Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Doi:10.1016/j.biopsych.2004.09.018
Males and Females Respond Differently to
Controllability and Antidepressant Treatment
Benedetta Leuner, Sabrina Mendolia-Loffredo, and Tracey J. Shors
Background: Women are much more likely to suffer from stress-related mental illness than men; yet few, if any, animal models for
such sex differences exist. Previously, we reported that exposure to an acute stressor enhances learning in male rats yet severely impairs
learning in female rats. Here, we tested whether these opposite effects in males versus females could be prevented by establishing control
over the stressor or by antidepressant treatment.
Methods: Learning was assessed using the hippocampal-dependent task of trace eyeblink conditioning. In the first experiment, groups
of male and female rats were exposed to controllable or uncontrollable stress and trained. In a second experiment, they were exposed
to an uncontrollable stressor after chronic treatment with the antidepressant fluoxetine (Prozac). In a final experiment, females were
exposed to uncontrollable stress after acute treatment with fluoxetine.
Results: Establishing control over the stressful experience eliminated the detrimental effect of stress on learning in females as well as
the enhancing effect of stress in males. Moreover, chronic but not acute treatment with fluoxetine prevented the learning deficit in
females after exposure to stress. Treatment with fluoxetine did not alter the male response to stress.
Conclusions: These data indicate that males and females not only respond in opposite directions to the same stressful event but also
respond differently to controllability and antidepressant treatments.
Key Words: Stress, memory, fluoxetine, sex differences, anxiety,
display little if any symptoms of helplessness. Thus, while males
show performance deficits after exposure to an uncontrollablestressful event, females do not One of the problems
Stressful life events can affect our abilities to learn and withthesefindingsisthatfemaleratsaregenerallymoreactive
remember, as well as our emotional state. It has been
than males and thus are more likely to move and "learn" to
proposed that establishing control over these stressors can
escape even after exposure to an inescapable stressful event.
ameliorate some of the effects on cognition and behavior. This
Simply put, the absence of a helplessness effect in females may
idea evolved from studies conducted in laboratory animals
reflect gender differences in performance and not learning, per
demonstrating that exposure to inescapable shock interfered
se This problem can be
with subsequent learning of operant tasks
minimized using classical eyeblink conditioning, in which the
The effect of stress was not
learned response is not dependent on gross motor activity. Using
evident following exposure to equivalent amounts of escapable
this task, we have observed sex differences in conditioning itself
shock, suggesting that the impaired learning was due to the
such that females acquire this task at a facilitated rate and overall
psychological nature of the stressor, namely controllability,
emit more learned responses
rather than the physical properties associated with the shocks.
Moreover, females and males respond in
This deficit in operant conditioning was termed learned helpless-
opposite directions after exposure to inescapable tail shocks or
ness and is regarded as a behavioral model of stress-related
mental illness such as posttraumatic stress disorder (PTSD) and
While exposure to the stressful event
major depression
enhances subsequent conditioning in male rats, exposure to the
Although there has always been some controversy about
same event dramatically impairs conditioning in females. These
whether or not helplessness in laboratory animals can model
effects appear indicative of learning, at least to the extent that
depression in humans the phenomenon does
stressor exposure does not alter the unconditioned motor re-
appear sensitive to treatment with antidepressants. Specifically,
sponse in either sex
chronic treatment with serotonergic antidepressants alleviates
They do not appear dependent on sex differences in
cognitive and emotional deficits in most depressed individuals
performance, since the effects persist even when performance is
and reduces some of the behavioral symptoms of helplessness in
similar between unstressed males and females
laboratory animals
Since females under these training conditions are particularly
Although these findings support the idea of helplessness as a
vulnerable to the negative consequences of stressful events, we
model of depression in humans, there are findings that do not.
questioned whether these effects might model or in some way
For example, it is well established that women are more likely to
inform us about sex differences in stress-related illness. In the
experience depression than men yet female rats
first experiment, we tested whether the effects of stress onlearning in males and females could be prevented if animalsestablished control over the stressor. In a second experiment, we
From the Department of Psychology and Center for Collaborative Neuro-
tested whether chronic treatment with the antidepressant fluox-
science, Rutgers University, Piscataway, New Jersey.
etine (Prozac) would prevent the effects of stress on learning. As
Address reprint requests to Tracey J. Shors, Ph.D., Rutgers University, De-
partment of Psychology, 152 Frelinghuysen Road, Piscataway, NJ 08854;
an indirect measure of anxiety associated with stress and antide-
pressant treatment, we also measured exploratory behavior in
Received March 15, 2004; revised August 18, 2004; accepted September 24,
the elevated plus maze. Lastly, we tested whether acute treat-
ment with fluoxetine would prevent the effects of stress on
BIOL PSYCHIATRY 2004;56:964 –970
2004 Society of Biological Psychiatry
BIOL PSYCHIATRY 2004;56:964 –970 965
learning and anxiety, since it is well known that humans only
this period and before the US were considered conditioned
respond to these drugs after weeks of treatment
responses (CRs). Every 10-trial sequence consisted of 1 CS alone
presentation, 4 paired presentations of the CS and US, 1 US alonepresentation, and 4 paired presentations of the CS and US. TheITI was randomized between 20 and 30 seconds. To detect
Methods and Materials
eyeblinks, the maximum EMG response occurring during a
Experiment 1
250-millisecond prestimulus baseline recording period was
Subjects and Surgery. Adult (⬃2– 4 months) female (250 –
added to four times its SD. Responses that exceeded that value
350 g) and male (300 – 450 g) Sprague-Dawley rats were individ-
and had a width of at least 3 milliseconds were considered
ually housed, had unlimited access to chow and water, and were
maintained on a 12-hour light/dark cycle. To implant electrodes
Analysis of variance (ANOVA) with
for measuring the eyeblink response, rats were anesthetized with
repeated measures was used to analyze escape latency data and
sodium pentobarbital anesthesia supplemented with Isoflurane
the percentage of CRs emitted during 600 trials of trace eyeblink
and oxygen. A headstage attached to four stainless steel elec-
conditioning. Newman-Keuls post hoc analysis was applied to
trodes was secured to the skull with screws and acrylic. Elec-
significant main effects.
