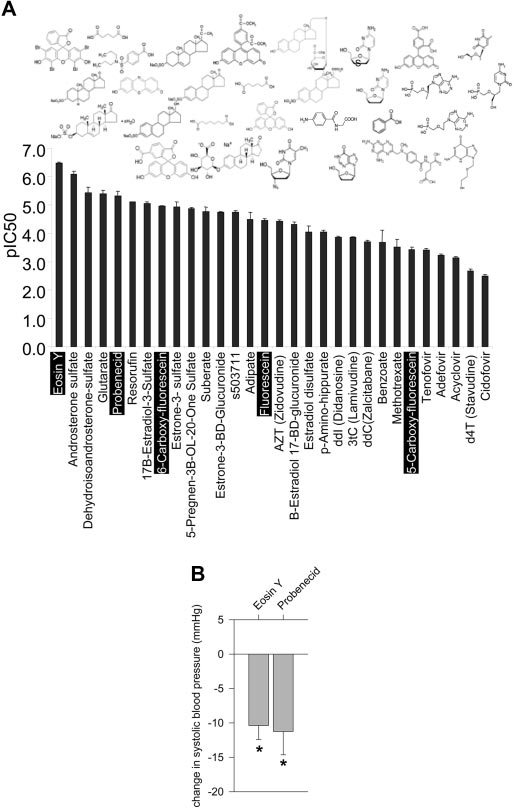
Identification of Oat3 Inhibitors
five tested), eosin-Y and probenecid (Figure 4B), but not of the
Although thymidine and FMN might be insufficiently potent to
three lower potency compounds (data not shown) consistently
inhibit Oat3
in vivo (see Figure 3B), more potent compounds
caused an acute decrease in BP. These results are consistent
might do so. Accordingly, we sought to identify other Oat3 inhib-
with our hypothesis that inhibition of Oat3 by sufficiently po-
itors, testing the capacity for Oat3 inhibition of approximately 30
tent inhibitors can lower BP. Nevertheless, it should be noted
small OA of diverse chemical structures. Tested compounds en-
that given the relatively high dosages used and that each of the
compassed fluorescein derivatives, steroid conjugates, nucleoside
tested compounds interacts with Oat1 with comparable
analog antivirals, and short-chain aliphatic dicarboxylates, among
potency,33a nonspecific effects cannot be excluded.
other small OA; data on the fluorescein derivatives and antiviralshas previously been reported.32 Inhibition by each compound was
Plasma Concentrations of Corticosterone,
determined as in the experiments presented in Figure 3B. The
Adrenocorticotropic Hormone, Aldosterone, and
tested compounds varied widely in inhibitory potency, ranging
Renin in Oat3 Knockout Mice
from submicromolar to millimolar (Figure 4A). In general, the
Given the high potency interaction of Oat3 with steroid con-
steroid conjugates were among the more potent of the com-
jugates demonstrated here (Figure 4A) as well as elsewhere
pounds tested and the antivirals among those less potent
(reviewed in reference6), we determined the concentrations ofthe BP-regulating adrenal steroids and of functionally related
Reduced BP after Administration of the Most Potent
hormones in Oat3 knockout compared with WT plasma. We
found lower BP in
Oat3⫺/⫺ mice to be associated with greater
We tested the effects on BP of a subset of the previously men-
plasma adrenocorticotropic hormone (ACTH) but unchanged
tioned compounds: The well-characterized OAT inhibitor
corticosterone concentrations (Figure 5A). ACTH stimulation
probenecid6,7,24,28,33 and the fluorescent OA eosin-Y, fluores-
increased plasma corticosterone by a factor of approximately
cein, 5-carboxyfluorescein (5CF), and 6-carboxyfluorescein
2.4 in both WT and
Oat3⫺/⫺ mice (Figure 5A), indicating
(the latter compounds were chosen because they allowed for
intact acute ACTH-responsiveness in
Oat3⫺/⫺ mice. These
fluorometric determination of inhibitor plasma levels). Ap-
results argue against an essential role for Oat3 in corticosterone
proximately 50 to 100 mg/kg of each of these compounds was
release from the adrenal gland and against a primary role for
intravenously infused into mice under anesthesia as described
defects in corticosterone handling in causing the lower BP in
already (resulting in initial plasma concentrations of approxi-
J Am Soc Nephrol 19: 1732–1740, 2008
Oat3 and BP Control
formed experiments with furosemide. Furosemide (40 mg/kgintraperitoneally) stimulates renal renin secretion by inhibit-ing the sensing of the luminal NaCl concentration at the mac-ula densa,34 thereby increasing macula densa release of prosta-glandin E2 (PGE2) across the basolateral membrane,35 amechanism that may involve Oat3.16 On the basis of our pre-vious studies,26 the applied dosage of furosemide is expected toinduce similar maximal inhibition of NaCl reabsorption inthick ascending limb and macula densa in both genotypes;however, furosemide in fact increased renin levels to a greaterdegree in Oat3⫺/⫺ than in WT mice (Figure 5A), indicatingenhanced rather than reduced PGE2 signaling in the knockout;as further outlined in the Discussion, this may reflect impairedPGE2 reuptake in the knockouts.
