Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Nsif.org.uk
Cell Transplantation, Vol. 23, pp. 1631–1655, 2014
0963-6897/14 $90.00 + .00
Printed in the USA. All rights reserved.
Copyright Ó 2014 Cognizant Comm. Corp.
Functional Regeneration of Supraspinal Connections in a Patient With
Transected Spinal Cord Following Transplantation of Bulbar Olfactory
Ensheathing Cells With Peripheral Nerve Bridging
Pawel Tabakow,* Geoffrey Raisman,† Wojciech Fortuna,*‡ Marcin Czyz,* Juliusz Huber,§ Daqing Li,†
Pawel Szewczyk,¶ Stefan Okurowski,# Ryszard Miedzybrodzki,**†† Bogdan Czapiga,* Beata Salomon,‡‡
Agnieszka Halon,§§ Ying Li,† Joanna Lipiec,§ Aleksandra Kulczyk,§ and Wlodzimierz Jarmundowicz*
*Department of Neurosurgery, Wroclaw Medical University, Wroclaw, Poland
†Spinal Repair Unit, Department of Brain Repair and Rehabilitation, UCL Institute of Neurology, London, UK
‡Ludwik Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wroclaw, Poland
§Department of Pathophysiology of Locomotor Organs, Karol Marcinkowski Medical University, Poznan, Poland
¶Department of General Radiology, Interventional Radiology and Neuroradiology, Wroclaw Medical University, Wroclaw, Poland
#Neurorehabilitation Center for Treatment of Spinal Cord Injuries AKSON, Wroclaw, Poland
**Bacteriophage Laboratory of the Ludwik Hirszfeld Institute of Immunology, and Experimental Therapy,
Polish Academy of Sciences, Wroclaw, Poland
††Department of Clinical Immunology of the Transplantation Institute, Medical University of Warsaw, Warsaw, Poland
‡‡University Clinical Hospital, Wroclaw, Poland
§§Department of Pathomorphology and Oncological Cytology, Wroclaw Medical University, Wroclaw, Poland
Treatment of patients sustaining a complete spinal cord injury remains an unsolved clinical problem because of
the lack of spontaneous regeneration of injured central axons. A 38-year-old man sustained traumatic transec-
tion of the thoracic spinal cord at upper vertebral level Th9. At 21 months after injury, the patient presented
symptoms of a clinically complete spinal cord injury (American Spinal Injury Association class A-ASIA A).
One of the patient's olfactory bulbs was removed and used to derive a culture containing olfactory ensheathing
cells and olfactory nerve fibroblasts. Following resection of the glial scar, the cultured cells were transplanted
into the spinal cord stumps above and below the injury and the 8-mm gap bridged by four strips of autologous
sural nerve. The patient underwent an intense pre- and postoperative neurorehabilitation program. No adverse
effects were seen at 19 months postoperatively, and unexpectedly, the removal of the olfactory bulb did not
lead to persistent unilateral anosmia. The patient improved from ASIA A to ASIA C. There was improved trunk
stability, partial recovery of the voluntary movements of the lower extremities, and an increase of the muscle
mass in the left thigh, as well as partial recovery of superficial and deep sensation. There was also some indica-
tion of improved visceral sensation and improved vascular autoregulation in the left lower limb. The pattern of
recovery suggests functional regeneration of both efferent and afferent long-distance fibers. Imaging confirmed
that the grafts had bridged the left side of the spinal cord, where the majority of the nerve grafts were implanted,
and neurophysiological examinations confirmed the restitution of the integrity of the corticospinal tracts and
the voluntary character of recorded muscle contractions. To our knowledge, this is the first clinical indication
of the beneficial effects of transplanted autologous bulbar cells.
Key words: Paraplegia; Cell transplantation; Repair; Regeneration
the nasal mucosa to make synaptic connections in the
Olfactory ensheathing cells (OECs) are a population of
olfactory bulb (OB) (9,28,34,36). Animal experiments
glial cells residing both in the peripheral and central ner-
have shown that transplantation of OECs and ONFs cul-
vous systems. Together with their accompanying enve-
tured from the OB mediate regeneration and functional
lope of olfactory nerve fibroblasts (ONFs), they enfold
reconnection of severed axons in spinal cord and brachial
the bundles of olfactory nerve fibers in their course from
plexus injuries (20,22,23,35,37), whereas cells cultured
Received February 18, 2014; final acceptance September 8, 2014. Online prepub date: October 21, 2014.
Address correspondence to Pawel Tabakow, M.D., Ph.D., Department of Neurosurgery, Wroclaw Medical University, Borowska str. 213, 50-556 Wroclaw, Poland. Tel: +48 606 137 846; Fax: +48 71 734 34 09; E-mail:
[email protected]
TABAKOW ET AL.
from the olfactory mucosa have only minor benefits in corticospinal tract injuries (46) and do not appear to mediate regeneration of severed axons (18,46). Clinical trials of transplantation of autologous OECs into spinal cord injuries (SCIs) have up to now been based on the more accessible cells obtained from the olfactory mucosa (6,14,26,27,30,43). While these studies serve to establish the safety of the procedure, there was, as in the animal studies, little (26,27,43) or no neurological improvement (6,30). In an experimental study of dorsal root repair in rats, we showed that bulbar, but not mucosal, OECs/ONFs can make a bridge for severed axons to cross from a peripheral nerve into the spinal cord (18). Based on this, we now describe the results from the treatment of an ASIA A patient (American Spinal Injury Association class A) with an almost total transection of the thoracic spinal cord, who underwent the operation of scar resec-tion, transplantation of cultured autologous bulbar OECs/ONFs, and reconnection of the spinal cord stumps with autologous sural nerve grafts, followed by a 19-month course of postoperative rehabilitation.
MATERIALS AND METHODS
A 38-year-old male with a complete chronic thoracic
SCI was qualified for the study. Thirteen months previ-ously, he had sustained a penetrating SCI at upper verte-bral level Th9, caused by a knife assault. Serial magnetic resonance imaging (MRI) studies of the thoracic spine showed an 8-mm-long gap between the spinal cord stumps. The stumps remained connected only by a 2-mm
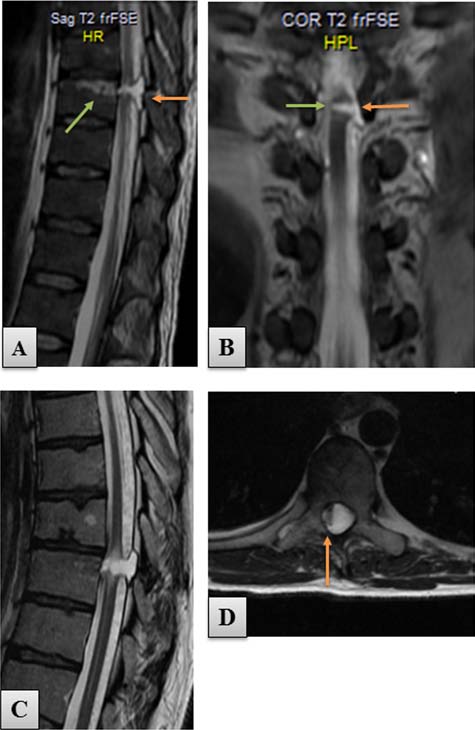
Figure 1. T2-weighted MRI scans of the thoracic spine per-
rim of spared tissue in the area of the right spinal cord lat-
formed preoperatively. (A) Sagittal view of the spinal cord
eral column (Fig. 1). Initial neurological examination of
transection at upper vertebral level Th9. A 5-mm gap of spi-
the patient showed a complete loss of sensory and motor
nal cord continuity (orange arrow) was present 1 month after
the injury. Green arrow indicates the area of the vertebral body
function below the injury (ASIA A), with a zone of partial
affected by the knife injury. (B) Coronal scan of the same study
sensory preservation at level Th9. Transcranial magnetic
showing the area of SCI (orange arrow). Note the thin rim of
motor-evoked potentials (MEPs) and electromyography
spared tissue connecting both spinal cord stumps (green arrow).
(EMG) excluded any response of the lower extremity
(C) Sagittal MRI scan performed 21 months after the injury. The
(LE) muscles to motor cortex activation and any volun-
posttraumatic gap increased its size to 8 mm because of progres-
sive spinal cord degeneration, seen as hyperdense myelopathic
tary muscle activity.
"caps" adjacent to the focus of injury. (D) Axial view of the area
The patient met all the general and neurological crite-
of spinal cord transection showing a 2-mm-thick tissue connect-
ria to be qualified for the OEC transplantation protocol
ing both spinal cord stumps (orange arrow). frFSE, fast relax-
as described in our recently completed phase I clinical
ation fast spin echo.
trial (43). However, a diagnosis of chronic allergic sinus-itis and nasal polyps (Fig. 2A) was a contraindication for using the olfactory mucosa for obtaining OECs. Initially, the patient was bilaterally anosmic, but after performance
one of his OBs for OEC isolation and subsequent trans-
of an endoscopic bilateral anterofrontoethmoid sphe-
plantation of cultured OECs/ONFs into the lesioned spi-
noidectomy, he regained his smell perception due to the
nal cord. The patient provided a written informed consent
improved airflow in the nasal cavity (Fig. 2B). In this
according to the Declaration of Helsinki and understood
situation, the only OEC-containing tissue not directly
the risk of the trial and the potential for no benefit. The
involved in the nasal pathology was the OB. The patient
study was approved by the Bioethics Committee of
was offered a new two-stage therapeutic approach con-
Wroclaw Medical University, according to the guidelines
sisting of the performance of a craniotomy for obtaining
of the National Health Council of Poland.
SPINAL CORD REPAIR: OECs AND NERVE GRAFTS
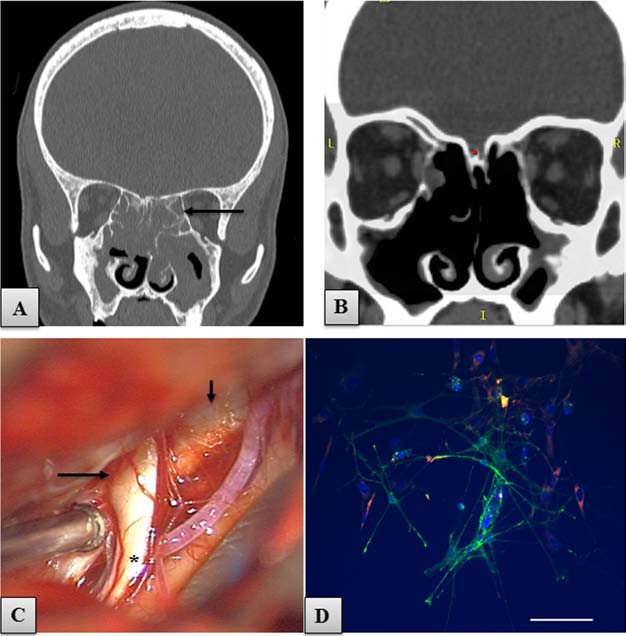
Figure 2. Imaging of head, nasal cavity. and cells to be transplanted. (A) Coronal CT scan of the head. An almost total obliteration
of the nasal cavity and paranasal sinuses due to sinusitis is apparent. Black arrow, affected ethmoid sinuses. (B) Coronal CT scan
performed after the operation of endoscopic anterofrontoethmoid sphenoidectomy, showing an improved air flow through the sinuses.
(C) Photo taken from the operating microscope, showing the region of left olfactory groove with the posterior part of the OB (long
arrow), olfactory tract (asterisk), and crista galli (short arrow). (D) Microphotographs of p75 low affinity nerve growth factor receptor-
positive (p75/NGFR+) bulbar OECs (green) and fibronectin (FN)-positive ONFs (red), taken before cell transplantation, on the 11th
day of culture. Hoechst, blue nuclear staining. Scale bar: 100 µm.
locomotor training, and 1 h of sensory training. The main
Before being qualified for the study, it was established
emphasis was set on locomotor training that included
that the patient had shown no neurological improvement
training of individual muscle groups of the lower limbs,
during a number of different rehabilitation programs
training for posture and balance, and overground walking
over the first 13 months after injury. These programs
exercises. For each exercise, the patient's legs were posi-
were incomplete and were interrupted for longer periods
tioned in a way to have maximal support, and a specific
because of the necessity for treatment of infections of the
load (using weights attached to ropes) was used to enable
respiratory tract, pressure ulcers, and inflammation of the
the movement in the joints of paralyzed limbs. Each
deep venous system of the lower limbs. For this reason,
exercise had a fixed number of repetitions. If the patient
the patient was subjected to an additional intense neu-
showed any improvement in the performed exercise, the
rorehabilitation program for 8 months before the planned
load was changed in a way to increase the difficulty of
experimental treatment to confirm that there would be
the task, as well as increase the number of repetitions.
no spontaneous recovery. This program was previously
The minimal increase of the load was 100 g. The walking
applied in our paraplegic patients qualified for the phase
exercise was performed with emphasis to the assessment
I clinical trial concerning transplantation of autologous
of the Walking Index (WI) (8), starting from the attempts
olfactory mucosal OECs (43). The patient was trained for
to stand and walk in parallel bars with braces and the
5 h/day, 5 days/week. The training agenda consisted of
assistance of two persons. Postoperative rehabilitation
1 h of range-of-motion and stretching exercises, 3 h of
was planned for at least 24 months.