trodes were threaded through the eyelid: two electrodes re-corded electromyographic (EMG) activity associated with an
Experiment 2
eyeblink and two electrodes delivered eyelid stimulation to elicit
Next, we tested whether the effects of uncontrollable stress on
the eyeblink reflex. Rats were allowed to recover for at least 3
trace conditioning in male and female rats could be alleviated
days before escape training and classical conditioning. Stages of
with antidepressant treatment. Fluoxetine, a selective serotonin
estrus were monitored with daily vaginal smears, as previously
reuptake inhibitor (SSRI), was chosen because of its efficacy in
described Only female rats with normal 4- to
treating humans with stress-related mental illness
5-day cycles were tested.
Adult male (300 – 450 g) and
Acclimation and Escape Training. On the first day of escape
female (250 –350 g) rats received daily injections (intraperitoneal
training, rats were taken from their home cages and acclimated for
[IP]) of fluoxetine (5 mg/kg) or vehicle (.9% saline) for a
1 hour to the chamber in which they would later undergo classical
minimum of 14 days while monitoring estrous cycles in females.
eyeblink conditioning. Headstages were connected to a shielded
When females had received at least 14 days of fluoxetine or
grounded cable that allowed free movement within the condition-
vehicle and were in the diestrus-2 stage of their cycle, they were
ing chamber. The chamber consisted of an illuminated (7.5 W bulb)
acclimated to the classical conditioning chamber and then ex-
inner chamber (22 cm ⫻ 26 cm ⫻ 35 cm) with metal walls and a
posed to an acute uncontrollable stressor consisting of restraint
grounded grid floor located within a sound-attenuated outer cham-
and 1 mA, 1-second shocks applied to the tail at a rate of one per
ber (51 cm ⫻ 52 cm ⫻ 35 cm). After acclimation, unstressed males
minute for 30 minutes or returned to their home cage (no stress).
(
n ⫽ 10) and females (
n ⫽ 10) were returned to their home cages.
Similarly, males were acclimated to the classical conditioning
Separate groups of males and females (
n ⫽ 8 per group) were taken
chamber and exposed to the same uncontrollable stressor or
into a different room and placed in one of two identical shuttle
returned to their home cage. Groups consisted of no stress males
boxes (46 cm ⫻ 19 cm ⫻ 18 cm) located within a sound-attenuated
(
n ⫽ 9) and females (
n ⫽ 9) injected with vehicle; stressed males
illuminated (7.5 W bulb) chamber (69 cm ⫻ 69 cm ⫻ 53 cm). A
(
n ⫽ 9) and females (
n ⫽ 9) injected with vehicle; no stress males
scrambled shock generator delivered 1 mA shocks through the grid
(
n ⫽ 10) and females (
n ⫽ 9) injected with fluoxetine; and
floor of the apparatus every minute for 30 minutes with an intertrial
stressed males (
n ⫽ 8) and females (
n ⫽ 8) injected with
interval (ITI) of 60 seconds. The shuttle boxes were electrically
linked so that when one rat received shock, the yoked animal
Twenty-four hours after the stressor, animals were returned to
received the same amount of shock. Rats in the controllable stress
the conditioning chamber and trace conditioned as in Experi-
condition learned to escape the shock by running through a
ment 1. Twenty-four hours later, rats were placed in the elevated
doorway (7.5 cm) and tripping a balance switch that shut off current
plus maze, which consisted of a cross-shaped platform made of
to both boxes simultaneously. The time to escape the footshock was
black Plexiglas elevated 50 cm from the floor. The apparatus was
recorded. Yoked animals could not escape and thus had no control.
located in a dimly lit room and consisted of four arms each 50 cm
Training occurred between 10:00 AM and 2:00 PM for 7 consecutive
in length: two were open and two were enclosed by walls 40 cm
days. Females in the same stage of estrus were yoked on the first
high. The rat was placed into the central area facing one of the
day of escape training.
open arms and allowed to explore for 10 minutes. Time spent
Conditioning Procedure. Twenty-four hours after the last
and entries into open versus closed arms were recorded. To
day of escape training, rats were returned to the classical
measure gross motor activity, rats were placed in a 30 cm2
eyeblink conditioning chamber. Spontaneous blinks and re-
Plexiglas chamber equipped with eight photo beams 4 cm apart.
sponses to 10 white noise stimuli (83 dB, 250 milliseconds,
Beam breaks were used to detect activity.
5 millisecond rise/fall time) were recorded. Rats were then
Analysis of variance followed by Newman-Keuls post hoc
exposed to 300 trials of eyeblink conditioning per day for 2
analysis were used to analyze percentage of CRs during trace
consecutive days. We used the hippocampal-dependent version
conditioning, time and entries in the plus maze, and beam breaks
of this task known as trace conditioning, which is sensitive to sex
for locomotor activity.
differences and stressor exposure In the paradigm, a 250-millisecond burst of white noise
Experiment 3
conditioned stimulus (CS) (83 dB, 5 millisecond rise/fall time)
Fluoxetine's effectiveness in treating human mental disorders
was followed by a 100-millisecond, .7 mA periorbital shock
emerges only after weeks of continuous administration
unconditioned stimulus (US). The two stimuli were separated by
To determine whether treatment with fluox-
a 500-millisecond stimulus-free interval, and eyeblinks during
etine would only prevent the effects of stress if delivered
966 BIOL PSYCHIATRY 2004;56:964 –970
chronically, we next tested the effects of acute fluoxetine treat-ment. Females in diestrus-2 were acclimated to the classicalconditioning chamber and then given a single injection of eitherfluoxetine or vehicle and returned to their home cage. One hourlater, half of each group was exposed to the stressor of restraintand brief tailshocks or remained in their home cage (no stress).
