Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Upright face-preferential high-gamma responses in lower-order visual areas: evidence from intracranial recordings in children

Contents lists available at
Upright face-preferential high-gamma responses in lower-order visualareas: Evidence from intracranial recordings in children
Naoyuki Matsuzaki Rebecca F. Schwarzlose , Masaaki Nishida Noa Ofen Eishi Asano a Department of Pediatrics, Children's Hospital of Michigan, Wayne State University, Detroit Medical Center, Detroit, MI 48201, USAb Department of Neurology, Children's Hospital of Michigan, Wayne State University, Detroit Medical Center, Detroit, MI 48201, USAc Institute of Gerontology, Wayne State University, Detroit, MI, USAd Department of Anesthesiology, Hanyu General Hospital, Hanyu City, Saitama 348-8505, Japane Trends in Cognitive Sciences, Cell Press, Cambridge, MA 02139, USA
Behavioral studies demonstrate that a face presented in the upright orientation attracts attention more rapidly
Accepted 5 January 2015
than an inverted face. Saccades toward an upright face take place in 100–140 ms following presentation. The
Available online 9 January 2015
present study using electrocorticography determined whether upright face-preferential neural activation, asreflected by augmentation of high-gamma activity at 80–150 Hz, involved the lower-order visual cortex within
the first 100 ms post-stimulus presentation. Sampled lower-order visual areas were verified by the induction
High-frequency oscillations (HFOs)
of phosphenes upon electrical stimulation. These areas resided in the lateral-occipital, lingual, and cuneus gyri
along the calcarine sulcus, roughly corresponding to V1 and V2. Measurement of high-gamma augmentation
during central (circular) and peripheral (annular) checkerboard reversal pattern stimulation indicated that
Pediatric epilepsy surgery
central-field stimuli were processed by the more polar surface whereas peripheral-field stimuli by the more
anterior medial surface. Upright face stimuli, compared to inverted ones, elicited up to 23% larger augmentationof high-gamma activity in the lower-order visual regions at 40–90 ms. Upright face-preferential high-gammaaugmentation was more highly correlated with high-gamma augmentation for central than peripheral stimuli.
Our observations are consistent with the hypothesis that lower-order visual regions, especially those for thecentral field, are involved in visual cues for rapid detection of upright face stimuli.
2015 Elsevier Inc. All rights reserved.
demonstrating thatdetection of an upright face occurs quite early in visual processing.
The human brain has a remarkable ability to efficiently recognize
Neural correlates of face recognition have been extensively exam-
faces. Behavioral studies have shown that picture stimuli containing
ined using functional MRI (fMRI)
the faces of living things, compared to those with other non-face
objects, more rapidly attract gazes from healthy participants, including
electrocorticography (ECoG)
infants (and adults
). These studies have consistently localized
). Among the most investigated
regions of face-selectivity around the fusiform and inferior occipital
features of human face recognition is its selectivity to upright orienta-
gyri. Disruption of the region around the inferior occipital gyrus using
repetitive transcranial magnetic stimulation (rTMS) at 60 and 100 ms
). A face presented in the upright orientation, compared to one
after presentation of face stimuli impairs performance on a discrimina-
flipped upside down, is more easily recognized and automatically
tion task with face parts, demonstrating the early involvement of face-
draws earlier and more sustained attention from healthy infants
selective areas in face processing (Combined with
the observed preferential saccades to upright faces within 100 and
Furthermore, preferential saccades toward
140 ms of stimulus presentation (
an upright face take place within 100–140 ms following presentation
these short timescales highlight the rapid nature of face de-tection and processing. Our knowledge of the time course of face pro-cessing is further informed by the findings from ECoG studies thatactivity for face stimuli, compared to non-face stimuli, elicit greater
⁎ Corresponding author at: Division of Pediatric Neurology, Children's Hospital of
high-gamma augmentation in the ventral occipital–temporal junction
Michigan, Wayne State University. 3901 Beaubien St., Detroit, MI 48201, USA. Fax: +1
313 745 0955.
–300 ms after the onset of stimulus presentation (
E-mail address: (E. Asano).