TABAKOW ET AL.
Obtaining the Olfactory Bulb
dishes were maintained in a humidified incubator at 37°C
Before the operation, standard laryngological tests
in 5% CO . On the fourth day in vitro (DIV), the superna-
for evaluation of nostril patency and for assessment of
tant containing the nonadherent cells was transferred into
respiratory epithelium function (the saccharine test) were
new PLL-coated dishes. The cells were fed every second
performed. Then, the patient's smell perception was
day by replacing half of the complete culture medium
tested using a scale for evaluation of smell perception
volume. On the 12th DIV, the cells became nearly conflu-
we described previously (43). For obtaining the OB, a
ent, and the cultures were harvested using CTSTM TrypLE
left-sided frontolateral craniotomy was performed under
(Gibco). The enzymatic digestion was stopped by add-
general anesthesia. As he had large frontal sinuses, they
ing complete culture medium, spun down at 300 × g for
were opened during craniotomy, cleared from the sinus
5 min, and resuspended. The tubes were placed on ice
mucosa, and cranialized at the end of the procedure with
in a fridge and transported immediately to the operating
autologous muscle and periosteum. Brain relaxation was
theater. After five subsequent rounds of washing using
achieved by a lumbar drainage and by opening of some
CTSTM DPBS, the cells were resuspended in appropriate
skull base arachnoid cisterns. Drilling of the skull base
volume of CTSTM DPBS and transferred to a glass syringe
ethmoid eminences was necessary because the OB was
(World Precision Instruments, Sarasota, FL, USA) for
localized deep inside the olfactory groove. The OB was
obtained in two pieces with microsurgical instruments
At the 10th DIV, a 5-ml aliquot of the cells was fixed
using the subfrontal microscopic endoscopy-assisted
with 4% paraformaldehyde (Sigma) in phosphate-buffered
approach (Fig. 2C). The choice of the left OB was based
saline (PBS; IITD, Wroclaw, Poland) for 30 min, washed
on the better smell perception on the left and the lesser
three times in PBS, permeabilized, and blocked with 2%
atrophy of the left OB in MRI. The tissue samples were
skim milk (Merck, Darmstadt, Germany) in PBS contain-
maintained at 4°C and transported to the culture facility
ing 0.1% Triton X-100 (Serva, Heidelberg, Germany).
in a complete culture medium consisting of 1:1, v:v, of
Primary antibodies in PBS containing 2% milk and 0.1%
Dulbecco's modified Eagle's medium and Ham's F12
Triton X-100 were applied overnight at 4°C. The cells
(CTSTM KnockOutTM DMEM/F12; Gibco, Grand Island,
were washed three times in PBS and incubated with fluo-
NY, USA), supplemented with 10% fetal bovine serum
rescent secondary antibodies in PBS containing 2% milk
(FBS ATMP-Ready, g irradiated; PAA, Pasching, Austria),
and 0.1% Triton X-100 for 90 min at room temperature
1 mM CTSTM KnockOutTM GlutaMAX-I Supplement
in the dark. After washing four times in PBS, cells were
(complete culture medium, Gibco), 100 U/ml penicillin,
counterstained with Hoechst 33342 (1 µg/ml, Sigma),
and 100 µg/ml streptomycin (Polfa Tarchomin Warszawa,
washed twice with PBS, and mounted using ProLong
Gold Antifade (Life Technologies, Rochester, NY, USA). Primary antibodies were 1:100 monoclonal mouse anti-
Cell Culture
low affinity nerve growth factor receptor (anti-p75;
Cultures of the patient's bulbar OECs were performed
clone NGFR5; Invitrogen) and 1:500 polyclonal rabbit
according to Miedzybrodzki et al. (31) with slight modifi-
anti-human fibronectin Ig (Dako, Glostrup, Denmark).
cations. The two fragments of OB tissue were transferred
Secondary antibodies were Alexa Fluor 488 goat anti-
to a 100-mm polystyrene Petri dish (Becton Dickinson,
mouse IgG (H + L) and Alex Fluor 546 goat anti-rabbit
Franklin Lakes, NJ, USA) and washed in 15 ml of CTSTM
IgG (H + L) (all 1:500; Molecular Probes, Invitrogen,
DPBS containing calcium and magnesium, without phe-
Carlsbad, CA, USA). In all assays, controls were per-
nol red (Gibco). The blood vessels and meninges were
formed by incubating cells with secondary antibodies.
carefully peeled off under a dissecting microscope and
Images of fluorescent-labeled cells were captured using
the tissue cut into pieces of 2 mm using a razor blade
a Floid fluorescence microscope (Life Technologies)
and incubated at 37°C in 0.25% trypsin solution (Sigma,
equipped with Floid Imagine Station Software. Images
St. Louis, MO, USA) for 15 min. The enzyme activity
were exported for further analysis to the ImageJ 1.46r
was stopped by adding complete culture medium, and
software (NIH, Bethesda, MD, USA).
the tissue was repeatedly triturated by passing through the tip of a 1-ml pipette (Costar, Corning, Amsterdam,
Sterility Tests
The Netherlands). The suspension was twice spun down
A 100-µl aliquot of supernatant from the culture
at 300 × g for 5 min and the pellet again gently triturated
medium was taken on the 5th, 9th, and 12th DIV for the
in 2 ml of complete culture medium. The dissociated cells
assessment of development of bacterial or fungal infec-
were seeded on two polystyrene dishes (9.6 cm2, Nunc,
tion. The samples were transferred to transport swabs
Roskilde, Denmark) and one four-well polystyrene plate
(Hagmed, Rawa Mazowiecka, Poland) and delivered to
(1.9 cm2, Nunc) coated with 0.1 mg/ml poly-l-lysine
the Microbiology Department of the Wroclaw Medical
hydrobromide (PLL, 30–70 kDa, Sigma). The culture
University (Wroclaw, Poland).
SPINAL CORD REPAIR: OECs AND NERVE GRAFTS
2 mm apart. The posterolateral sulcus was chosen as the entry point for cell microinjection. The cell suspension
Preoperative Preparation. The patient was readmitted
was planned to be delivered at five depths at each injec-
to the Department of Neurosurgery (Wroclaw Medical
tion site, 0.5 mm apart. Another target for cell microinjec-
University) for the operation of cell transplantation 12
tion was the rim of spared tissue connecting both spinal
days after OB retrieval. Based on data from the axial
cord stumps. We also planned to reconstruct the 8-mm
T2-weighted MRI scans, a virtual three-dimensional
gap between the spinal cord stumps with the patient's
model of the spinal cord lesion was built. Then a schematic
own peripheral nerve grafts.
grid for cell microinjection was elaborated with specific topographic reference points on the surface of the spi-
Surgical Technique. The patient was placed under gen-
nal cord for intraoperative navigation of the stereotactic
eral anesthesia in a prone position. After identification of
injection device, according to our previous experimental
the level of operation with fluoroscopy, a midline skin
protocol (43) (Fig. 3A). Briefly, the cell microinjections
incision was made, followed by dissection of the paraver-
were planned to be done in a matrix pattern into the lat-
tebral muscles and laminectomy of the thoracic vertebrae
eral columns of the spinal cord stumps, above and below
Th7-8-9. Under an operating microscope (OPMI Pentero,
the lesion epicenter. This matrix consisted of four rows,
Zeiss Company, Jena, Germany), an adhesion between the
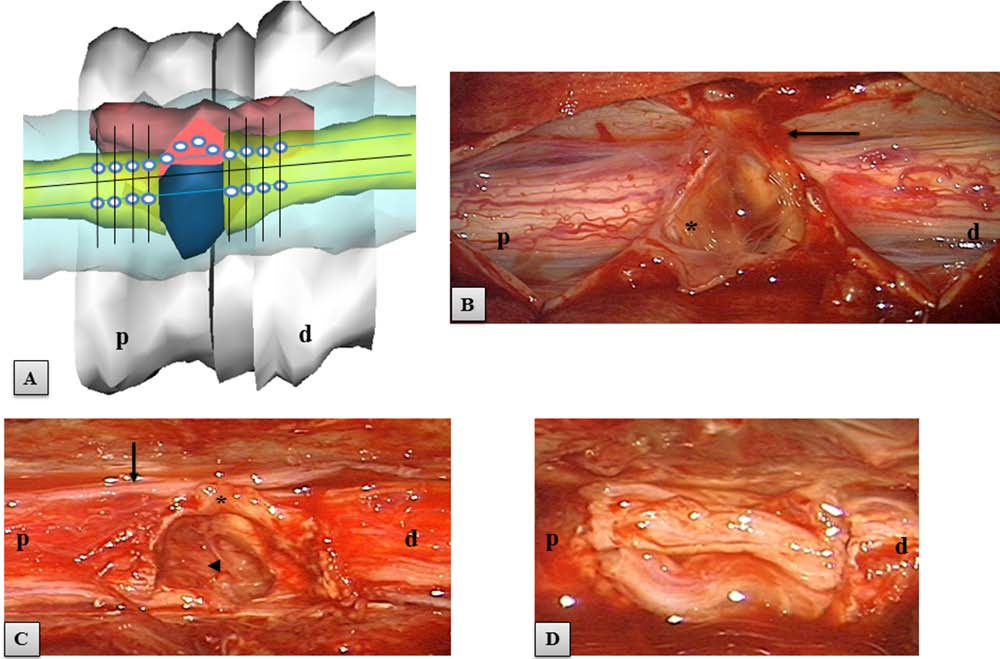
Figure 3. Area of the SCI. (A) A virtual 3D model of the spine and area of SCI. The spinal cord stumps in yellow, the posttraumatic
CSF-containing gap in blue, rim of tissue connecting stumps in dark red. For each spinal cord stump, the schematic grid for cell micro-
injection consisted of four rows (black lines), 2 mm apart. White dots, the sites of planned cell microinjection in the posterolateral
sulcus (blue lines) and in the rim of tissue connecting the stumps; p, proximal spinal cord stump; d, distal stump. (B–D) Intraoperative
microscopic images of the area of spinal cord transection. (B) Initial view of the spinal cord after opening of the dura mater. The area
of spinal cord transection was covered with yellowish scar tissue (asterisk). Arrow indicates an adhesion between the scar and dura.
(C) View of the spinal cord after myeloadhesiolysis and resection of the intraparenchymal scar. This maneuver led to an increase in
the initial gap from 8 to 10 mm. Asterisk, the rim of spared tissue connecting both stumps; arrow, a thoracic spinal nerve. Arrowhead,
the dura of the ventral surface of the spinal canal, which confirmed that the spinal cord stumps were totally disconnected in this area.
(D) View of the spinal cord gap reconstructed with four implanted strips of nerves (two strips were placed ventrally and two strips
dorsally), before their fixation with fibrin sealant.