Groups consisted of no stress females injected with vehicle(n ⫽ 7); stressed females injected with vehicle (n ⫽ 7); no stressfemales injected with fluoxetine (n ⫽ 10); and stressed femalesinjected with fluoxetine (n ⫽ 7). Twenty-four hours later, animalswere trace conditioned. Twenty-four hours after trace condition-ing, anxiety behavior in the plus maze and gross motor activitywere measured.
Experiment 1
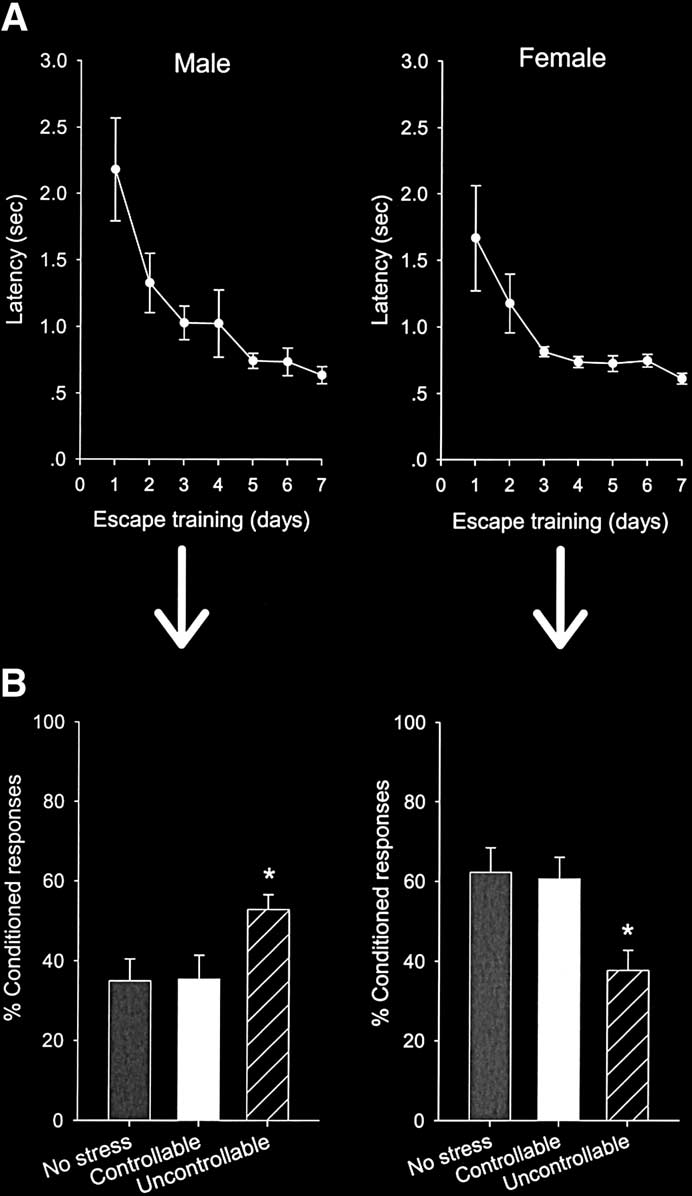
No Sex Differences in Escape Performance. During escape
training, latency to escape the footshock and enter the other sideof the shuttlebox was recorded and used as the measure ofperformance. As expected, latencies decreased dramatically overthe 7 days of training. In males, the mean latency decreased from2.2 seconds on the first day to .64 second on day 7 [F (6,42) ⫽7.41; p ⫽ .00002] In female rats, the mean latencydecreased from 1.7 seconds on the first day to .61 second on day7 [F (6,42) ⫽ 4.46; p ⫽ .001] We observed nodifference in escape latencies between male and female rats overthe 7 days of training (p ⫽ .17).
Sex Differences in Trace Conditioning. Analyzing perfor-
mance (percentage of CRs) in unstressed animals alone, therewas a main effect of sex (male or female) on the percentage ofCRs emitted during trace conditioning [F (1,18) ⫽ 10.85, p ⫽.004]. As shown previously, females emitted a greater percentageof CRs than males
Controllability Prevents the Effects of Stress on Trace Con-
ditioning in Males and Females. One day after escape training,
animals underwent trace conditioning using an eyeblink re-
sponse. Performance (percentage of CRs) was analyzed with
type of stressor (controllable, uncontrollable, no stress) as the
independent variable. In males, there was a main effect of
stressor type on the percentage of CRs [F (2,23) ⫽ 3.66; p ⫽ .04]
Males exposed to uncontrollable shock emitted
Figure 1. Controllability prevents the effects of stress on learning in males
more CRs than those exposed to the same number and amount
and females. Groups of male and female rats were trained to escape afootshock in a shuttle box apparatus in which one rat had to transverse the
of controllable shock (p ⫽ .03). There was no difference in the
cage to escape footshock (controllable). Yoked controls were exposed to the
percentage of CRs between unstressed males left in their home
same numbers and amount of stress but could not escape (uncontrollable).