In addition to increased renin, Oat3⫺/⫺ mice manifested
modestly greater plasma aldosterone (Figure 5A), which is ex-pected to help stabilize BP by enhancing renal NaCl reabsorp-tion. To test the efficiency of that system in Oat3⫺/⫺ mice, weassessed the response to a low NaCl diet given for 7 d. Underbasal conditions, no significant differences were observed be-tween WT and Oat3⫺/⫺ mice in body weight and food or fluidintake (data not shown). A low NaCl diet, however, induced asignificantly greater loss of body weight in Oat3⫺/⫺ comparedwith WT mice (Figure 5B), which could not be explained bylower food intake. This diet increased plasma renin to similarlevels in both genotypes, but aldosterone upregulation was im-paired in Oat3⫺/⫺ mice (Figure 5A). It is possible that thisimpaired upregulation led to salt and fluid loss, lowering bodyweight and inducing thirst and increased fluid intake, as shownin Figure 5B.
Interaction of Aldosterone and Its Potential Precursorswith Oat3 in Xenopus Oocytes
Given that Oat3 is expressed in adrenal tissue and is able to
Reduced BP after administration of Oat3 inhibitors. (A)
The inhibitory potency for Oat3 of each of approximately 30 OA
mediate transport of cortisol,36 aldosterone dysregulation in
was determined as in the experiments presented in Figure 3B.
Oat3 knockouts might be due to loss of interaction of aldoste-
Among these, data on the fluorescein derivatives and antivirals
rone or related compounds with Oat3. We found aldosterone
has previously been reported.32 Data are means ⫾ SEM of deter-
and three of its potential precursors, progesterone, corticoste-
minations of pIC50 (the negative log of IC50; thus, higher values
rone, and desoxycorticosterone,37,38 to each dosage-depen-
indicate greater inhibitory potency). The chemical structures of
dently inhibit Oat3-mediated tracer uptake in vitro, with me-
the tested compounds are depicted above their corresponding
dian inhibition concentration (IC50) values of 12, 29, 10, and 9
pIC50 values. (B) Selected compounds from Figure 4A (f) were
M, respectively (Figure 6). Although these affinities are com-
intravenously infused into WT mice under anesthesia. These com-
parable to that of the prototypical Oat3 substrate estrone-3-
prised the well-characterized OAT inhibitor probenecid and the
sulfate (Figure 3B), they are much greater than physiologic free
fluorescent OA eosin-Y, fluorescein, 5CF, and 6- carboxyfluores-
plasma concentrations of these compounds (this study)39,40
cein, each administered at a dosage of 50 to 100 mg/kg. Aconsistent decrease in BP was noted after the infusion of the two
(although local concentrations around the adrenal glomeru-
higher potency compounds, eosin-Y and probenecid, but not
losa cells might be considerably higher). Thus, the physiologic
after infusion of the three lower potency compounds (data not
significance of these interactions is uncertain.
shown). Data are means ⫾ SEM of measurements in three to fourindependent experiments each. *P ⬍ 0.05 versus vehicle.
Oat3⫺/⫺ mice had modestly greater plasma renin concen-
Recent studies localized Oat3 to the basolateral membrane of
trations (Figure 5A) and greater renal expression of renin ver-
macula densa cells, and it was proposed that Oat3 at that site
sus WT mice (data not shown). To test for a potential role of
might contribute to the local release or reuptake of PGE2.16,41
Oat3 in macula densa stimulation of renin secretion,16 we per-
Basolateral release of PGE2 mediates the stimulation of renin
Journal of the American Society of Nephrology
J Am Soc Nephrol 19: 1732–1740, 2008
Figure 5. Plasma vasoactive hormones and the
response to low-salt diet in Oat3 knockout mice.