TABAKOW ET AL.
injured dura and the spinous process Th8 was removed. A
30 min), followed by a constant infusion of 5.4 mg/kg/h ×
midline durotomy was performed, followed by sharp dis-
23 h, together with a prophylactic antacid therapy.
section of the posttraumatic adhesions between the spinal cord surface and the dura. The area of SCI consisted of
two separated spinal cord stumps, covered with yellowish
Immunohistochemical examination was performed on
scar tissue (Fig. 3B). This tissue was removed and speci-
tissue samples taken for routine diagnostic purposes from
mens taken for histology. This maneuver increased the
scar tissue in the spinal cord. Formalin-fixed, paraffin-
gap between the stumps from 8 mm to 10 mm (Fig. 3C).
embedded tissue was freshly cut (4 µm). Immuno-
A 2-mm thin rim of spared tissue connected the margin
histochemistry was performed as previously described
of the cord stumps on the right. After completion of the
(17) using the following antibodies diluted in Antibody
myeloadhesiolysis (spinal cord untethering), the system
Diluent, Background Reducing (Dako): epithelial mem-
for stereotactic cell microinjection was mounted on the
brane antigen (EMA; clone E29; monoclonal mouse,
operating table, as described in our previous study (43).
dilution 1:100, Dako), S100 (rabbit polyclonal, dilution
Briefly, it was composed of an automatic micropump
1:400, Dako), neurofilament (NF; clone 2F11; mono-
(UltraMicro Pump III, World Precision Instruments) and a
clonal mouse, dilution 1:100, Dako), vimentin (clone V9;
three-dimensional micromanipulator (SM-15, Narishige,
monoclonal mouse, dilution 1:100, Dako), and glial fibril-
Tokyo, Japan). The injector device was fitted with a 25-µl
lary acidic protein (GFAP; rabbit polyclonal, dilution 1:500,
glass syringe (World Precision Instruments). The autolo-
Dako). Hematoxylin–eosin (Sigma) counterstaining was
gous OEC/ONF mixture was suspended in serum-free
culture medium, centrifuged, and added to an Eppendorf vial, and a 25-µl glass syringe with a 26-gauge beveled
Initial and Repeated Assessments
needle was filled with the cells. The cell mixture was
The patient's medical condition was evaluated regu-
injected through the posterolateral sulcus between the
larly pre- and postoperatively. This included general
dorsal nerve rootlets into the lateral columns of the spi-
medical assessment, otorhinolaryngological, neurologi-
nal cord, proximally and distally for a distance of 8 mm
cal, physiotherapeutic, and psychological tests, as well as
on either side of the lesion epicenter. The remaining cell
radiological and neurophysiological studies. All studies
suspension was injected into the rim of tissue connecting
were performed by the same assessors. They were not
both spinal cord stumps. The parameters of cell micro-
injection are summarized in Table 1. At the end of the
A detailed general medical assessment was conducted
procedure of microinjection, a small aliquot of cell sus-
preoperatively and in the first 5 weeks postsurgery and
pension remaining in the Hamilton syringe was taken for
included hematology, blood chemistry, and urine analy-
sterility tests and monitored in continued cell culture. In
sis. Tests for the presence of anti-HIV antibodies were
the last stage of the operation, a 6-cm-long fragment of
performed preoperatively, and hepatitis B and C status
the patient's left sural nerve was harvested. Four 12-mm
was assessed. Standard electrocardiographic studies and
strips of nerve grafts were used for reconnection of the
chest X-rays (Philips, Amsterdam, Netherlands) were
spinal cord stumps (Fig. 3D). The nerves were positioned
also performed. Microbiological studies of blood, urine,
along the long axis of the spinal cord tracts and were fixed
or cerebrospinal fluid (CSF) were planned to be con-
to the spinal cord stumps with fibrin sealant (Tisseel Lyo,
ducted only in case of suspicion of infection.
Baxter AG, Vienna, Austria). The dura was closed with
Laryngological examination included smell percep-
absorbable sutures. No duraplasty was performed. A wound
tion tests, as well as evaluation of computed tomography
drain was placed under the muscle layer, and the wound
(CT) and MRI scans of the head, nasal cavity, and para-
was closed in layers. During the operation, the patient
nasal sinuses.
received intravenous methylprednisolone (Solu-Medrol,
Neurological examination was performed monthly
Pfizer, Kent, UK) in a bolus of 30 mg/kg (over 15 to
and included ASIA examination, examination of deep
Table 1. Parameters of Cell Microinjection
Single Injection
OECs, olfactory ensheathing cells.
SPINAL CORD REPAIR: OECs AND NERVE GRAFTS
sensation, evaluation of spasticity using the Ashworth
of uroflowmetry, pressure flow studies, and EMG of the
scale, and assessment of reflex activity and the Medical
anal sphincter. It was performed before surgery and at 1,
Research Council (MRC) muscle scale.
5, and 12 months postoperatively.
A spinal injuries physiotherapist undertook regularly
a Functional Independence Measure (FIM), evaluated the
WI, and recorded the achievements in each physical exer-
Statistical analyses were performed using Statistica
cise during the locomotor training.
10 (StatSoft, Inc., Tulsa, OK, USA) A value of p = 0.05
Psychological evaluation was performed preopera-
was considered significant. The Mann–Whitney U test
tively and at 1, 12, and 19 months after surgery. A clini-
was applied for assessment of statistical differences in
cal interview as well as the following psychological tests
measured variables. The Spearman Rank test was used
were conducted: the Minnesota Multiphasic Personality
to test correlations between specific measurements and
Inventory-2 test (MMPI-2) (2), the Generalized Self-
features. Statistically significant correlation coefficients
Efficacy Scale (GSES) (19), the State-Trait Anxiety
greater than 0.50 were considered important.
Inventory test (STAI) (42), and the Eysenck Personality Questionnaire-Revised (EPQ-R) (10). A cultural adapta-
tion of these tests was routinely applied.
Recovery After the Operation of
MRI images were undertaken on a 1.5-Tesla MR unit
Olfactory Bulb Retrieval
(GE Signa HDx, Milwaukee, WI, USA) and included
The operation for obtaining the OB via craniotomy
T2-weighted images, T2-weighted fat saturation (FAT-
was safe. No neurological or general complications were
SAT) images, and T1-weighted images before and after
noted postoperatively. The head CT scan performed 2
administration of contrast medium (gadolinium; Multihance,
days after the surgery did not show any abnormalities.
Bracco Diagnostics, Singen, Germany) in the sagittal, cor-
Shortly after the operation, the patient lost his smell per-
onal, and axial planes. Diffusion tensor imaging (DTI)
ception on the left, where the bulbectomy was performed,
included an assessment of the topography of the water
but unexpectedly, follow-up showed a later, partial recov-
diffusion tracts in the spinal cord on tractography and the
ery of olfaction on the bulbectomized side. This persisted
estimation of the values of fractional anisotropy (FA) of
for the period of 19 months of observation (Fig. 4).
the spinal cord on the FA maps, at 0.5 and 2 cm above and below the lesion epicenter. MRI and DTI studies
Cell Culture
were performed preoperatively and at 1, 5, 8, 12, and 17
The cultures of cells isolated from the OB were not
months postsurgery according to the previously described
purified and contained mainly ONFs and OECs and
protocol (43).
comprised more than 95% of the Hoechst-stained cell
Neurophysiological examinations included transcra-
population. OECs were identified as bi- or multipolar
nial magnetic MEPs, electroneurography (ENG), and
p75-NGFR-positive cells, with small cytoplasm and thin,
EMG using the KeyPoint Diagnostic System (Medtronic,
long processes. They formed a network on a monolayer
Copenhagen, Denmark), as previously described (43).
of flattened FN-positive ONFs (Fig. 2D). The percent-
Neurophysiological testing was performed twice before
age of p75-NGFR-positive cells was 16%. After 12 days,
surgery and at 1, 5, 8, 11, 14, and 17 months postopera-
when OECs reached a confluent monolayer in culture,
tively. During the MEP study, three positive recordings
they were detached from the culture flasks and prepared
with similar amplitudes and latencies of potentials were
for transplantation. The sterility tests of the cell culture,
recorded from rectus abdominis, rectus femoris, and
performed every 5 days, showed no evidence of bacte-
extensor digiti muscles on both sides to show the integ-
rial or fungal contamination within the whole period of
rity of long efferent neural transmission. Beside well-
cell culture. The residual volume of cells remaining in the
known parameters of amplitudes and latencies in MEP
Hamilton syringe at the end of the operation of cell micro-
recordings, the duration of potentials measured from the
injection was seeded onto culture flasks and cultured for
onset to the end with the reference to isoelectric line were
about 10 days for identification of cell populations and
also recorded. Bilateral EMG recordings from the rectus
for assessment of culture sterility. In all cultures used for
abdominis, rectus femoris, gastrocnemius, anterior tibial,
transplantation, p75-NGFR-positive cells could be iden-
and extensor digiti muscles were assessed using surface
tified in culture, and there was no evidence of microbial
electrodes, and needle electrodes were used to increase
the measurement precision when the patient was asked to perform the voluntary contractions lasting 5 s.
Assessment of the Safety of the Operation of Cell
The urodynamic study was conducted according to
Transplantation and Spinal Cord Reconstruction
the protocol of the International Continence Society (44)
The operation of myeloadhesiolysis and glial scar
using the Duet Logic G2 system (Medtronic). It consisted
resection followed by cell microinjection and bridging of
TABAKOW ET AL.
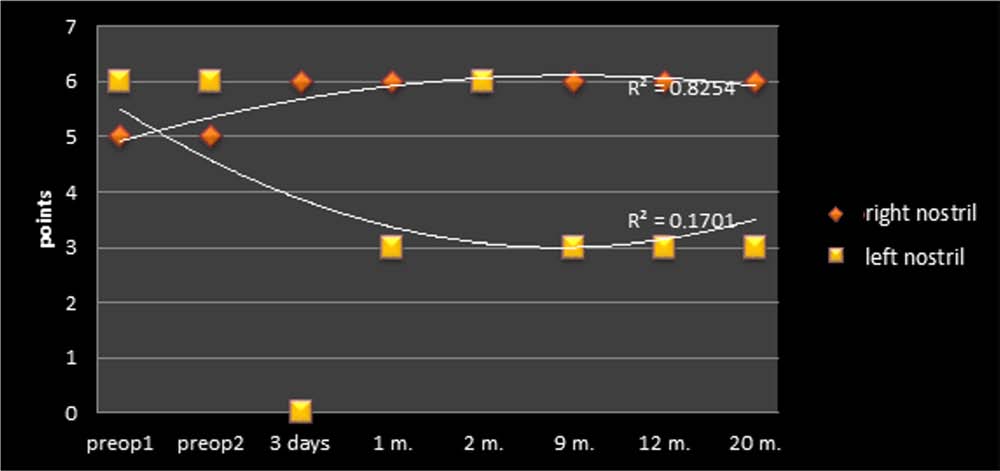
Figure 4. Summary of the smell perception test performed for the right and left nostril pre- and postoperatively. The maximal achieve-
ment that can be assigned for each tested side was six points. Note that after removing the left OB, the patient did not totally lose
olfaction on the left but remained hyposmic.
the spinal cord with autologous peripheral nerve grafts
spinal cord stumps. Yet tractography studies at 17 months
was safe over the period of 19 months of observation. We
after operation again showed a gap in the continuity of
did not observe neurological deterioration, neuropathic
diffusion tracts, which did not correlate with the contin-
pain, clinical or laboratory evidence of neuroinfection,
ued neurological and neurophysiological recovery of the
nor any general medical complications attributed to the
patient (Fig. 6). Assessment of the values of FA did not
surgical intervention. The patient spent the first 2 days
show significant changes between pre- and postoperative
after the surgery in the intensive care unit to have opti-
studies (Table 2).
mal control of his medical condition. In the first 3 days after the surgery, he was suffering mainly from pain from
the operative wound, requiring treatment for a period
Histopathological examination of the specimen obtained
of several days with morphine (Morphini Sulfas, Polfa,
from the resected intraparenchymal scar tissue showed
Warszawa, Poland) administered subcutaneously at a
no astroglial reactivity but fibrous connective tissue inter-
dose of 5 mg every 6 h. There was no spine instability or
mingled with bundles of peripheral nerve fibers and
stenosis, as well as no evidence of myelomalacia, edema,
Schwann cells (Fig. 7).
inflammation, or tumors of the spinal cord at the injection site, as documented in the five postoperative MRI studies
(Fig. 5). MRI showed a good integration of the implanted
Preoperatively, the patient presented, in serial exami-
nerve strips with the host spinal cord tissue. The nerve
nations, symptoms of a complete SCI (ASIA A), with a
implants were well vascularized (Fig. 5B) and retained
zone of partial preservation (ZPP) at dermatomal level
their size (Fig. 5G). T2-weighted scans showed some
Th9 and complete loss of any type of sensation below
mild degenerative changes in the spinal cord stumps that
this dermatome, including the S4–S5 dermatomes. The
were not progressive and were not associated with any
patient was assigned 31 points for each side in the ASIA
negative influence on the clinical state of the patient and/
Light Touch and Pin Prick Score. There was a paralysis
or the results of his electrophysiological studies (Fig. 5).