cage before trace conditioning and those exposed to controllable
(A) Latencies to escape the shock decreased over the 7 days of training and
shock (p ⫽ .96). Therefore, exposure to controllable but not
did not differ between males and females. (B) Learning to escape prevented
uncontrollable stress enhanced trace conditioning in males.
the effects of stress on subsequent trace eyeblink conditioning in both
Neither stressor affected the numbers of spontaneous blinks (p ⫽
males and females. Thus, conditioning was enhanced in males exposed touncontrollable stress but not in those exposed to the same amount of
.65) or sensitized responses to the white noise stimulus (p ⫽ .66).
controllable stress. Conditioning was impaired in females that were ex-
In females, we also observed that uncontrollable but not
posed to uncontrollable stress but not in those exposed to the same amount
controllable stress affected subsequent trace conditioning; how-
of controllable stress. Values represent mean ⫾ SE. Significant differences
ever, the effect was in the opposite direction to that in males.
are noted with asterisks, p ⬍ .05.
There was an overall effect of stressor type on trace conditioning[F (2,23) ⫽ 5.5, p ⫽ .01] Females exposed to the
of stress on spontaneous blinking (p ⫽ .54) or responses to the
uncontrollable shock emitted fewer CRs when compared with
white noise stimulus (p ⫽ .11).
females that were exposed to the same number and amount ofcontrollable shock (p ⫽ .01). The percentage of CRs did not differ
Experiment 2
between unstressed females left in their home cage before trace
Sex Differences in Trace Conditioning Are Not Affected by
conditioning and those exposed to controllable shock (p ⫽ .84).
Fluoxetine. As in the first experiment, there were sex differ-
Therefore, exposure to uncontrollable but not controllable stress
ences in trace conditioning itself. Examining only the unstressed
reduced trace conditioning in females with no detectable effect
animals, females trained during proestrus emitted a greater
BIOL PSYCHIATRY 2004;56:964 –970 967
The Protective Effect of Fluoxetine in Females Is Not Neces-
sarily Associated with Anxiety.
After trace conditioning, we
assessed the effects of stressor exposure with and without antide-pressant treatment on anxiety-related behavior in the elevated plusmaze. This test creates a conflict between the exploratory drive ofthe rat and its innate fear of open spaces. Thus, increased open armexploration is thought to reflect a decrease in anxiety. In males,neither stressor exposure nor antidepressant treatment affectedpercent time in the open arms of the elevated plus maze (p ⫽ .64;p ⫽ .28, respectively) however, in females, there was amain effect of stress as well as a main effect of antidepressanttreatment on percent time in the open arms Specifically,both stressor exposure [F(1,31) ⫽ 4.45, p ⫽ .04] and fluoxetine[F(1,31) ⫽ 8.08, p ⫽ .008] decreased percent time in the open armsThese effects were not attributable to changes in activityin the plus maze, since neither stress nor fluoxetine affected thenumber of closed arm entries (p ⫽ .12; p ⫽ .13, respectively). Also,there was no effect of stress or fluoxetine on gross motor activity(p ⫽ .51; p ⫽ .81, respectively).
Figure 2. Chronic treatment (ⱖ 2 weeks) with the antidepressant fluoxetine
Experiment 3
prevents the effect of stress on learning in females but not males. (A) Exposure
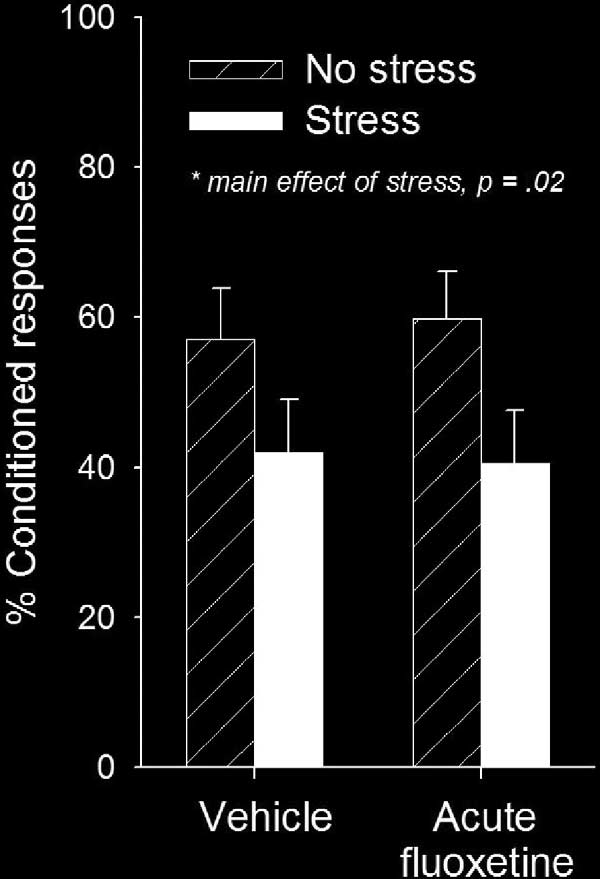
Acute Treatment with Fluoxetine Is Ineffective. A single
to the acute stressor of brief uncontrollable tailshocks enhanced conditioning
in males, irrespective of chronic treatment with fluoxetine. (B) Exposure to the
injection of fluoxetine before the stressful experience did not
tailshock stressor impaired conditioning in vehicle-treated females but not in
lessen the impact of stress on trace conditioning. With or without
those that received chronic fluoxetine. Values represent mean ⫾ SE. Significant
fluoxetine, females exposed to the stressor emitted fewer CRs
differences are noted with asterisks, p ⬍ .05.