(A) Lower BP in Oat3⫺/⫺ mice was associated with
increased plasma concentrations of ACTH, renin,
plasma corticosterone (ng/ml)
plasma renin (ng
plasma aldosterone (pg/ml)
and aldosterone, whereas plasma corticosterone
concentrations were not different. The ACTH-in-duced increase in plasma corticosterone was un-
affected in the knockouts, whereas the furosemide
(FURO)-induced renin response was enhanced,
and the low-NaCl diet–induced aldosterone in-
crease was blunted (n ⫽ 6 to 9 per genotype). (B)
The blunted aldosterone response to a low-NaCl
diet in Oat3⫺/⫺ compared with WT mice was
associated with greater body weight loss and in-
creased fluid intake but similar food intake (n ⫽ 6
per genotype). Data are means ⫾ SEM. *P ⬍ 0.05,
cumulative food intake (g/g bw) 0.0
cumulative fluid intake (ml/g bw 0.0
**P ⬍ 0.01 versus WT.
release (in turn leading to aldosterone activation) that occurs
compounds identified via metabolomic analyses and subse-
in response to low salt concentrations at the macula densa or to
quent transport assays as potential endogenous Oat3 sub-
inhibition of macula densa transport by furosemide.35 We find
strates: Thymidine and FMN. Whereas FMN displayed no ef-
that the renin response to furosemide is enhanced in Oat3⫺/⫺
fect, thymidine exhibited a consistent dosage-dependent
mice, suggesting that Oat3 might contribute to the reuptake of
lowering of BP. Normal serum concentrations of thymidine
PGE2 (loss of such reuptake might also have contributed to
are approximately 1 M, and there is a thymidine pool that is
greater basal renin levels in Oat3⫺/⫺ mice, helping ameliorate
in rapid equilibrium with blood thymidine and that is at least
hypotension). Of note, the aldosterone response to salt depri-
10 times larger than the pool of extracellular thymidine.42 It is
vation was blunted in Oat3⫺/⫺ mice; however, aldosterone
difficult to know what the local concentrations of the exog-
dysregulation cannot explain the observed hypotension under
enously administered thymidine were; however, assuming it
basal conditions because basal plasma aldosterone concentra-
was rapidly distributed into the aforementioned pools, a con-
tions were increased in mice lacking Oat3.
centration of 20 M would be achieved at 10 mg/kg injection,
The aforementioned results raise the possibility that Oat3
where the BP effect was first noted. Within a roughly 10-fold
mediates the transport of other endogenous regulators of BP.
higher concentration, an effect comparable to that observed in
To test this possibility, we examined the effects of two of the
the Oat3 knockout was observed.
IC50, µM
(% of control)
Figure 6. Interaction of aldosterone and its potential precursors, progesterone, corticosterone, and desoxycorticosterone, with Oat3.
Uptake of 5-CF in mOat3-expressing Xenopus oocytes was determined in the presence of the indicated concentrations of the steroidhormones to calculate the respective IC50 values. Each compound caused a dosage-dependent decrease in uptake, consistent withcompetitive inhibition of uptake of the fluorescent tracer. Data are presented as percentage of control uptake and are the means ⫾ SEMof measurements in four groups of four to five oocytes each. IC50 values were determined by curve-fitting the points using nonlinearregression.
J Am Soc Nephrol 19: 1732–1740, 2008
Oat3 and BP Control
Although a number of endogenous OA compounds trans-
0.97% K⫹) for quantitative collection of urine, immediately followed
ported by Oat3 may be involved in regulating BP, at the very least,
by collection of plasma via puncture of the retrobulbar plexus as de-
these results suggest that some of them can, perhaps in concert, act
scribed previously.43
to regulate BP under physiologic conditions. This also suggeststhat inhibitors of Oat3, regardless of whether they are actual sub-
strates for the transporter, might lower BP. This could lead to a
GC/MS was performed as described previously.28 Briefly, plasma and
novel approach to the design of antihypertensive agents. We
urine samples (100 l or volumes equivalent to 0.25 to 1.00 mol of
therefore tested a set of Oat3 inhibitors (selected from a larger
creatinine, respectively) were reacted with pentafluoro-benzylhy-
screen) for effects on BP, finding that administration of two of the
droxylamine to form oximes of ketones, oxo-acids, and aldehydes.
five tested compounds resulted in lowering of BP.