of the leg muscles (0 points according to the MRC scale)
The preoperative DTI study revealed that a gap in con-
and weakness of the lower trunk muscles causing trunk
tinuity of diffusion tracts in the spinal cord was at the level
instability (positive Beevor's sign) during the attempts of
of the SCI (Fig. 6). An early postoperative DTI study,
the patient to stand. Increased spasticity was present in
performed at 5 weeks, showed restitution of the tracts of
the LEs between 4 and 5 on the Ashworth scale, with a
water diffusion across the area of implanted nerve grafts
bilaterally positive Babinski sign and LE hyper-reflexia,
and also across the rim of spared tissue connecting the
except in the ankles, where there was no Achilles tendon
SPINAL CORD REPAIR: OECs AND NERVE GRAFTS
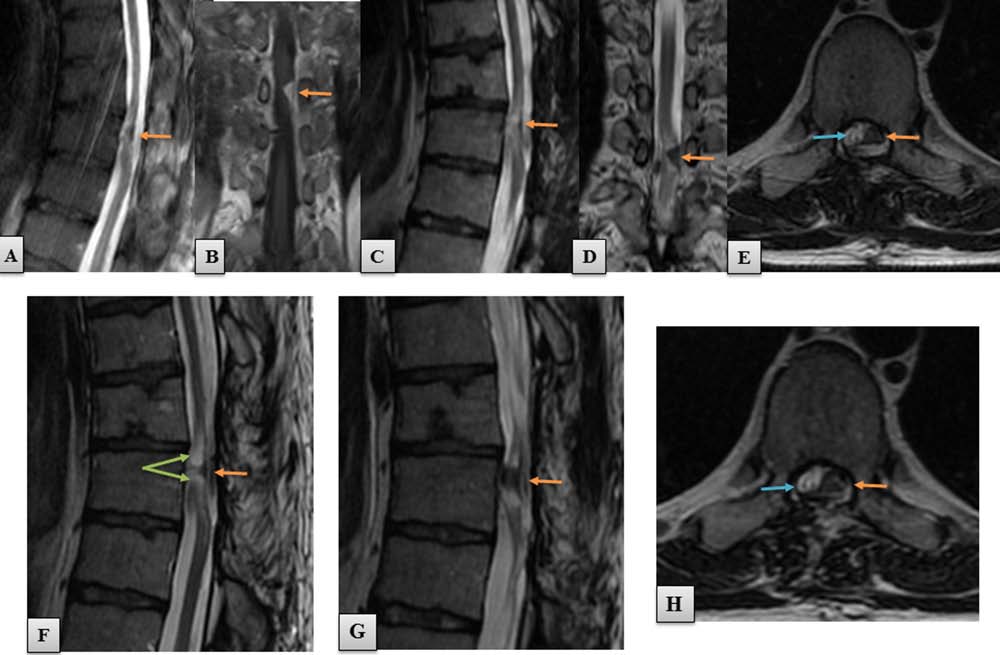
Figure 5. MRI studies of the spinal cord at set time points after treatment. MRI studies performed at 5 weeks (A,B), 5 months (C,D,E),
and 17 months (F–H) after the spinal cord operation. Orange arrows, implanted sural nerve strips. (A) On the sagittal T2-weighted
image, the nerve implant appeared as a hypodense structure that reconnected effectively the sectioned spinal cord stumps. (B) Coronal
scan of the same area showing that after contrast administration the nerves were hyperdense, which confirms their good vasculariza-
tion. Implant size was 1.3 × 0.7 cm. T2-weighted sagittal (C) and coronal scans (D) after 5 months confirmed the integrity of the nerve
grafts with the host tissue. (E) Axial T2-weighted scan shows that the nerve grafts (orange arrow) filled most of the surface connecting
the spinal cord stumps, while the rim of spared tissue was hardly visible (blue arrow), when compared with preoperative MRI scans
(Fig. 1D). (F) Sagittal T2-weighted image at 17 months showed a hyperdense area of mild spinal cord degeneration that was present
in both pre- as well as all postoperative studies (green arrows). (G) The sural nerve autografts retained their size when compared with
the early MRI study. (H) At 17 months postsurgery, the nerve grafts still were the dominant structure found in the area of spinal cord
reconstruction (orange arrow). The spared tissue was also identifiable (blue arrow).
reflex. Additionally, the legs were constantly cold due to an
achieved a higher score in the ASIA Pin Prick Test (42
impaired vascular autoregulation. A small pressure ulcer
for the right leg and 34 for the left) but was statistically
was present on the lateral surface of the right hip, which
significant only for the right leg (p < 0.05) (Fig. 8). For
was not painful because of the sensory impairment.
the first time, the patient reported pain evoked by irrita-
After the operation, the patient's neurological state did
tion of the small pressure ulcer present on the right hip.
not change significantly during the first 4 months. Five
We also noticed a recovery of the deep sensation in the
months after surgery, the first signs of recovery of sensa-
legs. At 6 months postsurgery, the patient began to feel
tion in dermatomes S4–S5 were present, becoming more
the tension applied to his leg muscles during training and
evident at 6 months. A gradual recovery of superficial
the movement of his joints. Between the 10th and 19th
sensation, particularly in the right LE, was observed dur-
months after surgery, the recovery of deep sensation was
ing the whole postsurgical period of 19 months, increas-
confirmed with specific tests such as the vibration test or
ing in pace after the 10th month. The ASIA Light Touch
tests for evaluation of limb position. On about 30 trials,
score reached 35 points for the right leg, while it was 32
the patient could determine, with 75–85% accuracy, the
in the left leg. The increase in the light touch score for the
direction of movement of his feet with his eyes closed
period after 10 months postsurgery was statistically sig-
and even could discriminate the movement of his toes
nificant for both LEs (p < 0.05) (Fig. 8). The patient also
from the movement of the whole foot.
TABAKOW ET AL.
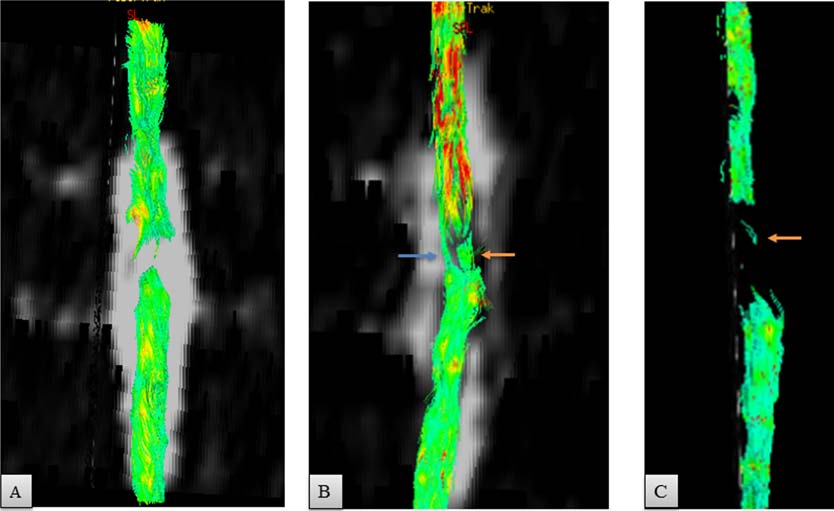
Figure 6. Scans of spinal cord tractography. (A) An 8-mm gap of continuity of diffusion tracts was observed preoperatively. (B) Five
weeks after the operation, the tracts of water diffusion crossed through the area of implanted nerves (orange arrow) and the rim of
spared tissue connecting the stumps (blue arrow). (C) At 17 months, a gap of 1.8 cm was present. Orange arrow points out either thin
tracts at the level of the implant or an artifact.
Simultaneously with the improvement of superficial
42 cm in the right thigh and increased gradually from 50
and deep sensation, we observed an evident recovery
to 54 cm within a period of 18 months. The increase in
of voluntary motor function in the previously paralyzed
muscle mass in the right thigh did not exceed 2 cm in
muscle groups. This recovery appeared in a segmental
the same period. There was no change in the mass of the
pattern, starting from the lower part of the abdominal and
calf muscles. As the patient had sustained inflammation
other trunk muscles. This was first evident 5 months after
and thrombosis of the LE deep venous system in the pre-
surgery. The recovery of voluntary function of selected
operative period, Doppler ultrasonography (Vivid 7, GE
LE muscles was preceded by a marked increase in muscle
Healthcare, Horten, Norway) of the veins and arteries of
mass of the left thigh 4 months postoperatively, causing
the LE was performed. The independent angiologic study
visible leg asymmetry. The circumference of the left thigh,
excluded venostatic edema or postthrombotic syndrome
measured in the middle of the line connecting the ante-
as a cause of the increased circumference of the left leg.
rior superior iliac spine and the patella, was 50 cm versus
The motor recovery was more prominent in the left LE.
The first voluntary adduction of the left leg was observed 5 months after surgery and increased with time, reach-ing level 3 according to the MRC scale at 11 months.
Table 2. Summary of the Values of Fractional Anisotropy
Ten months postoperatively, a voluntary adduction of
the right leg (MRC 2) was noted and slight hip flexion
on the left (MRC 1). The increase in the strength of the
left and right adductor muscles was statistically signifi-
cant for the period after 7 months postsurgery (p < 0.05)
(Fig. 9). The first voluntary knee extension was recorded
on the left (MRC 2) at 1 year and was confirmed in later
studies. A slight knee extension in the right leg was also
FA values were measured in the spinal cord above and below the lesion
noted at 14 months postsurgery (MRC 1). As a result, the
epicenter. Levels 0.5 cm and 2 cm refer to the proximal spinal cord
patient's neurological state turned 6 months after surgery
stump, whereas levels −0.5 cm and −2 cm to the distal stump. Preop., preoperatively.
from ASIA A to ASIA B and at 11 months to ASIA C
SPINAL CORD REPAIR: OECs AND NERVE GRAFTS
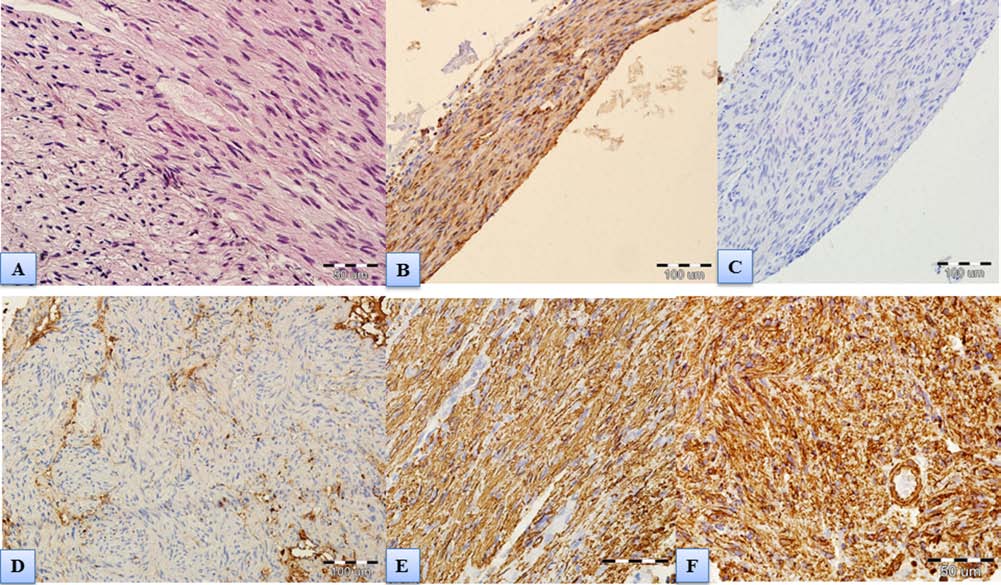
Figure 7. Spinal cord scar. (A) Scar composed of peripheral nerve fibers and fibrous connective tissue without CNS tissue; hema-
toxylin and eosin staining. (B) Immunohistochemical expression of S100 protein confirming aligned clusters of Schwann cells
with typical ovoid nuclei within the scar. (C) Lack of immunohistochemical expression of EMA excluding presence of menin-
geal tissue. (D) Negative immunohistochemical staining for glial fibrillary acidic protein (GFAP) proving no glial components.
(E) Immunohistochemical expression of NF typical for nerve fibers (scale bar: 50 µm). We assumed that these are the central branches
of dorsal rootlets lying in the scarred area. (F) Immunohistochemical expression of vimentin corresponding with connective tissue.