than unstressed females [F (1,27) ⫽ 6.28, p ⫽ .02] There was no main effect of fluoxetine (p ⫽ .92) or interactionbetween stress and fluoxetine (p ⫽ .75) on the percentage of
percentage of CRs than males [F (1,33) ⫽ 15.50, p ⫽ .0004]. The
CRs. A single injection with fluoxetine did not affect anxiety
enhanced performance of females during trace conditioning
behavior in the elevated plus maze (p ⫽ .21). As in the previous
occurred irrespective of fluoxetine treatment.
experiment, exposure to the acute stressor decreased percent
Fluoxetine Prevents the Effect of Stress in Females but Not
time in the open arms [F (1,27) ⫽ 7.10, p ⫽ .01] (Vehicle no stress
The percentage of CRs was used as the dependent
⫽ 14 ⫾ 2; Vehicle stress ⫽ 7 ⫾ 3; Fluoxetine no stress ⫽ 22 ⫾ 4;
measure of performance. Stressor exposure (stress, no stress)
Fluoxetine stress ⫽ 9 ⫾ 4). The number of closed arm entries was
and antidepressant treatment (fluoxetine, vehicle) were indepen-
also decreased in response to stress (p ⫽ .002), but gross motor
dent variables. In males, there was a main effect of stress on the
activity was unaffected (p ⫽ .37). Overall, these data indicate that
percentage of CRs [F (1,32) ⫽ 4.7, p ⫽ .04] Irrespec-
acute exposure to fluoxetine does not prevent the effects of
tive of treatment with fluoxetine, males emitted more CRs after
stress on trace conditioning or anxiety-related behaviors in
stressor exposure when compared with unstressed males.
Chronic treatment with fluoxetine did not affect conditioningitself, since the percentage of CRs emitted by unstressed males
treated with fluoxetine did not differ from that of unstressedmales injected with vehicle (p ⫽ .22). There was no effect of
Previous studies have shown that exposure to an uncontrol-
stress or antidepressant treatment on spontaneous blinking (p ⫽
lable stressful event impairs associative learning in females, but
.07; p ⫽ .36, respectively) or responses to the white noise
enhances learning in males
stimulus (p ⫽ .56; p ⫽ .64, respectively).
Thus, females appear especially sensitive
In females, there was an interaction between antidepressant
to the detrimental consequences of stressful experience. This
treatment and stressor exposure on the percentage of CRs
observation is consistent with the clinical literature in whichwomen are at higher risk for stress-related illness such as
[F (1,31) ⫽ 8.16, p ⫽ .008] Exposure to the stressor
depression and posttraumatic stress disorder In
reduced the percentage of CRs in females injected with vehicle (p⫽ .01); however, stressor exposure did not alter the percentage
Table 1. Anxiety Behavior in the Elevated Plus Maze
of CRs in females treated with fluoxetine (p ⫽ .64). Importantly,treatment with fluoxetine prevented the effect of stress on trace
% Time in Open Arms
conditioning in females, since those injected with fluoxetine and
stressed emitted more CRs than those injected with vehicle andstressed (p ⫽ .02). As in males, there was no detectable effect of
Vehicle No Stress
23 ⫾ 8 (n ⫽ 9)
33 ⫾ 8 (n ⫽ 9)
fluoxetine on trace conditioning itself, since the percentage of
22 ⫾ 8 (n ⫽ 8)
22 ⫾ 6 (n ⫽ 9)
CRs emitted by unstressed females treated with fluoxetine was
Fluoxetine No Stress
17 ⫾ 8 (n ⫽ 10)
18 ⫾ 5 (n ⫽ 9)
not different from that of unstressed females injected with vehicle
Fluoxetine Stress
12 ⫾ 3 (n ⫽ 8)
7 ⫾ 2 (n ⫽ 8)
(p ⫽ .42). There was no effect of stress or antidepressant
In males, neither stressor exposure (p ⫽ .64) nor fluoxetine treatment (p
treatment on spontaneous blinking (p ⫽ .33; p ⫽ .55, respec-
⫽ .28) affected percent time in the open arms. In females, exposure to the
tively) or responses to the white noise stimulus (p ⫽ .49; p ⫽ .93,
stressor (p ⫽ .04) and fluoxetine (p ⫽ .008) decreased percent time in the
open arms, reflecting increased anxiety. Values represent mean ⫾ SEM.
968 BIOL PSYCHIATRY 2004;56:964 –970
However, the effect in males was unexpectedsince a "positive" response (that of enhanced learning) wasprevented by establishing control. To our knowledge, this is thefirst demonstration that controllability can prevent an enhance-ment of subsequent learning. As noted, learned helplessness andthe selective effects of uncontrollable stress on subsequentlearning have been promoted as an animal model of depression.
Since males without control were not helpless but were insteadfacilitated, these findings raise questions about the use of learnedhelplessness as an animal model for depression in humans. Infact, one might have expected the opposite result from thatpresented here–that "learning" how to control the stressor wouldfurther enhance their subsequent ability to learn. However,learning in males was essentially unaffected by controllablestress and not different from animals that were not exposed toany stressor experience. It could be argued that animals exposedto the uncontrollable stress experience the stressor differentlythan those exposed to the controllable stress. This is difficult torefute, although it has been shown that stress hormones such ascorticosterone are not different in animals exposed to uncontrol-lable versus controllable stress
In females, the effect of controllability was more expected in
that controllability prevented the detrimental effect of stress onnew learning. Helplessness effects have been notoriously diffi-cult to observe in female animals A recent study did report helplessnesseffects during specific stages of the estrous cycle, although theylikely reflect changes in performance rather than learning The detrimental effect of stress on classicalconditioning, on the other hand, appears to reflect a learningdeficit, at least to the extent that exposure to uncontrollable stress
Figure 3. Acute (1 day) fluoxetine treatment did not alter the stress effect on
learning in females. Females injected with vehicle and exposed to the acute
does not decrease the animal's sensitivity to the CS or the US
tailshock stressor produced fewer conditioned responses as did those in-
nor alter pain sensitivity or general
jected once with fluoxetine. Values represent mean ⫾ SE. Significant differ-
activity at the time of eyeblink conditioning
ence noted with asterisk.