The reaction products were lyophilized and extracted in 42% t-amyl
Together, the data suggest that (1) Oat3 regulates BP, (2) Oat3
alcohol:chloroform over a column of silicic acid. The dried eluate was
affects the regulation of plasma aldosterone (although this cannot
reacted with BSTFA/TRISIL to form trimethylsilyl derivatives. The
explain the hypotensive phenotype of Oat3-null mice), (3) at least
latter were injected (0.5 to 1.0 l) on a bonded phase (DB5) capillary
some endogenous compounds that accumulate in the absence of
column (30 m ⫻ 0.25 mm) in an Agilent 6890/5973 GC/MS. Electron
Oat3 are substrates with the potential to regulate BP, and (4) ad-
impact mass spectra were obtained in scan mode (50 to 650 amu at 2.4
ministration of sufficiently potent inhibitors might result in low-
cycles/s). The species were quantified using calibrated response curves
ering of BP. Although much more work needs to be done, the data
of selected ions and 4-nitrophenol and 2-oxocaproic acid as internal
suggest novel concepts regarding the physiologic role of Oat3 sub-
strates and raise the possibilities that Oat3 inhibitors might be ofuse in the treatment of hypertension and that polymorphisms in
Global Metabolomics
human OAT3 might contribute to variability in the propensity for
Urine and plasma samples were extracted in methanol and lyophi-
lized, and the equivalents of 0.4 to 1.4 l of extracted urine (afternormalization to the previously measured concentrations of creati-nine) or 1 l of extracted plasma were applied to a capillary reverse-
phase column with dimensions 150 ⫻ 0.3 mm (diameter; ZorbaxSB-300, Agilent) at a flow rate of 4 l/min. Buffer A was H O ⫹ 0.1%
formic acid, and Buffer B was acetonitrile with 0.1% formic acid.
Oat3⫺/⫺ mice24 were back-crossed to C57BL/6J mice for eight gen-
Plasma samples were eluted with a gradient from 5 to 95% B over 45
erations. Heterozygous mice from the last back-cross were bred to
min; for urine samples, the gradient was 5% B from 0 to 10 min then
each other to generate gene knockout and WT mice. All of the animals
5 to 95% from 10 to 55 min, then held at 95% to 60 min. The column
used in the experiments described descended from those mice. Mice
was interfaced to an electrospray ionization time-of-flight mass spec-
were genotyped by PCR as described previously.24 Experimental pro-
trometer (Agilent). Data were collected from approximately 100 to
tocols were in accordance with the Guide for Care and Use of Labo-
1000 m/z in continuum mode and converted from the instrument
ratory Animals (National Institutes of Health, Bethesda, MD) and
format (.wiff) to the common format (.cdf). Data were analyzed using
were approved by the Institutional Animal Care and Use Committee.
the analysis and nonlinear alignment program XCMS,47 in which theintegrated intensities of all observed ions were compared between the
Oat3⫺/⫺ and WT groups. These procedures resulted in the detection
[3H]-estrone sulfate (ES) and [3H]-thymidine were purchased from
of approximately 4000 features in urine and approximately 5000 in
Perkin-Elmer Life Sciences (Boston, MA), and 5CF, a fluorescent OA
plasma, which would include any metabolites with m/z values be-
transported by mOat332 and other OA test compounds (all ⬎98%
tween 100 and 1000 that ionize under positive electrospray conditions
pure and in the L-enantiomer) were purchased from Sigma-Aldrich
and were within the detection limit of the instrument. The data were
(St. Louis, MO).
then analyzed using the recently developed software package XCMS,which performs a nonlinear alignment of the chromatograms and
BP MeasurementsMice were anesthetized for terminal experiments with 100 mg/kg in-
integration of the intensities for each ion.
actin intraperitoneally and 100 mg/kg ketamine intramuscularly, thejugular vein was cannulated for infusion of maintenance fluids and
Xenopus Oocyte Transport Experiments
test compounds, and the femoral artery was cannulated for BP and
Xenopus oocyte assays were performed as described previously.28
heart rate measurement as described previously.43,44 In separate ex-
Briefly, capped cRNA was generated via in vitro transcription from
periments, BP and heart rate in unanesthetized mice were determined
linearized plasmid DNA, mOat3 (Image clone ID 4239544), or mOat1
by the tail-cuff method in trained awake mice as described previ-
(Image clone ID 4163278), using mMessage mMachine in vitro tran-
scription kit (Ambion, Austin, TX). Stage V and VI Xenopus oocyteswere isolated, maintained in Barth's growth medium, injected with
Urine and Plasma Samples
approximately 20 nl/oocyte cRNA solution (1 g/l), and allowed to
Mice were placed in metabolic cages (Tecniplast, Hohenpeissenberg,
mature for 3 d after injection. Oat3-expressing and control nonin-
Germany) with free access to tap water and standard diet (0.44% Na⫹,
jected oocytes were then incubated in Barth's buffer for 1 h at 25°C
Journal of the American Society of Nephrology
J Am Soc Nephrol 19: 1732–1740, 2008
with Oat3 tracer substrate (50 M 5CF, 0.017 M 3H-ES, or 0.097
M 3H-thymidine) in the presence of various concentrations of testcompounds or in their absence. Transport was terminated by washes
1. Robertson EE, Rankin GO: Human renal organic anion transporters:
in ice-cold Barth's buffer, and the tracer content of groups of four to
Characteristics and contributions to drug and drug metabolite excre-tion. Pharmacol Ther 109: 399 – 412, 2006
five oocytes was determined by fluorometry or scintillation counting
2. Eraly SA, Blantz RC, Bhatnagar V, Nigam SK: Novel aspects of renal
in the case of experiments using 5CF and the radiolabeled tracers,
organic anion transporters. Curr Opin Nephrol Hypertens 12: 551–
respectively. The background uptake of tracer was determined in
noninjected oocytes and subtracted from that in Oat3-injected oo-
3. Eraly SA, Bush KT, Sampogna RV, Bhatnagar V, Nigam SK: The mo-
cytes to calculate the Oat3-mediated component of uptake. The IC50
lecular pharmacology of organic anion transporters: From DNA toFDA? Mol Pharmacol 65: 479 – 487, 2004
were determined by nonlinear regression using Prism software 5.0
4. Koepsell H, Endou H: The SLC22 drug transporter family. Pflugers
(GraphPad, San Diego, CA).