(Fig. 10). The observed increase of ASIA motor scores
patient tilting and made walking in braces in parallel bars
for the left and right LEs was statistically significant
impossible. WI was 0.
for the period after 10 months when compared with the
Increased strength of the trunk muscles, the first volun-
preoperative state and the first 10 months after surgery
tary movements of adduction and abduction of the left leg,
(p < 0.05) (Fig. 9).
decrease in muscle spasticity, and the evident recovery of
A decrease of spasticity in both LEs was noted postop-
proprioception in the LE, which started to be visible from
eratively but was significant statistically only for the right
the sixth month after surgery, increased the quality of per-
(p < 0.05). The mean Ashworth score decreased from 5
formed exercises and enabled the introduction of more
to 3.8 for the left LE and from 4 to 3 for the right. At
difficult tasks. The increase of the muscle strength and
5 months postoperatively, the Babinski sign disappeared
coordination enabled better trunk, pelvis, and hip stabili-
bilaterally, and the Achilles tendon reflex reappeared in
zation and could prepare the patient for the first exercises
the left LE. The left LE seemed also to have better vascu-
of walking reeducation. In the period from 9 to 11 months
lar autoregulation when compared to the right.
after surgery, there was an evident improvement in the technique of exercise performance and an increase in the
values of the loads in exercises requiring a high degree
During the period of 8 months of intense preoperative
of voluntary function of abdominal and back muscles,
rehabilitation, the patient did not show any improvement
gluteal muscles, adductors and abductors, hip flexors,
in performed physical exercises. There was asymmetri-
and knee extensors. The left leg was dominant in muscle
cally increased spasticity of the trunk and LE, paralysis
mass, number of voluntary controlled muscles, and time
and loss of sensation of the LE, and the equinovarus
of appearance of first symptoms of motor recovery. This
positioning of the left foot. This hindered any attempts of
enabled the introduction of separate exercises for the left
TABAKOW ET AL.
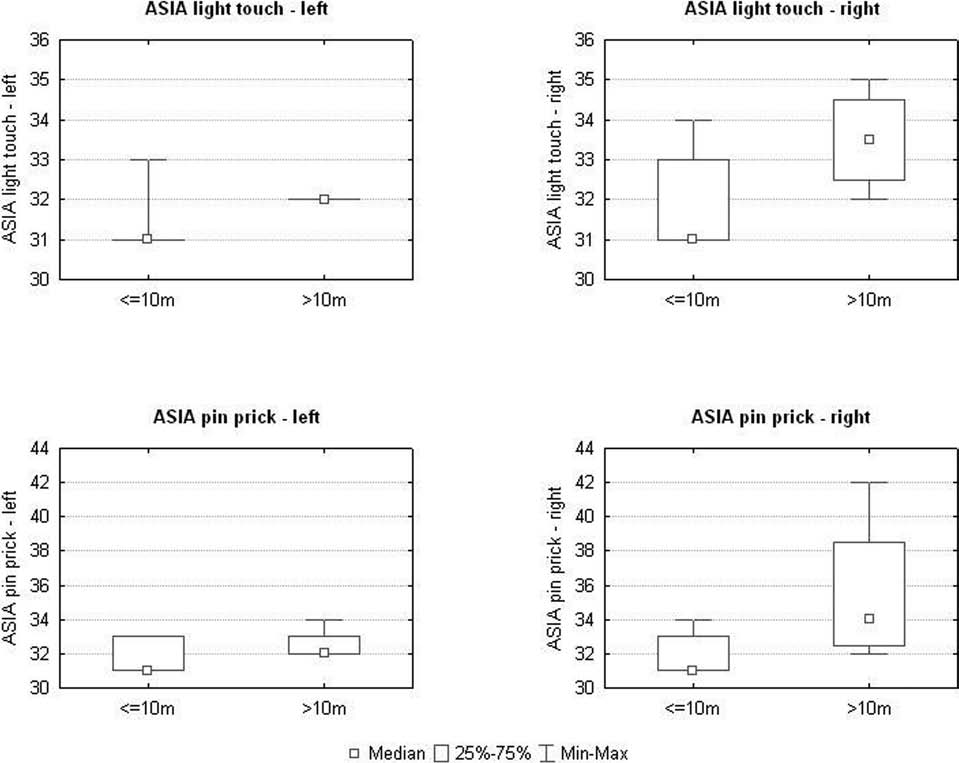
Figure 8. Summary of the results from the light touch and pin prick tests. The observed improvement in these tests in the period
after the 10th postoperative month was statistically significant (p < 0.05) when compared to the preoperative state and the first 10
months postsurgery. There was also more evident recovery of superficial sensation in the right LE when compared with the left. ASIA,
American Spinal Injury Association.
and right leg. We did not observe any notable improve-
Measurements of functional activity using the FIM
ment in the exercises testing muscles of the posterior part
scale showed a significant increase in the FIM values
of the thigh and the calf. Table 3 summarizes the results
from 102 to 104 points before surgery to 116–120 points
from physiotherapy.
in the period between the 6th and 11th months, to reach
The observed improvement in different static physi-
123–124 points between the 12th and 19th months.
cal exercises led gradually to the first attempts to walk. Six months postsurgery, the patient was able to ambu-
Correlations Between the Results From Neurological
late 10 m in parallel bars with long leg braces and the
and Physiotherapeutic Studies (Table 4)
physical assistance of one person (WI = 3); at 13 months,
A statistically significant strong positive correlation
WI increased to 5 (parallel bars, no physical assistance),
(p < 0.05) was found between the ASIA motor scores for
and starting from 14 months, the patient was able for the
both LEs, the scores obtained for leg adduction, and the
first time to ambulate with a walker, long braces, and the
light touch and pin prick scores in the right and left LE;
assistance of one person (WI = 6). Additionally, in the last
between the left and right ASIA motor scores and the WI
months of observation, the patient started to walk both in
and the right light touch and pin prick scores and WI; and
parallel bars and with a walker with short braces, locked
between the WI and the FIM score. A negative correla-
only at the ankles. The observed increases in the WI val-
tion was noted between the leg adduction tests and the leg
ues after surgery were statistically significant (p < 0.05).
spasticity measured with the Ashworth score.
SPINAL CORD REPAIR: OECs AND NERVE GRAFTS
Figure 9. Graphs showing the results from motor tests of the LE. A voluntary muscle activity could be observed in both the left and
right LE but was more evident after the 10th postoperative month and achieved higher MRC scores in the left one.
Figure 10. Chart showing the changes in the ASIA score in the postoperative period. ASIA Score increased to B around 6 months and
C around 11 months.
TABAKOW ET AL.
Table 3. Summary of the Achievement From Selected Rehabilitation Exercises
Roman numerals refer to the month. Positive numbers show the value of applied load (in grams). Negative numbers show the value of load relief. Minus sign indicates that the patient was unable to perform the exercise. 1. Forward bends from a supine position—a test of abdominal muscle strength. In this case, the patient was initially holding a bar connected with a suspended load and started the exercise from an angle of 30°. Starting from 9 months after surgery, he could bend forward without any help (w.h.), beginning the movement from a completely supine position (0°). 2. Hip extension—an exercise testing trunk muscles and gluteal muscles and performed in a supine position with suspended straight legs. Preoperatively, the patient was able only to initiate a simultaneous downward movement of both legs when blocked in long braces. This leg movement was triggered by the use of trunk musculature, but further movement was prevented by increased spasticity and muscle paralysis. After surgery, he was able to perform a full range of hip extension without leg immobilization in braces. In addition, the patient became able to perform the same exercise with his legs suspended separately (No. 3, right leg; No. 4, left leg). The left leg was able to overcome much higher loads than the right. 5. Leg abduction in a sitting position. 6. Leg adduction in a sitting position. 7. Drawing of suspended knees toward the abdomen using hip flexors (iliopsoas muscles and rectus femoris). 8. Cycling in supine position—test of hip flexors and knee extensors (quadriceps muscle). The feet are placed into a rotor, and the patient is trying to perform alternating pedaling movements. This could be performed first at 5 months postsurgery with the use of high load relief. In the period after the 12th month, the patient could cycle without any support. The left leg was the dominant one in this exercise. 9. Knee flexion in a prone position—test for the posterior group of LE muscles (semitendinosus, semimembranosus, biceps femoris, and gastrocnemius, innervated by the sacral spinal cord segments). There was no improvement in this muscle group.
Neurophysiological Evaluation (Fig. 11, Table 5)
of contraction of rectus femoris muscles. An increase in
During the first and second preoperative examinations,
the amplitudes in MEPs recorded in subsequent trials was
MEP and EMG studies detected efferent transmission
prominent for the left rectus femoris muscle and reached
from motor cortex centers to spinal cord motoneurons
300 µV at 14 months. MEPs from the right rectus femo-
only to the level of innervation of the rectus abdominis
ris muscle were comparable to the one taken from the
muscles. Postoperatively, MEPs recorded from abdomi-
left rectus femoris at 5 months but decreased at 8 and 11
nal muscles on both sides were close to the normal
months and could not be recorded at 14 and 17 months.
recorded in healthy volunteers (more than 1,500 µV). At
Activation of the corticospinal connections to L5–S1
5 months after the operation, excitation reached L1 to L4
motoneurons following the magnetic field excitation
spinal motoneurons followed by clear signs of recovery
was only recorded rudimentarily from the distal muscles
Table 4. Summary of the Most Important Correlations Found During the Performed Tests of
Neurological and Physiotherapeutic Achievement
ASIA motor score–right and ASIA motor score–left
Leg adduction–left and leg adduction–right
ASIA Light Touch–left and ASIA Light Touch–right
ASIA Pin Prick–left and ASIA Pin Prick–right
ASIA motor score–left and Walking Index
ASIA motor score–right and Walking Index
ASIA Light Touch–right and Walking Index
ASIA Pin Prick–right and Walking Index
FIM and Walking Index
Leg adduction–right and Ashworth spasticity–right
Leg adduction–left and Ashworth spasticity–left
ASIA, American Spinal Cord Injury Association; FIM, Functional Independence Measure.
SPINAL CORD REPAIR: OECs AND NERVE GRAFTS
Figure 11. Pre- and postoperative comparison of MEPs and EMG recordings. Comparison of MEP recordings (upper traces) and
EMG recordings (lower traces) performed pre- (A, B) and postoperatively (C, 1 month postsurgery, D, 5 months, E, 8 months, F, 11
months, G, 14 months, H, 17 months). Recordings were performed from the left LE. Note different amplifications of recordings and
increase in MEP amplitudes recorded from both rectus abdominis and rectus femoris muscles (spinal efferent transmission recovery)
as well as the improvement of motor unit activity in rectus abdominis and rectus femoris muscles observed in EMG recordings. The
calibration bars of amplification (vertical, in millivolts or microvolts) and time base (horizontal, in milliseconds) for all MEP and EMG
recordings are shown in (A).
TABAKOW ET AL.
Postoperative Recordings
-Evoked Potential (MEP) Parameters
Electromyography (EMG) Parameters
Electroneurography (ENG) of Peroneal Nerve Parameters
Preoperative Recordings
-Evoked Potentials, Electromyographic Recordings, and Electroneurographic Findings Over
SPINAL CORD REPAIR: OECs AND NERVE GRAFTS
1 months, 14 months, and 17 months).
-Evoked Potential (MEP) Parameters
Postoperative Recordings (Continued)
Electromyography (EMG) Parameters
Electroneurography (ENG) of Peroneal Nerve Parameters
They were recorded bilaterally at eight stages of observation (preop 1, preop 2, 1 month, 5 months, 8 months, 1
, nonrecorded.
of peroneal nerves.