Like those of the effect of stress onlearning is sensitive to stages of estrus and most evident when
the present set of experiments, we asked whether these effects of
estrogen levels are increasing
stress on learning in rats were sensitive to treatment strategies
Whether estrogen mediates the controllability effect reported
used in these patient populations, namely controllability and
here is unknown, since females were exposed to the escape
antidepressant treatment. There are a number of findings to
training procedures each day for 1 week and would have been
report from these studies but two stand out. The first is that the
stressed at least once during each stage of their cycle.
effects of stress on learning in both males and females werecompletely eliminated if the animals could establish control overthe stress, even though they were exposed to the very same
Antidepressants and Learning in Males Versus Females
numbers and amounts of shock. Thus, the opposite effects of
In a second experiment, we observed that daily treatment
stress on conditioning in males versus females are mediated by
with the serotonergic antidepressant fluoxetine (i.e., Prozac)
psychological aspects of the stressful experience, namely con-
prevented the effect of stress on trace conditioning in females but
trollability, and not the physical nature of the manipulation. The
not males. Treatment did not alter the overall rate of learning in
second notable finding reported here is that chronic treatment
either males or females, despite the presence of sex differences
with the commonly prescribed antidepressant fluoxetine pre-
in learning itself. Together, these findings may inform us about
vented the negative effect of stress on learning in females but had
the mechanisms whereby stress reduces performance in females
no effect in males. These data reveal sex differences in an
The effects of stress on learning
animal's response to antidepressant treatment, effects that may
can be interpreted in one of two ways; first, that stress directly
be important for understanding the prevalence of stress-related
impairs conditioning or second, that stress prevents the enhance-
mental disorders such as depression and PTSD in women. Each
ment in learning that normally occurs during proestrus. The
of these findings is discussed in turn below.
present data are consistent with the first explanation, sinceunstressed females (trained in proestrus) as well as those that
Controllability and Learned Helplessness in Males Versus
were stressed in the presence of fluoxetine responded more than
stressed females. Thus, treatment with fluoxetine did not prevent
The present findings are generally consistent with most
the enhanced learning that occurs in females during proestrus
findings using "learned helplessness" procedures, that is, con-
but rather prevented the effects of stress on learning.
trollability prevents the effects of stress from being expressed
The sex differences reported here are likely mediated by
hormonal substrates that mediate the effects of stress on learning.
BIOL PSYCHIATRY 2004;56:964 –970 969
For example, glucocorticoids are necessary for the enhancing
and associated with the acquisition of trace memories
effect of stress on conditioning in males, yet do not contribute
significantly to the impairment in females, which is insteaddependent on the ovarian hormone estrogen
Women and Stress-Related Mental Illness
Estrogen is considered a contributing
Although certainly not definitive, the present findings may
factor to the high incidence of stress-related mental illness in
model some aspects of depression and stress-related mental
women and interacts with serotonergic
illness in women. Not only are women twice as likely to
processes These interactions may explain
experience depression as men, they are also most vulnerable
why fluoxetine prevented the effects of stress in females but not
after stressful life events Their depression is
males. It is also possible that fluoxetine selectively targets
often accompanied by problems with declarative learning and
negative consequences of stress that just happen to be more
memory, which is responsive to antidepressant treatment
prevalent in females. One of the predictions from these results is
that women should be more responsive to SSRIs than men; this
trace conditioning is considered a declarative memory task
is indeed the case in depressed patients
our results may reflect aspects of these
learning deficits in humans Minimally, they indicate major sex differences in the
Antidepressants and Anxiety in Males Versus Females
response to uncontrollable stress and in response to antidepres-
As an indirect measure of anxiety, we evaluated behavior in
sants. These differences may be important for understanding
the elevated plus maze Three days after
why women are so susceptible to stress-related mental illness
stressor exposure, females were more anxious whereas males
such as depression, PTSD, and generalized anxiety disorder.
were not, suggesting a sustained effect of stress in females. Theincrease occurred irrespective of treatment with fluoxetine andwas not attributable to alterations in motor activity. Thus, the
This work was supported by the National Institute of Mental
effect of stress on anxiety appears dissociated from that on
Health (MH59970) and the National Alliance for Research on
learning and is generally consistent with studies in humans
Depression and Schizophrenia (TJS). BL was supported by a
For example, fluoxetine reportedly
enhances memory performance in depressed patients indepen-
(MH65368). We thank D. MacNeil for technical assistance and
dent of their emotional response or mood
Drs. L. Matzel and M. Friedman for comments.
Interestingly, chronic treatment (ⱖ 2 weeks) with fluoxetinealone produced an increase in anxiety in females. One might
Alves SE, Hoskin E, Lee SJ, Brake WG, Ferguson D, Luine V, et al (2002):
assume that antidepressants would reduce anxiety, but humans
Serotonin mediates CA1 spine density but is not crucial for ovariansteroid regulation of synaptic plasticity in the adult rat dorsal hippocam-
and laboratory animals are often more anxious during the first
pus. Synapse 45:143–151.
few weeks of treatment In
Austin MP, Mitchell P, Goodwin GM (2001): Cognitive deficits in depression:
conclusion, the data on stress and anxiety add to the evidence
Possible implications for functional neuropathology. Br J Psychiatry 178:
that females are especially vulnerable to the detrimental conse-
quences of stressful experience.