Arch 447: 666 – 676, 2004
5. Sweet DH, Bush KT, Nigam SK: The organic anion transporter family:
Plasma Concentrations of Aldosterone, Renin,
From physiology to ontogeny and the clinic. Am J Physiol Renal
Corticosterone and ACTH
Physiol 281: F197–F205, 2001
Mice were placed under short-term isoflurane anesthesia, and blood
6. Burckhardt BC, Burckhardt G: Transport of organic anions across the
was taken from the retrobulbar plexus for determination of hemato-
basolateral membrane of proximal tubule cells. Rev Physiol BiochemPharmacol 146: 95–158, 2003
crit and basal plasma concentrations of aldosterone (Diagnostic Sys-
7. Wright SH, Dantzler WH: Molecular and cellular physiology of renal
tems Laboratories, Webster, TX; cross-reactivity with corticosterone
organic cation and anion transport. Physiol Rev 84: 987–1049, 2004
0.02%), renin (see below in this paragraph), corticosterone, and
8. Nigam SK, Bush KT, Bhatnagar V: Drug and toxicant handling by the
ACTH (both MP Biomedicals, Orangeburg, NY). To determine a po-
OAT organic anion transporters in the kidney and other tissues. Nat
tential role of Oat3 in corticosterone release from the adrenal gland,36
Clin Pract Nephrol 3: 443– 448, 2007
9. Burckhardt G, Bahn A, Wolff NA: Molecular physiology of renal p-
we performed an ACTH stimulation test. To this end, plasma corti-
aminohippurate secretion. News Physiol Sci 16: 114 –118, 2001
costerone was determined 45 min after intraperitoneal application of
10. Sweet DH, Wolff NA, Pritchard JB: Expression cloning and character-
1 U of 1-24 ACTH (Cortrosyn®; Organon, Roseland, NJ).48 To deter-
ization of ROAT1: The basolateral organic anion transporter in rat
mine a potential role of Oat3 in macula densa stimulation of renin
kidney. J Biol Chem 272: 30088 –30095, 1997
secretion,16 we determined plasma renin concentration 45 min after
11. Lopez-Nieto CE, You G, Bush KT, Barros EJ, Beier DR, Nigam SK:
Molecular cloning and characterization of NKT, a gene product re-
intraperitoneal application of furosemide (40 mg/kg).34 In addition,
lated to the organic cation transporter family that is almost exclusively
the response in body weight and food and fluid intake as well as in
expressed in the kidney. J Biol Chem 272: 6471– 6478, 1997
plasma concentrations of renin and aldosterone to a low-NaCl diet for
12. Lopez-Nieto CE, You G, Barros EJ, Beier DR, Nigam SK: Molecular
8 d was assessed. Plasma renin concentration was measured by RIA
cloning and characterization of a novel transport protein with very high
(GammaCoat Plasma Renin Activity, CA-1533; DiaSorin, Stillwater,
expression in the kidney [Abstract]. J Am Soc Nephrol 7: 1301, 1996
13. Brady KP, Dushkin H, Fornzler D, Koike T, Magner F, Her H, Gullans S,
MN) as the generation of angiotensin I after addition of excess angio-
Segre GV, Green RM, Beier DR: A novel putative transporter maps to
tensinogen substrate from bilaterally nephrectomized rats.49 Concen-
the osteosclerosis (oc) mutation and is not expressed in the oc mutant
trations of Na⫹ and K⫹ in plasma and urine were determined using a
mouse. Genomics 56: 254 –261, 1999
flame photometer (Cole-Parmer Instrument Co., Vernon Hills, IL).