TABAKOW ET AL.
of the left LE. The first observed low-amplitude MEP
Urodynamic studies did not show any difference in
recordings were associated with an increased latency
control examinations. A hyper-reflexia of the bladder
and duration of potentials, but a tendency for shortening
detrusor and dyssynergy between the bladder detrusor
was detected during recordings at 11, 14, and 17 months
and urethral sphincter activity was noted both pre- and
(Fig. 12). Postoperative EMG recordings from LE showed
postoperatively. There was no EMG evidence of volun-
voluntary muscle contractions recorded as action poten-
tary functional activation of the anal sphincter. However,
tials from the rectus femoris muscles and the left digital
an improvement of bladder sensation helped the patient
extensor during the attempt of maximal muscle contrac-
determine the timing for voiding. After surgery, the
tion, which were not present preoperatively. EMG record-
patient reported that he had regained the ability to obtain
ings (both surface and needle performed) confirmed the
and maintain erection without the need for pharmacologi-
voluntary contraction of muscles when recorded mainly
cal support.
on the left side. The frequency of positive EMG record-ings in the proximal LE muscles was higher on the left
side. Low frequency of the positive EMG recordings
Clinical observation performed for 19 months after
from distal muscles of the left LE, as well as absent or
the operative treatment revealed positive changes in
very weak responses, were found for the distal muscles
the psychological and social profile of the patient. This
of the right LE. ENG studies suggested this was related to
included a change of the style of interpersonal relations
degenerative changes in the peripheral motor fibers in the
from passive (before treatment) to dominant (increase of
peroneal nerves. ENG studies showed their axonal type
MMPI-2 Leary index from 4 to 7), a better resolution of
basing on recorded low amplitudes of M-wave potentials.
anger and aggression (increase of Leary index from 4 to
Needle EMG recordings performed from active muscles
7), a decrease in anxiety level (EPQ-R: a decrease from 8
during voluntary contraction showed at rest also positive
sten to 5 sten), an increase in the sense of self-control and
sharp potentials in almost all of the recordings (20/24 tri-
efficacy in life (GSES: increase from 5 sten to 7 sten),
als), indicating a degenerative process at the neuromus-
and an increased satisfaction in interpersonal relations
cular junctions. The above observations were confirmed
(including sexual). Negative observations concerned the
by three independent neurophysiologists involved in the
patient's lower threshold of tolerance of frustration and
higher irritability. Conclusively, an improvement of the
Figure 12. Variability of the MEP duration, latency, and amplitude parameters, recorded at stages during the neurophysiological
assessment of the left rectus femoris muscle. There was no muscle response to motor cortex activation preoperatively. Note the con-
tinued muscle response after the fifth postoperative month and the improvement in spinal cord efferent transmission, registered as a
gradual decrease of the latency and duration of the potential. A smaller MEP response of the right rectus femoris muscle (shown only
numerically in Table 5) was present at 5–11 months but was not observed in the following two trials.
SPINAL CORD REPAIR: OECs AND NERVE GRAFTS
mental state of the patient was noted, especially with
Unexpectedly, the patient regained some smell per-
respect to satisfying the most important needs like the
ception on the side of the bulbectomy 3 weeks after the
sense of safety, social acceptability, and quality of life.
craniotomy. Our first explanation was that following the
According to the patient, his improved quality of life was
sustained operation of anterofrontoethmoid sphenoidec-
influenced by both the neurological improvement, giv-
tomy, new anatomical connections between the left and
ing him a higher degree of independence, and the fact of
right nostrils for the airway passage could have been
participation in the therapeutic project.
created (as in septostomy, etc.), enabling the right OB to be excited by the odor stimuli entering the left nos-
tril. Yet CT scans and laryngological fiberoscopy could
Safety of the Experimental Procedure
not confirm this hypothesis. A possibility of an efficient
The operation of the craniotomy for unilateral bul-
bilateral flow of the air through the posterior nares was
bectomy, followed by isolation and culture of OECs and
also not confirmed. For example, we noted in some of
ONFs and their transplantation into the spinal cord with
our patients treated for anterior skull base fracture, in
simultaneous bridging of the gap with autologous sural
whom the lesioned OB had to be removed, that they
nerves, was safe over the period of 19 months of postsur-
did not regain olfaction on the side of the bulbectomy
gical observation. There was no general and neurological
(unpublished data). As those patients underwent crani-
deterioration, neuropathic pain, infection, spine instabil-
alization of the skull base with the use of a periosteal
ity, stenosis, tumors, or progressive myelopathy in all
flap that separated the fila olfactoria from the olfactory
observational periods. Sterility tests of cell cultures and
cortex, and our patient did not, we suggest that a plastic
the CSF did not show any bacterial or fungal infection.
response or a regenerative response within the olfactory
The implanted nerve strips retained their structure and
system may have occurred in the present case. Although
integrated well with the host spinal cord tissue, making
the phenomenon of direct reinnervation of the olfactory
efficient contact with the sectioned cord stumps. MRI
cortex by olfactory axons, after bulbectomy, has been
revealed both pre- and postoperatively the development
described in mammals (15), further investigation of
of small areas of myelopathy in the spinal cord adjacent
our observation is required and is beyond the scope of
to the lesion area. They remained radiologically stabile
this article.
and did not influence the neurological and neurophysi-ological state of the patient (Fig. 5). We used measure-
The Pattern of Neurological Recovery:
ments of the FA to assess the degree of integrity of spinal
Spontaneous Versus Induced Recovery?
cord white matter tracts in the spinal cord (Table 2) (40).
In this article, we show an essential neurological recov-
Values of FA measured at two levels above and below the
ery of an ASIA A patient with chronic 21-month paraple-
lesion focus were typical for cases of spinal cord lesion
gia to ASIA C grade (sensory and motor incomplete)
(29,41) and did not deteriorate in the subsequent studies,
within a period of 19 months after an operative interven-
which confirms the assumption that the observed spinal
tion consisting of spinal cord scar resection, intraparen-
cord degeneration was not progressive. Five weeks post-
chymal bulbar cell microinjection, and reconnection of
surgery, a DTI study showed a realignment of the tracts
the spinal cord stumps with sural nerve grafts. The first
of water diffusion through the area of spinal cord recon-
issue that has to be discussed is whether the observed
struction, followed in the next studies by a gap of water
recovery could have been spontaneous and triggered by
diffusion, as in the preoperative studies (Fig. 6). The early
rehabilitation rather than a result of the transplantation
realignment of the tracts in DTI is unlikely to be indica-
procedure. Preoperative MRI images, as well as intra-
tive of fiber regeneration because this process would be
operative exploration, showed an almost total physical
expected to need longer periods of time. It rather showed
disconnection of the spinal cord stumps at upper verte-
a good integrity of degenerated spinal cord and nerve
bral level Th9. Only a thin 2-mm-thick spur of tissue was
endoneural channels. The gap of water diffusion, occur-
connecting the stumps (Figs. 1 and 3). Serial preopera-
ring in the later DTI studies, was not associated with any
tive MEP and EMG studies indicated that this tissue was
worsening of the patient's neurophysiological and neuro-
not conducting electrical stimuli to the lumbar group of
logical state, and conventional MRI images showed that
motoneurons below the area of injury. We did not remove
the nerve implants remained well integrated with the host
it because we believed that it may be able to act as a scaf-
spinal cord tissue. We believe that either the performed
fold to guide new regrowing axons and also might help to
study was not sensitive enough to show thin regenerat-
mechanically stabilize our grafted nerve strips.
ing fibers crossing the implant, or the pattern of fiber
The preoperative clinical, electrophysiological, radio-
regrowth might not have been longitudinal and changed
logical, and intraoperative data showed that the knife entry
the direction of water diffusion in the reconstructed area.
produced a rare type of severe spinal cord lesion seen in
TABAKOW ET AL.
humans, resembling an experimental situation of an iso-
scale) (Fig. 9). This motor recovery was preceded by a
lated spinal cord transection with minimal involvement of
marked increase in muscle mass in the left thigh as a sign
vertebral structures. In our opinion, the prognostic signs
of ongoing reinnervation. The recovery concerned not
made spontaneous recovery unlikely. A panel of leading
only the ZPP but also several motor segments below this
experts in the field of treatment of SCIs, who analyzed
zone and has been considered to be a sign of spinal cord
the data from three large double-blind placebo-controlled
repair (13). The observed appearance of new voluntary
trials on neuroprotection in SCI—the National Acute
muscle function, coming from reinnervated motor seg-
Spinal Cord Injury Study (NASCIS), Sygen (monosia-
ments below the level of spinal cord transection, could
lotetrahexosylganglioside; GM ganglioside), and GK-11
also be confirmed more objectively by the performance
(gacyclidine) trials—concluded that a spontaneous recov-
of MEP and EMG studies. Repeated electrophysiological
ery of motor and sensory in an ASIA A patient may be
studies were predictive for the observed motor recovery
possible in 20% of the cases in the first year after injury
in the ASIA motor investigation and indicated again a
but mainly in the first 3 months (13). Another study showed
dominant motor recovery in the left LE up to the L5–S1
that most spontaneous motor recovery in ASIA A patients
segments (Fig. 11 and Table 5). Difficulties in MEP
occurred in the first 6 months after injury and was almost
recordings from the proximal muscles of the right LE, the
absent after 12 months (45). Additional data on the late
lack of MEP recordings and low amplitudes of the EMG
spontaneous recovery of 987 SCI patients showed that
response in its distal muscles, and the worsening of the
between the first and fifth year after injury, only 5.6%
ENG values in the right peroneal nerve (Table 5) may be
of the ASIA A patients may recover, from which 3.5%
explained by an ongoing peripheral nerve degeneration
recover to ASIA B grade and only 1.05% to ASIA C and
of central origin (33). This phenomenon was described in
1.05% to ASIA D (21). The data of Kirshblum et al. (21)
patients after stroke (16) and does not arise from a direct
are strong, even though they were not supported by elec-
lesion of the peripheral nerves, but from a long-term lack
trophysiological studies and information about how the
of activation of spinal cord motoneurons from neurons of
patients were rehabilitated. To be sure that our patient
supraspinal origin. In our case, such lack of activation or
would not improve spontaneously, and to exclude the
weak activation may have occurred in the motoneurons
"nocebo" effect seen in inappropriately rehabilitated
innervating the right LE.
patients, he underwent an additional 8-month intense
The improvement in efferent spinal cord transmission
preoperative rehabilitation program in one of the Polish
was characterized by increasing MEP amplitudes in the
reference centers for rehabilitation of SCI patients. MEP
abdominal muscles and mainly in the left LE muscles and
and EMG studies performed at the beginning and end of
also as gradual increase in the velocity of nerve signal
this 8-month training, together with regular neurological
conduction, registered as shortening of the latency of sig-
assessments, did not show neurological recovery. For this
nal conduction and the duration of motor potential (Table
reason, we consider that the probability of spontaneous
5, Fig. 12). The first latency (46.5 ms) and duration of the
recovery in our patient was lower than 1%.
MEP response (74.3 ms) of the left femoris muscle, reg-
We observed a gradual recovery of both sensory and
istered at 5 months postoperatively, were pathologically
motor function that started after the fourth month postop-
protracted. With time, a gradual normalization of these
eratively. This recovery was nonlinear, having two criti-
parameters was observed. Hence, the latency of the MEP
cal periods: the fifth through sixth months, when the first
response at 14 and 17 months postsurgery reached values
evident signs of sacral sparing and voluntary muscle con-
typical for the healthy population (23.4 ms and 21 ms,
tractions from the first motor segments below the level
respectively). In conclusion, the improvement in efferent
of injury appeared (the patient became ASIA B), and
spinal cord transmission gave evidence of ongoing long-
the period after the 10th month, when the patient turned
distance motor fiber regeneration (amplitude increase),
to ASIA C. The motor recovery occurred in a segmen-
possibly from the corticospinal tracts, and remyelination
tal pattern, starting at 5 months from an increase of the
of part of these tracts (latency and potential duration nor-
strength of the lower abdominal muscles and other trunk
malization), occurring mainly from the part of the spinal
muscles (lower thoracic motor segments), followed by
cord that had been bridged with the nerve strips and was
a gradual increase of voluntary LE adduction (L1 seg-
a good prognostic factor for the observed neurological
ments), reaching 3 points according to the MRC scale in
the left LE and 2 points in the right LE. The voluntary
Starting from the fifth month after surgery, as in the
control of the musculature proceeded in time downward,
case of the motor function tests, we observed a timely
predominantly to the left LE, and included hip flexion
nonlinear pattern of recovery of superficial sensation, with
(L2 segments, 1 point according to MRC) and knee
higher scores achieved in the right LE both in the ASIA
extension (L3 segments, 2 points according to the MRC
Light Touch and Pin Prick scores in dermatomes from L5
SPINAL CORD REPAIR: OECs AND NERVE GRAFTS
to S5, depending on the type of tested modality (Fig. 8).