Bangasser DA, Shors TJ (2004): Acute stress impairs trace eyeblink condition-
ing in females without altering the unconditioned response. NeurobiolLearn Mem 82:57– 60.
Potential Neural Mechanisms of Antidepressant Effects
Bethea CL, Pecins-Thompson M, Schutzer WE, Gundlah C, Lu ZN (1999):
Fluoxetine was only effective in preventing the detrimental
Ovarian steroids and serotonin neural function. Mol Neurobiol 18:87–
effect of stress on learning in females if it was administered for
weeks. These data are consistent with the well-established
Beylin AV, Gandhi CC, Wood G, Talk AC, Matzel LD, Shors TJ (2001): The role
of the hippocampus in trace conditioning: Temporal discontiguity or
therapeutic delay seen in humans, an effect that has not been
task difficulty? Neurobiol Learn Mem 76:447– 461.
shown very often in the laboratory
Beylin AV, Shors TJ (2003): Glucocorticoids are necessary for enhancing the
Such a delayed response suggests that the
acquisition of associative memories after acute stressful experience.
effects of antidepressants are mediated by long-term changes in
Horm Behav 43:124 –131.
neuronal plasticity and/or anatomy One
Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS (2000):
brain region that may be involved is the hippocampal formation.
Hippocampal volume reduction in major depression. Am J Psychiatry157:115–118.
Clinical brain imaging studies have reported that hippocampal
Brown ES, Rush AJ, McEwen BS (1999): Hippocampal remodeling and dam-
volume is decreased in depressed patients
age by corticosteroids: Implications for mood disorders. Neuropsychop-
In rats, dendritic spines in the female
harmacology 21:474 – 484.
hippocampus decrease in number after stressor exposure and
Clark RE, Squire LR (1998): Classical conditioning and brain systems: The role
thus correlate with the decrease in learning after stress
of awareness. Science 280:77– 81.
Dendritic spines are
Cryan JF, Markou A, Lucki I (2002): Assessing antidepressant activity in
rodents: Recent developments and future needs. Trends Pharmacol Sci
sources of synaptic connectivity that are associated with the
23:238 –245.
formation of new memories, including those acquired during
Denti A, Epstein A (1972): Sex differences in the acquisition of two kinds of
classical eyeblink conditioning Since these
active avoidance behavior in rats. Physiol Behav 8:611– 615.
structures are sensitive to antidepressant treatment and manipu-
Foa E, Zinbarg RE, Olasov-Rothbaum B (1992): Uncontrollability and unpre-
lations of serotonin
dictability in post-traumatic stress disorder: An animal model. Psychol
fluoxetine may prevent the effects of stress on learning by
Bull 112:218 –238.
Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ (1999): Learning enhances
its effects on spine density. Antidepressants are also known to
adult neurogenesis in the hippocampal formation. Nat Neurosci 2:260 –
affect the production of new neurons in the hippocampus
which are sensitive to stress and anxiety-
Gould E, Tanapat P (1999): Stress and hippocampal neurogenesis. Biol Psy-
related behaviors
970 BIOL PSYCHIATRY 2004;56:964 –970
Grillon C, Hill J (2003): Emotional arousal does not affect delay eyeblink
Requirement of hippocampal neurogenesis for the behavioral effects of
conditioning. Brain Res Cogn Brain Res 17:400 – 405.
antidepressants. Science 301:805– 809.
Jenike MA, Buttolph L, Baer L, Ricciardi J, Holland A (1989): Open trial of
Seligman MEP (1975): Helplessness. San Francisco: Freeman.
fluoxetine in obsessive compulsive disorder. Am J Psychiatry 146:909 –
Seligman MEP, Maier SF (1967): Failure to escape traumatic shock. J Comp
Psysiol Psychol 74:1–9.
Jenkins JA, Williams P, Kramer GL, Davis LL, Petty F (2001): The influence of
Servatius RJ, Brennan FX, Beck KD, Beldowicz D, Coyle-DiNorcia K (2001):
gender and the estrous cycle on learned helplessness in the rat. Biol
Stress facilitates acquisition of the classically conditioned eyeblink re-
sponse at both long and short interstimulus intervals. Learn Motiv 32:
Joyce PR, Mulder RT, Luty SE, McKenzie JM, Rae AM (2003): A differential
response to nortriptyline and fluoxetine in melancholic depression: The
Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW (1996): Hip-
importance of age and gender. Acta Psychiatr Scand 108:20 –23.
pocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A
Kendler KS (1998): Gender differences in the genetic epidemiology of major
93:3908 –3913.
depression. J Gend Specif Med 1:28 –31.
Shors TJ (2004): Learning during stressful times. Learn Mem 11:137– 44.
Kendler KS, Thornton LM, Gardner CO (2000): Stressful life events and previ-
Shors TJ, Chua C, Falduto J (2001a): Sex differences and opposite effects of
ous episodes in the etiology of major depression in women: An evalua-
stress on dendritic spine density in the male versus female hippocam-
tion of the "kindling" hypothesis. Am J Psychiatry 157:1243–1251.
pus. J Neurosci 21:6292– 6297.
Kirk RC, Blampied NM (1985): Activity during inescapable shock and subse-
Shors TJ, Falduto J, Leuner B (2004): The opposite effects of stress on den-
quent escape avoidance learning: Female and male rats compared. NZ
dritic spines in male versus female rats are NMDA receptor dependent.
J Psychol 14:9 –14.
Eur J Neurosci 19:145–150.