14. Hosoyamada M, Sekine T, Kanai Y, Endou H: Molecular cloning and
functional expression of a multispecific organic anion transporter from
Statistical Analysis
human kidney. Am J Physiol 276: F122–F128, 1999
Data are presented as means ⫾ SEM. Statistical differences between
15. Ljubojevic M, Herak-Kramberger CM, Hagos Y, Bahn A, Endou H,
Burckhardt G, Sabolic I: Rat renal cortical OAT1 and OAT3 exhibit
gene-knockout and WT mice were analyzed by the unpaired t test.
gender differences determined by both androgen stimulation and
P ⬍ 0.05 was considered to be statistically significant.
estrogen inhibition. Am J Physiol Renal Physiol 287: F124 –F138, 2004
16. Bahn A, Ljubojevic M, Lorenz H, Schultz C, Ghebremedhin E, Ugele B,
Sabolic I, Burckhardt G, Hagos Y: Murine renal organic anion trans-
porter 1 (mOAT1) and organic anion transporter 3 (mOAT3) facilitatethe transport of neuroactive tryptophan metabolites. Am J Physiol CellPhysiol 289: C1075–C1084, 2005
This work was supported by National Institute of Diabetes and Diges-
17. Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goto M,
tive and Kidney Diseases grants DK56248 and DK28602 (to V.V.),
Fukatsu A, Ogawa O, Inui K: Gene expression levels and immunolo-
DK064839 and DK075486 (to S.A.E.), and AI057695, DK079784, and
calization of organic ion transporters in the human kidney. J Am Soc
HL35018 (to S.K.N.); the Department of Veterans Affairs (to V.V.);
Nephrol 13: 866 – 874, 2002
18. Kojima R, Sekine T, Kawachi M, Cha SH, Suzuki Y, Endou H: Immuno-
and Deutsche Forschungsgemeinschaft RI 1535/3-1 and RI 1535/3-2
localization of multispecific organic anion transporters, OAT1, OAT2,
and OAT3, in rat kidney. J Am Soc Nephrol 13: 848 – 857, 2002
We thank Duke A. Vaughn, Shamara Closson, Kerstin Richter,
19. Hasegawa M, Kusuhara H, Sugiyama D, Ito K, Ueda S, Endou H,
and Jana Schroth for expert technical assistance.
Sugiyama Y: Functional involvement of rat organic anion transporter 3(rOat3; Slc22a8) in the renal uptake of organic anions. J PharmacolExp Ther 300: 746 –753, 2002
20. Cha SH, Sekine T, Fukushima JI, Kanai Y, Kobayashi Y, Goya T, Endou
H: Identification and characterization of human organic anion trans-
J Am Soc Nephrol 19: 1732–1740, 2008
Oat3 and BP Control
porter 3 expressing predominantly in the kidney. Mol Pharmacol 59:
35. Harris RC, Zhang MZ, Cheng HF: Cyclooxygenase-2 and the renal
1277–1286, 2001
renin-angiotensin system. Acta Physiol Scand 181: 543–547, 2004
21. Tojo A, Sekine T, Nakajima N, Hosoyamada M, Kanai Y, Kimura K,
36. Asif AR, Steffgen J, Metten M, Grunewald RW, Muller GA, Bahn A,
Endou H: Immunohistochemical localization of multispecific renal or-
Burckhardt G, Hagos Y: Presence of organic anion transporters 3
ganic anion transporter 1 in rat kidney. J Am Soc Nephrol 10: 464 –
(OAT3) and 4 (OAT4) in human adrenocortical cells. Pflugers Arch 450:
22. Sweet DH, Chan LM, Walden R, Yang XP, Miller DS, Pritchard JB:
37. Vinson GP: Glomerulosa function and aldosterone synthesis in the rat.
Organic anion transporter 3 (Slc22a8) is a dicarboxylate exchanger
Mol Cell Endocrinol 217: 59 – 65, 2004
indirectly coupled to the Na⫹ gradient. Am J Physiol Renal Physiol
38. Ishimura K, Fujita H: Light and electron microscopic immunohisto-
284: F763–F769, 2003
chemistry of the localization of adrenal steroidogenic enzymes. Mi-
23. Bakhiya A, Bahn A, Burckhardt G, Wolff N: Human organic anion
crosc Res Tech 36: 445– 453, 1997
transporter 3 (hOAT3) can operate as an exchanger and mediate
39. Schneider JS, Stone MK, Wynne-Edwards KE, Horton TH, Lydon J,
secretory urate flux. Cell Physiol Biochem 13: 249 –256, 2003
O'Malley B, Levine JE: Progesterone receptors mediate male aggres-
24. Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK:
sion toward infants. Proc Natl Acad Sci U S A 100: 2951–2956, 2003
Impaired organic anion transport in kidney and choroid plexus of
40. Vincent M, Gomez-Sanchez CE, Bataillard A, Sassard J: Steroids dur-
organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J Biol
ing development of genetic hypertension in rats of Lyon strain. Am J
Chem 277: 26934 –26943, 2002
Physiol 257: H506 –H510, 1989
25. Vanwert AL, Bailey RM, Sweet DH: Organic anion transporter 3 (Oat3/
41. Nilwarangkoon S, Anzai N, Shiraya K, Yu E, Islam R, Cha SH, Onozato
Slc22a8) knockout mice exhibit altered clearance and distribution of
ML, Miura D, Jutabha P, Tojo A, Kanai Y, Endou H: Role of mouse
penicillin G. Am J Physiol Renal Physiol 293: F1332–F1341, 2007
organic anion transporter 3 (mOat3) as a basolateral prostaglandin
26. Vallon V, Rieg T, Ahn SY, Wu W, Eraly SA, Nigam SK: Overlapping in
E(2) transport pathway. J Pharmacol Sci 103: 48 –55, 2007
vitro and in vivo specificities of the organic anion transporters OAT1
42. Lee DJ, Prensky W, Krause G, Hughes WL: Blood thymidine level and
and OAT3 for loop and thiazide diuretics. Am J Physiol Renal Physiol
iododeoxyuridine incorporation and reutilization in DNA in mice given
294: F867–F873, 2008
long-acting thymidine pellets. Cancer Res 36: 4577– 4583, 1976
27. Vanwert AL, Sweet DH: Impaired clearance of methotrexate in organic
43. Vallon V, Grahammer F, Volkl H, Sandu CD, Richter K, Rexhepaj R,
anion transporter 3 (Slc22a8) knockout mice: A gender specific impact
Gerlach U, Rong Q, Pfeifer K, Lang F: KCNQ1-dependent transport in
of reduced folates. Pharm Res 25: 453– 462, 2008
renal and gastrointestinal epithelia. Proc Natl Acad Sci U S A 102:
28. Eraly SA, Vallon V, Vaughn DA, Gangoiti JA, Richter K, Nagle M,
17864 –17869, 2005
Monte JC, Rieg T, Truong DM, Long JM, Barshop BA, Kaler G, Nigam
44. Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V:
SK: Decreased renal organic anion secretion and plasma accumulation
Mice lacking P2Y2 receptors have salt-resistant hypertension and facili-
of endogenous organic anions in OAT1 knock-out mice. J Biol Chem
tated renal Na⫹ and water reabsorption. FASEB J 21: 3717–3726, 2007
281: 5072–5083, 2006
45. Vallon V, Wyatt AW, Klingel K, Huang DY, Hussain A, Berchtold S,
29. Mori K, Ogawa Y, Ebihara K, Aoki T, Tamura N, Sugawara A, Kuwahara
Friedrich B, Grahammer F, Belaiba RS, Gorlach A, Wulff P, Daut J,
T, Ozaki S, Mukoyama M, Tashiro K, Tanaka I, Nakao K: Kidney-
Dalton ND, Ross J Jr, Flogel U, Schrader J, Osswald H, Kandolf R, Kuhl
specific expression of a novel mouse organic cation transporter-like
D, Lang F: SGK1-dependent cardiac CTGF formation and fibrosis
protein. FEBS Lett 417: 371–374, 1997
following DOCA treatment. J Mol Med 84: 396 – 404, 2006
30. Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH,
46. Vallon V, Huang DY, Grahammer F, Wyatt AW, Osswald H, Wulff P,
Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y,
Kuhl D, Lang F: SGK1 as a determinant of kidney function and salt
Oda T, Ichida K, Hosoya T, Shimokata K, Niwa T, Kanai Y, Endou H:
intake in response to mineralocorticoid excess. Am J Physiol Regul
Molecular identification of a renal urate anion exchanger that regu-
Integr Comp Physiol 289: R395–R401, 2005
lates blood urate levels. Nature 417: 447– 452, 2002
47. Smith CA, Want EJ, O'Maille G, Abagyan R, Siuzdak G: XCMS: Process-
31. Eraly SA, Vallon V, Rieg T, Gangoiti JA, Wikoff WR, Siuzdak G, Barshop
ing mass spectrometry data for metabolite profiling using nonlinear peak
BA, Nigam SK: Multiple organic anion transporters contribute to net
alignment, matching, and identification. Anal Chem 78: 779–787, 2006