bulbar OECs/ONFs, and reconnection of the stumps with
This may be consistent with regeneration of the primary
four strips of sural nerves and was followed by a long and
afferent fibers crossing the midline below the injury site
intense neurorehabilitation program. Each single inter-
and regenerating along the nerves implanted predomi-
vention had its importance but, in our opinion, could not
nantly in the left half of the spinal cord as spinothalamic
be in itself sufficient if applied without the others.
tracts. Additionally, the recovery of deep sensation on
The spinal cord untethering (myeloadhesiolysis) may
both sides, which was stronger in the feet, may be consis-
have improved the vascular supply in the area of spinal
tent with regeneration of primary afferent proprioceptive
cord reconstruction and could be beneficial for the inte-
fibers from lower lumbar and sacral segments, ascending
gration of the nerve grafts with the spinal cord tissue.
in the dorsal columns closest to the midline and crossing
Studies on a large group of patients with SCI undergoing
the bridged injury on both sides. The lesser recovery of
late myeloadhesiolysis did not show any significant influ-
motor function in the right LE and superficial sensation
ence of this intervention on the sensorimotor recovery
in the left LE might have been due to either plasticity of
(11). The removal of the intraparenchymal scar tissue was
fibers that have regenerated across the nerve grafts on
beneficial because it eliminated some of the physical and
the left or minimal regrowth of fibers crossing the OEC-
chemical barriers for axonal regeneration contained in the
infiltrated scar tissue on the right side of the spinal cord.
scar (12) and turned the chronic SCI into an acute one,
Taken together, all observed recovery of motor and
enabling a better interaction of the transplanted OECs
sensory function had a partial Brown–Sequard pattern
and ONFs with host astrocytes. We consider it most likely
and fitted exactly with the location of the repair, suggest-
that the crucial interventions during the operation were
ing that the bridge of peripheral nerve grafts that preferen-
the intraparenchymal transplantation of OECs/ONFs and
tially reconnected the left half and the medial part of the
the reconnection of the spinal cord stumps with strips of
right half of the spinal cord stumps (Fig. 13). Additional
peripheral nerves. Transplanted bulbar cells could have
clinical observations concerned the reappearance of the
been responsible for realignment of the astrocytic pro-
left Achilles tendon reflex and the disappearance of the
cesses and "opened the door" for regrowth of central
Babinski sign. The observed normalization of reflex activ-
ity may also indicate an improved supraspinal control of
The specific clinical condition of our patient, suffer-
local spinal cord neuronal circuits. We also noticed some
ing from a chronic inflammatory condition of the nasal
symptoms of improved autonomic function, such as an
mucosa, allowed us to use for the first time OECs isolated
improvement of bladder sensation, confirmed in urody-
from the human OB. There is growing evidence in the
namic studies; an improvement of erection control with-
literature that bulbar OECs have stronger regeneration-
out the need for pharmacological support; and improved
promoting capacity than mucosal OECs (18,32,39,46).
vascular autoregulation mainly in the left LE.
We also observed in our previous trial on ASIA A para-
The observed statistically significant improvement
plegic patients that mucosal OECs/ONFs gave minimal
of sensorimotor function had a positive impact on the
neurological improvement in all operated patients (43).
achievements in physical exercises during rehabilitation
The only completed phase I clinical study on applica-
and significantly influenced the results from the FIM
tion of purified OECs in the treatment of human paraple-
tests and the ability of the patient to walk, measured as
gia did not show any efficacy of transplanted purified
increased WI (Table 4). The improved walking was not
autologous mucosal OECs (30). As in our previous study,
only due to the increasing strength of trunk and LE mus-
we transplanted cultures containing mixtures of OECs
cles but also to the recovery of deep sensation in both LEs
and ONFs. The ONFs form an intimate outer cover on
and superficial sensation mainly in the right LE. The sen-
the outer surface of the OECs, with the nerve fibers on the
sory recovery enabled better coordination and perception
inner surface. After transplantation into the corticospinal
of leg movements and improved the quality of the walk.
tract or optic nerve lesions, the advancing ONFs precede and establish a channel for the advance of the OECs
Aspects of the Operation Contributing to the
(23,25). In all these situations, OECs and ONFs seem to
act together as essential and complementary components
Because our approach, as oriented to give the patient
of a proregenerative tissue, and our experience (unpub-
the "best medical treatment" was complex, it is difficult
lished) is that purified OECs do not survive well after
to determine which aspects of the interventions contrib-
transplantation in rat spinal cord lesions. The mechanism
uted to the observed neurological recovery. The surgical
of these complex interactions between OECs and ONFs
intervention included spinal cord untethering, resection of
is unknown. It certainly involves intimate surface-to-
the intraparenchymal scar tissue, injection into the spinal
surface contact, implying a dependence on the interac-
cord stumps and the rim of spared tissue of a mixture of
tions between membrane-bound molecules and leading to a
TABAKOW ET AL.
SPINAL CORD REPAIR: OECs AND NERVE GRAFTS
basal lamina forming on the surface of the OECs facing
a variety of neurotrophic factors (1). The administration
of methylprednisolone may have led to an increase in the
The use of peripheral nerve grafts to bridge the sec-
number of myelinated CNS axons growing through the
tioned spinal cord in experimental animals has been
implants and also may have given better integration of
described three decades ago (7,38). In these studies, only
the transplant with the host tissue as described by Chen
some populations of sensory neurons and intrinsic spinal
et al. (3). Taken together, our experimental approach of
cord neurons were seen to elongate their axons within the
combining Schwann cell bridges and bulbar OECs was
implants and enter, for short distances, the spinal cord.
very similar to the one described in rats by Ramon-Cueto
Newer modifications of the described methods claimed,
et al. (37). In that study, the authors demonstrated that
both in preclinical and clinical studies, that if the nerve
supraspinal axons regenerating through the transplant
grafts connect the white matter above the level of injury
reentered the spinal cord and made synaptic connections.
with the gray matter below and the white matter below the
Thus, we believe that the neurological recovery in our
injury with the gray matter above, with local administra-
patient indicates regeneration of central nervous system
tion of acidic fibroblast growth factor, a regrowth of long-
fibers that crossed the host/peripheral nerve interfaces
distance supraspinal axons may be possible (4,5) and may
and grew for a considerable distance to make functional
be beneficial for the patient (5). Cheng et al. (4,5) claimed
connections in the cord (Fig. 13).
that they rerouted the regenerative pathway from the non-permissive white matter to the more permissive gray mat-
ter that is devoid of factors hindering regeneration.
The results from the treatment of the first patient with
In our technique, multiple microinjections of a mixture
a complete SCI receiving transplantation of bulbar OECs/
of OECs and ONFs into the lateral columns of the spinal
ONFs and simultaneous reconstruction of the spinal cord
cord may have enabled the necessary interaction with
gap with peripheral nerve implants are very encouraging
host astrocytes to make possible the regeneration of both
but have to be confirmed in a larger group of patients
efferent and afferent axons throughout the bridged nerves
sustaining similar types of SCI. Further laboratory stud-
into the caudal and rostral stumps. The nerve strips were
ies will be needed to elucidate the properties of human
placed along the long axis of both white and gray matter
bulbar OECs/ONFs and their interaction with periph-
and enabled the central axons to grow within their natu-
eral nerve bridges or artificial implants in vitro as well
ral anatomical compartments. Implanted peripheral nerve
as their reparative potential in vivo. We are investigating
grafts served as guidance tubes with the appropriate
surgical techniques for minimally invasive access to the
extracellular environment for axon adhesion and elonga-
human OB. There remains a possibility that sources of
tion and also as a large source of Schwann cells, known to
other, more readily obtainable, reparative cells may be
be able to myelinate fibers of central origin and to secrete
FACING PAGE
Figure 13. Schematic diagram showing the proposed pattern of fiber regeneration. The injury consisted of a complete transection
with 8-mm separation over the left three fourths of the spinal cord at the vertebral T9 level, resulting in complete loss of motor and
sensory function from and including the spinal L1 segment downward. Cultured OECs/ONFs were microinjected into the lateral
parts of the upper and lower stumps adjacent to the injury and into the rim of nonfunctional spared tissue on the right. Four strips of
sural nerve (green) were used to bridge the gap between the stumps over the remaining three fourths of the cord. Within 5 months,
motor recovery (red) had started around the left hip (L1/2 cord segments) and superficial sensation (SS, green) over dermatomal
levels S3–5 (sacral sparing). By 12 months, motor recovery on the left had extended to the leg (L2,3), and deep sensation (DS,
blue) had returned on both sides down to the feet (L5/S1). On the right side of the body, there was a slight but much inferior and
incomplete recovery of motor control, while there was a substantial recovery of SS. In contrast, on the left, there was a predominant
motor recovery but minimal recovery of SS. The patient has been restored from complete paraplegia to a condition resembling an
incomplete Brown–Sequard syndrome. Together with the neurophysiological data, this is consistent with regeneration of descend-
ing motor control (corticospinal) fibers (red) across the injury and their progressive descent with time on the left side of the distal
cord. Similarly, the primary fibers carrying SS (green) from the right side of the body, which cross below the injury level, have also
regenerated across the injury site and made contact with local spinothalamic relay neurons on the left side of the cord above the
injury. The fibers of DS (blue) seem to have been able to cross the medial cord regions (the dorsal columns), which were bridged
by sural nerve strips alone, but in the absence of local relay neurons in the cord, the DS fibers require a longer time for the greater
distance of regeneration needed for sensation to reach the brain. Unlike the progressive downward extension of the motor fibers, the
ascending sensory fibers from all caudal levels are severed at the level of the injury, and there is no preferential recovery of upper
versus lower segmental fibers. The ability of motor and SS fibers to cross the injury requires both OECs/ONFs and sural nerve
bridging. There is an indication that DS fibers may have regenerated across sural nerve bridges without OECs/ONFs. Infiltrating
the nonfunctional residual tissue on the right of the cord with OECs/ONFs, but without interposed peripheral nerve tissue, gives a
much inferior motor and SS result.
TABAKOW ET AL.
ACKNOWLEDGMENTS: The authors are very grateful to Dr.
14. Feron, F.; Perry, C.; Cochrane, J.; Licina, P.; Nowitzke, A.;
Dariusz Szarek from the Department of Neurosurgery Wroclaw
Urquhart, S.; Geraghty, T.; Mackay-Sim, A. Autologous
Medical University for the help during the operation of OEC
olfactory ensheathing cell transplantation in human spinal
transplantation and to Dr. Krzysztof Fortuna for the performed
cord injury. Brain 128(Pt 12):2951–2960; 2005.
statistical analysis of the results. This work was supported by
15. Graziadei, P. P.; Levine, R. R.; Graziadei, G. A. Regeneration
funds from the Wroclaw Medical University in the years 2009–
of olfactory axons and synapse formation in the forebrain
2012 (study number ST 406) and the Nicholls Spinal Injury
after bulbectomy in neonatal mice. Proc. Natl. Acad. Sci.
Foundation and the UK Stem Cell Foundation. The authors
USA 75(10):5230–5234; 1978.
declare no conflict of interest.
16. Hallet, M.; Chokroverty, S. Magnetic stimulation in clini-
cal neurophysiology: Philadelphia PA: Elsevier; 2005.
17. Halon, A.; Materna, V.; Drag-Zalesinska, M.; Nowak-
Markwitz, E.; Gansukh, T.; Donizy, P.; Spaczynski, M.;
1. Bunge, M. B. Bridging the transected or contused adult rat
Zabel, M.; Dietel, M.; Lage, H.; Surowiak P. Estrogen
spinal cord with Schwann cell and olfactory ensheathing
receptor alpha expression in ovarian cancer predicts longer
glia transplants. Prog. Brain Res. 137:275–282; 2002.
overall survival. Pathol. Oncol. Res. 17(3):511–518; 2011.
2. Butcher, J. N.; Graham, J. R.; Ben-Porath, Y. S.; Tellegen,
18. Ibrahim, A.; Li, D.; Collins, A.; Tabakow, P.; Raisman, G.;
A.; Grant, W.; Dahlstrom, W. G.; Kaemmer, B. The
Li, Y. Comparison of olfactory bulbar and mucosal cultures
Minnesota Multiphasic Personality Inventory (MMPI-2).
in a rat rhizotomy model. Cell Transplant. 23(11):1465–
Minneapolis, MN: University of Minnesota Press; 2001.