Leuner B, Falduto J, Shors TJ (2003): Associative memory formation in-
Shors TJ, Leuner B (2003): Estrogen-mediated effects on depression and
creases the observation of dendritic spines in the hippocampus. J Neu-
memory formation in females. J Affect Disord 74:85–96.
rosci 23:659 – 665.
Shors TJ, Lewczyk C, Pacynski M, Matthew PR, Pickett J (1998): Stages of
Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors
estrous mediate the stress-induced impairment of associative learning
TJ (2004): Learning enhances the survival of new neurons beyond the
in the female rat. Neuroreport 9:419 – 423.
time when the hippocampus is required for memory. J Neurosci 24:
Shors TJ, Miesegaes G (2002): Testosterone in utero and at birth dictates how
stressful experience will affect learning in adulthood. Proc Natl Acad Sci
U S A 99:13955–13960.
Leuner B, Shors TJ (2004): New spines, new memories. Mol Neurobiol 29:117–
Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001b): Neuro-
genesis in the adult is involved in the formation of trace memories.
Levkovitz Y, Caftori R, Avital A, Richter-Levin G (2002): The SSRI drug fluox-
etine, but not the noradrenergic tricyclic drug desipramine, improves
Shors TJ, Seib TB, Levine S, Thompson RF (1989): Inescapable versus escap-
memory performance during acute major depression. Brain Res Bull 58:
able shock modulates long-term potentiation in the rat hippocampus.
Science 244:224 –226.
Maier SF, Jackson RL (1979): Learned helplessness: All of us were right (and
Shors TJ, Weiss C, Thompson RF (1992): Stress-induced facilitation of classi-
wrong): Inescapable shock has multiple effects. In: Bower B, editor. Ad-
cal conditioning. Science 257:537–539.
vances in Learning and Motivation. New York: Academic Press, 155–215.
Silva MTA, Alves CRR, Santarem EMM (1999): Anxiogenic-like effect of acute
Maier SF, Ryan SM, Barksdale CM, Kalin NH (1986): Stressor controllability
and chronic fluoxetine on rats tested on the elevated plus maze. Braz
and the pituitary adrenal system. Behav Neurosci 100:669 – 674.
J Med Biol Res 32:333–339.
Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000): Chronic antidepressant
Steenbergen HL, Heinsbroek RPW, Van Hest A, Van de Poll NE (1990): Sex-
treatment increases neurogenesis in adult rat hippocampus. J Neurosci
dependent effects of inescapable shock administration on shuttlebox-
20:9104 –9110.
escape performance and elevated plus maze behavior. Physiol Behav
Martenyi F, Dossenbach M, Mraz K, Metcalfe S (2001): Gender differences in
the efficacy of fluoxetine and maprotiline in depressed patients: A dou-
Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER,
ble-blind trial of antidepressants with serotonergic or norepinephriner-
Snow J, et al (2004): Hippocampal volume, memory and cortisol status in
gic reuptake inhibition profile. Eur Neuropsychopharmacol 11:227–232.
major depressive disorder: Effects of treatment. Biol Psychiatry 56:101–
Martin P, Soubrie P, Puech AJ (1990): Reversal of helpless behavior by sero-
tonin uptake blockers in rats. Psychopharmacology (Berl) 101:403– 407.
Wall PM, Messier C (2001): Methodological and conceptual issues in the use
Minor TR, Dess NK, Overmier JB (1991): Inverting the traditional view of
of the elevated plus maze as a psychological measurement instrument
"learned helplessness". In: Denny MR, editor. Fear, Avoidance and Pho-
of animal anxiety-like behavior. Neurosci Biobehav Rev 25:275–286.
bias: A Fundamental Analysis. Hillsdale, NJ: Lawrence-Erlbaum Assoc.,
Willner P (1990): Animal models of depression: An overview. Pharmacol Ther
45:425– 455.
Nestler EJ, Gould E, Manji H, Bucan M, Duman RS, Gershenfeld HK, et al
Wong M-L, Licinio J (2001): Research and treatment approaches to depres-
(2002): Preclinical models: Status of basic research in depression. Biol
sion. Nature Rev Neurosci 2:343–361.
Wood GE, Beylin A, Shors TJ (2001): The contribution of adrenal and repro-
Norrholm SD, Ouimet CC (2001): Altered dendritic spine density in animal
ductive hormones to the sexually opposed effects of stress on trace
models of depression and in response to antidepressant treatment.
conditioning. Behav Neurosci 115:1–13.
Wood GE, Shors TJ (1998): Stress facilitates classical conditioning in
Overmier JB, Seligman MEP (1967): Effects of inescapable shock on subse-
males, but impairs classical conditioning in females through activa-
quent escape and avoidance learning. J Comp Physiol Psychol 63:23–33.
tional effects of ovarian hormones. Proc Natl Acad Sci U S A 95:4066 –
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al (2003):
Source: http://njc.rockefeller.edu/PDF_BN08/topic%203-%20shors-controlability.pdf
HOUSING POLICIES IN THE EUROPEAN UNION Christian Donner / Vienna 2000 Financing of housing (section A 5) is one of the most important areas in housing policy. Long-term fi- nancing for developers and investors or for buyers The present study consists of four parts. Part A of (owner-occupied) housing is dominated by contains a compact theory of housing markets and
The PVEout project within the European 5th framework, NRU T. Dyrby, 06-07-03 The pipeline program for PVElab PARTIAL VOLUME ERROR .3 THE PIPELINE PROGRAM .4 THE USER-INTERFACE .4 Restrictions using project .7 Syntax and indices.8 The project field .9 The pipeline field and sub-fields .10 The taskDone field and its sub-fields .12