renal excretion of uric acid. Physiol Genomics 33: 180 –192, 2008
48. Meiner VL, Cases S, Myers HM, Sande ER, Bellosta S, Schambelan M,
32. Truong DM, Kaler G, Khandelwal A, Swaan PW, Nigam SK: Multi-level
Pitas RE, McGuire J, Herz J, Farese RV Jr: Disruption of the acyl-CoA:
analysis of organic anion transporters 1, 3, and 6 reveals major differ-
cholesterol acyltransferase gene in mice: Evidence suggesting multi-
ences in structural determinants of antiviral discrimination. J Biol
ple cholesterol esterification enzymes in mammals. Proc Natl Acad Sci
Chem 283: 8654 – 8663, 2008
U S A 93:14041–14046, 1996
33. Dantzler WH, Wright SH: The molecular and cellular physiology of
49. Rieg T, Schnermann J, Vallon V: Adenosine A(1) receptors determine
basolateral organic anion transport in mammalian renal tubules. Bio-
effects of caffeine on total fluid intake but not caffeine appetite. Eur
chim Biophys Acta 1618: 185–193, 2003
J Pharmacol 555: 174 –177, 2007
33a.Kaler G, Truong DM, Khandelwal A, Nagle M, Eraly SA, Swaan PW,
Nigam SK: Structural variation governs substrate specificity for organicanion transporter (OA1) homologs. J Biol Chem 282: 23841–23853, 2007
34. Kim SM, Mizel D, Huang YG, Briggs JP, Schnermann J: Adenosine as
a mediator of macula densa-dependent inhibition of renin secretion.
Supplemental information for this article is available online at http://www.
Am J Physiol Renal Physiol 290: F1016 –F1023, 2006
Journal of the American Society of Nephrology
J Am Soc Nephrol 19: 1732–1740, 2008
Source: https://masspec.scripps.edu/publications/public_pdf/139_art.pdf
Neither lavender oil nor tea tree oil can be linked to breast growth in young boys Robert Tisserand Introduction In 2007, a correlation was alleged between commercial products containing lavender and tea tree oils and breast growth in young boys. Three cases were seen in boys aged 4-7, who had all been using such products. In each case, the breast growth reduced to normal parameters within several months of ceasing to use the products. Subsequent laboratory testing showed that both essential oils had estrogen-like properties (Henley et al 2007). In the report, no information was given about any of the product ingredients, and there is scant information on product use. No analysis was carried out to confirm or rule out the presence of essential oil constituents. Case one In the first case, "The patient's mother reported applying a "healing balm" containing lavender oil to his skin starting shortly before the initial presentation." No further details of the product or its use are given, but a healing balm sounds like something that might only be applied to a small area of skin. If so, then it is unlikely that any ingredient could have entered the boy's blood in sufficient concentration to cause gynecomastia within a short time period. Case two In the second case, a styling hair gel was applied to the hair and scalp every morning, along with regular use of a shampoo. Both tea tree oil and lavender oil are cited on the ingredient list of both products. In a subsequent website report, it is claimed that the two hair products used in this case were manufactured by Paul Mitchell®, and that these were analyzed by a competitor. The shampoo was said to contain "very low concentrations" of tea tree oil, and the content in the hair gel was "virtually undetectable". Lavender oil concentration was not checked (Neustaedter 2007). Dermal absorption of fragrance from shampoo application has been estimated to be 80 times less than that from body lotion (Cadby et al 2002) and tea tree oil constituents are poorly absorbed by human skin. In one study, only 3% of the essential oil volume, applied as a 20% concentration in ethanol, was absorbed in a 24 hour period (Cross and Roberts 2006). If the website report is reliable, considering that shampoo is a wash-off product, and that there was only a negligible amount of tea tree oil in the hair gel, tea tree oil can be ruled out as a possible cause of this boy's gynecomastia. However, liberal use of a hair gel rich in lavender oil could result in moderate dermal absorption of lavender oil constituents (Cal 2006).
EQUINE VETERINARY JOURNAL Equine vet. J. (2011) •• (••) ••-••doi: 10.1111/j.2042-3306.2010.00313.x Comparative efficacy of inhaled albuterol between twohand-held delivery devices in horses with recurrentairway obstruction F. R. BERTIN, K. M. IVESTER and L. L. COUËTIL* Department of Veterinary Clinical Sciences, School of Veterinary Medicine, Purdue University, Indiana, USA.