3. Chen, A.; Xu, X. M.; Kleitman, N.; Bunge, M. B. Methyl-
19. Jerusalem, M.; Schwarzer, R. Generalized Self-Efficacy
prednisolone administration improves axonal regeneration
Scale GSE. Berlin, Germany: Freie Universitat; 1993.
into Schwann cell grafts in transected adult rat thoracic spi-
20. Keyvan-Fouladi, N.; Raisman, G.; Li, Y. Functional repair
nal cord. Exp. Neurol. 138(2):261–276; 1996.
of the corticospinal tract by delayed transplantation of
4. Cheng, H.; Cao, Y.; Olson, L. Spinal cord repair in adult
olfactory ensheathing cells in adult rats. J. Neurosci.
paraplegic rats: Partial restoration of hind limb function.
Science 273(5274):510–513; 1996.
21. Kirshblum, S.; Millis, S.; McKinley, W.; Tulsky, D. Late
5. Cheng, H.; Liao, K. K.; Liao, S. F.; Chuang, T. Y.; Shih,
neurologic recovery after traumatic spinal cord injury.
Y. H. Spinal cord repair with acidic fibroblast growth factor
Arch. Phys. Med. Rehabil. 85(11):1811–1817; 2004.
as a treatment for a patient with chronic paraplegia. Spine
22. Li, Y.; Decherchi, P.; Raisman, G. Transplantation of olfac-
tory ensheathing cells into spinal cord lesions restores
6. Chhabra, H. S.; Lima, C.; Sachdeva, S.; Mittal, A.; Nigam,
breathing and climbing. J. Neurosci. 23(3):727–731; 2003.
V.; Chaturvedi, D.; Arora, M.; Aggarwal, A.; Kapur, R.;
23. Li, Y.; Field, P. M.; Raisman, G. Regeneration of adult
Khan, T. A. Autologous olfactory mucosal transplant in
rat corticospinal axons induced by transplanted olfactory
chronic spinal cord injury: An Indian Pilot Study. Spinal
ensheathing cells. J. Neurosci. 18(24):10514–10524; 1998.
Cord 47(12):887–895; 2009.
24. Li, Y.; Li, D.; Raisman, G. Interaction of olfactory ensheath-
7. David, S.; Aguayo, A. J. Axonal elongation into periph-
ing cells with astrocytes may be the key to repair of tract
eral nervous system "bridges" after central nervous system
injuries in the spinal cord: The ‘pathway hypothesis'.
injury in adult rats. Science 214(4523):931–933; 1981.
J. Neurocytol. 34:343–351; 2005.
8. Ditunno, Jr., J. F.; Ditunno, P. L.; Scivoletto, G.; Patrick,
25. Li, Y.; Sauve, Y.; Li, D.; Lund, R. D.; Raisman, G.
M.; Dijkers, M.; Barbeau, H.; Burns, A. S.; Marino, R. J.;
Transplanted olfactory ensheathing cells promote regen-
Schmidt-Read, M. The Walking Index for Spinal Cord
eration of cut adult rat optic nerve axons. J. Neurosci.
Injury (WISCI/WISCI II): Nature, metric properties, use
and misuse. Spinal Cord 51(5):346–355; 2013.
26. Lima, C.; Escada, P.; Pratas-Vital, J.; Branco, C.; Arcangeli,
9. Doucette, J. R. The glial cells in the nerve fiber layer of the
C. A.; Lazzeri, G.; Maia, C. A.; Capucho, C.; Hasse-
rat olfactory bulb. Anat. Rec. 210(2):385–391; 1984.
Ferreira, A.; Peduzzi, J. D. Olfactory mucosal autografts
10. Eyseneck, H. J.; Eyseneck, S. B. G. Manual of the Eyseneck
and rehabilitation for chronic traumatic spinal cord injury.
Personality Questionnaire. London, UK: Hodder and
Neurorehabil. Neural Repair 24(1):10–22; 2010.
Stoughton; 1975.
27. Lima, C.; Pratas-Vital, J.; Escada, P.; Hasse-Ferreira, A.;
11. Falci, S. P.; Indeck, C.; Lammertse, D. P. Posttraumatic
Capucho, C.; Peduzzi, J. D. Olfactory mucosa autografts
spinal cord tethering and syringomyelia: Surgical treatment
in human spinal cord injury: A pilot clinical study. J. Spinal
and long-term outcome. J. Neurosurg. Spine 11(4):445–
Cord Med. 29(3):191–203; discussion 204–206; 2006.
28. Lindsay, S. L.; Riddell, J. S.; Barnett, S. C. Olfactory
12. Fawcett, J. W.; Asher, R. A. The glial scar and central ner-
mucosa for transplant-mediated repair: A complex tissue
vous system repair. Brain Res. Bull. 49(6):377–391; 1999.
for a complex injury? Glia 58(2):125–134; 2010.
13. Fawcett, J. W.; Curt, A.; Steeves, J. D.; Coleman, W. P.;
29. Loy, D. N.; Kim, J. H.; Xie, M.; Schmidt, R. E.; Trinkaus,
Tuszynski, M. H.; Lammertse, D.; Bartlett, P. F.; Blight,
K.; Song, S. K. Diffusion tensor imaging predicts hyper-
A. R.; Dietz, V.; Ditunno, J.; Dobkin, B. H.; Havton, L. A.;
acute spinal cord injury severity. J. Neurotrauma 24(6):979–
Ellaway, P. H.; Fehlings, M. G.; Privat, A.; Grossman,
R.; Guest, J. D.; Kleitman, N.; Nakamura, M.; Gaviria,
30. Mackay-Sim, A.; Feron, F.; Cochrane, J.; Bassingthwaighte,
M.; Short, D. Guidelines for the conduct of clinical trials
L.; Bayliss, C.; Davies, W.; Fronek, P.; Gray, C.; Kerr, G.;
for spinal cord injury as developed by the ICCP panel:
Licina, P.; Nowitzke, A.; Perry, C.; Silburn, P. A.; Urquhart,
Spontaneous recovery after spinal cord injury and statisti-
S.; Geraghty, T. Autologous olfactory ensheathing cell
cal power needed for therapeutic clinical trials. Spinal Cord
transplantation in human paraplegia: A 3-year clinical trial.
Brain 131(Pt9):2376–2386; 2008.
SPINAL CORD REPAIR: OECs AND NERVE GRAFTS
31. Miedzybrodzki, R.; Tabakow, P.; Fortuna, W.; Czapiga, B.;
mammalian central nervous system. Anat. Rec. B New
Jarmundowicz, W. The olfactory bulb and olfactory mucosa
Anat. 271(1):77–85; 2003.
obtained from human cadaver donors as a source of olfac-
40. Schwartz, E. D.; Chin, C. L.; Shumsky, J. S.; Jawad,
tory ensheathing cells. Glia 54(6):557–565; 2006.
A. F.; Brown, B. K.; Wehrli, S.; Tessler, A.; Murray, M.;
32. Paviot, A.; Guerout, N.; Bon-Mardion, N.; Duclos, C.; Jean,
Hackney, D. B. Apparent diffusion coefficients in spinal
L.; Boyer, O.; Marie, J. P. Efficiency of laryngeal motor
cord transplants and surrounding white matter correlate
nerve repair is greater with bulbar than with mucosal olfactory
with degree of axonal dieback after injury in rats. AJNR
ensheathing cells. Neurobiol. Dis. 41(3):688–694; 2011.
Am. J. Neuroradiol. 26(1):7–18; 2005.
33. Preston, D. C.; Shapiro, B. E. Electromyography and
41. Shanmuganathan, K.; Gullapalli, R. P.; Zhuo, J.; Mirvis,
Neuromuscular Disorders, Clinical-Electrophysiologic Cor-
S. E. Diffusion tensor MR imaging in cervical spine trauma.
relations, 2nd ed. Philadelphia, PA: Elsevier; 2005.
AJNR Am. J. Neuroradiol. 29(4):655–659; 2008.
34. Raisman, G. Specialized neuroglial arrangement may
42. Spielberger, C. D.; Gorusch, R. L.; Lushene, R. E. Manual
explain the capacity of vomeronasal axons to reinnervate
for the State-Trait Anxiety Inventory. Mountain View, CA:
central neurons. Neuroscience 14(1):237–254; 1985.
Consulting Psychologists Press; 1970.
35. Ramon-Cueto, A.; Cordero, M. I.; Santos-Benito, F. F.;
43. Tabakow, P.; Jarmundowicz, W.; Czapiga, B.; Fortuna,
Avila, J. Functional recovery of paraplegic rats and motor
W.; Miedzybrodzki, R.; Czyz, M.; Huber, J.; Szarek, D.;
axon regeneration in their spinal cords by olfactory ensheath-
Okurowski, S.; Szewczyk, P.; Gorski, A.; Raisman, G.
ing glia. Neuron 25(2):425–435; 2000.
Transplantation of autologous olfactory ensheathing cells
36. Ramon-Cueto, A.; Nieto-Sampedro, M. Glial cells from
in complete human spinal cord injury. Cell Transplant.
adult rat olfactory bulb: Immunocytochemical properties
of pure cultures of ensheathing cells. Neuroscience 47(1):
44. van Mastrigt, R.; Griffiths, D. J. ICS standard for digital
213–220; 1992.
exchange of urodynamic study data. Neurourol. Urodyn.
37. Ramon-Cueto, A.; Plant, G. W.; Avila, J.; Bunge, M. B.
Long-distance axonal regeneration in the transected adult
45. Waters, R. L.; Adkins, R. H.; Yakura, J. S.; Sie, I. Motor
rat spinal cord is promoted by olfactory ensheathing glia
and sensory recovery following complete tetraplegia. Arch.
transplants. J. Neurosci. 18(10):3803–3815; 1998.
Phys. Med. Rehabil. 74(3):242–247; 1993.
38. Richardson, P. M.; McGuinness, U. M.; Aguayo, A. J.
46. Yamamoto, M.; Raisman, G.; Li, D.; Li, Y. Transplanted
Peripheral nerve autografts to the rat spinal cord: Studies with
olfactory mucosal cells restore paw reaching function with-
axonal tracing methods. Brain Res. 237(1):147–162; 1982.
out regeneration of severed corticospinal tract fibres across
39. Santos-Benito, F. F.; Ramon-Cueto, A. Olfactory ensheath-
the lesion. Brain Res. 1303:26–31; 2009.
ing glia transplantation: A therapy to promote repair in the
Source: http://www.nsif.org.uk/wp-content/uploads/Tabakow-Cell-2014-Transplantation-spinal-cord-transection.pdf
Report of the Scientific Committee of the Spanish Agency for Consumer Affairs, Food Safety and Nutrition (AECOSAN) on the risk of using Tribulus terrestris in food supplements Section of Food Safety and Nutrition Reference number: AECOSAN-2015-003 Elena Alonso Lebrero, José Manuel Barat Baviera, María Report approved by the Section of Food Safety and
RIA / Vol. 37 / N.À 2 Study and evolution of the qualityof raw milk from dairy farms in thenorthwest of the province of Santa Feand south of the province of Santiagodel Estero, Argentina (1993 – 2009) REVELLI, G.R.1; SBODIO, O.A.2; TERCERO, E.J.2 A total of 10,704 raw milk samples from a bulk tank were collected on 55 dairy farms associated to the Coo- perativa Tambera y Agropecuaria Nueva Alpina Ltda. between 1993 and 2009. Physicochemical, microbiologicaland sanitary parameters were analyzed within the framework of the Comprehensive Milk Quality ImprovementProgram to determine the mean values that characterize the zone. The following values were found: Acidity:16.30 ± 0.96 °D, pH: 6.68 ± 0.04, Fat: 3.48 ± 0.24%, True Protein: 3.11 ± 0.12%, Lactose: 4.74 ± 0.16%, Ash:0.70 ± 0.09%, Total Solids: 12.18 ± 0.42%, Freezing Point: -0.530 ± 0.02 °C, Total Bacterial Count: 9.6 x 104± 2.2 x 105 CFU/ml, Somatic Cell Count: 407,000 ± 230,000 cells/ml and Antibiotic Residues: 99.64% Negative.The most significant correlations were: Fat vs Total Solids (r = 0.784; P < 0.001) and True Protein vs TotalSolids (r = 0.557; P < 0.001). The compositional quality of raw milk from dairy farms in the northwest of SantaFe and south of Santiago del Estero were studied over a period of 17 years and showed a significant improve-ment, particularly regarding the indicators that infer a high industrial value. Optimization of the producers' ma-nagement capacity and the operational quality of dairy farmers contributed to achieve these results.