1053-8119/ 2015 Elsevier Inc. All rights reserved.
N. Matsuzaki et al. / NeuroImage 109 (2015) 249–259
The roles of lower-order visual cortex in face recognition have been
; ). The regions of interest in the present study included:
relatively under-reported. Taking into account that speeded saccadic
(i) lingual gyrus, (ii) cuneus gyrus, (iii) lateral occipital region, and
responses toward an upright face take place as early as 100 ms, it is fea-
(iv) fusiform gyrus posterior to the midbrain ).
sible to hypothesize that the lower-order visual cortex is involvedin low-level visual cues for saccades toward upright face stimuli at
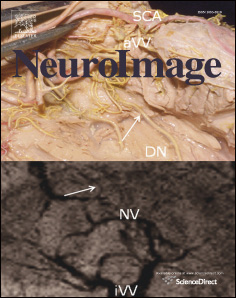
Localization of lower-order visual function by electrical stimulation
b100 ms (). In thepresent study of patients with focal epilepsy who underwent chronic
We localized lower-order visual areas with electrical stimulation
ECoG recording, we investigated this idea using event-related augmen-
mapping performed as a part of presurgical evaluation of medically
tation of high-gamma activity at N80 Hz as a surrogate marker of in-situ
uncontrolled focal seizures (
cortical activation. The strengths of measurement of ECoG high-gamma
In short, we defined the lower-order visual areas
augmentation include: (i) 20 to N100 times better signal-to-noise ratio
as the sites at which stimulation constantly resulted in phosphene, or
compared to noninvasive neurophysiology modality ),
percepts of simple shape, color, or flush light
(ii) a temporal resolution of b20 ms , and (iii) direct
). Areas were not treated as lower-order visual areas if
signal sampling from deep structures such as medial and inferior
their stimulation elicited other types of visual symptoms, such as distor-
surfaces of the occipital lobes (
tion (). We expected that lower-order visual function
In this study, we specifically determined if upright compared to
would involve the lingual, cuneus, and lateral occipital regions close to
inverted face stimuli would elicit larger high-gamma augmentation in
the calcarine sulcus.
lower-order visual sites at b100 ms following stimuli presentation.
A train of repetitive electrical stimuli was delivered to a pair of
We hypothesized that upright face-preferential high-gamma augmen-
subdural electrodes using the Grass stimulator (Astro-Med, Inc, West
tation would involve the lower-order visual regions, especially the
Warwick, RI), and clinical symptoms elicited by stimulation were
portions of these regions dedicated to the central rather than the pe-
observed by at least two investigators including a neuropsychologist.
ripheral field, as humans tend to fixate face parts (such as eyes, nose,
The stimulus frequency was 50 Hz, the pulse duration was 300 μs, and
and mouth) rather than the facial contour or peripheral background
the train duration ranged up to 2 s. To determine the presence of after-
during natural viewing
discharges, video-ECoG was recorded continuously during the entiremapping session. When a clinical symptom was elicited, the train of stim-
uli was immediately terminated. Stimulus intensity was initially set to3 mA and was increased to 6 and 9 mA in a stepwise manner until a clin-
ical symptom or after-discharge was observed. Once the after-dischargethreshold was determined, stimulus intensity above that threshold was
The inclusion criteria of the present study included: (i) extraoperative
no longer utilized. Sites at which stimulation consistently (at least
ECoG recording as a part of clinical management of medically-
twice) elicited a clinical symptom were classified as eloquent areas specif-
uncontrolled seizures at Children's Hospital of Michigan in Detroit,
ic to a given symptom. Sites were declared ‘not proven to be eloquent' if
(ii) ECoG sampling from the occipital and occipital–temporal regions,
after-discharges were elicited without a symptom, or a symptom failed to
(iii) completion of a visual task described below, and (iv) age of
be elicited by maximal stimuli. To minimize worry or fear on the part of
5 years and above. The exclusion criteria included: (i) brain malforma-
the patient, each individual was informed prior to the stimulation study
tions or seizure onset zone involving the occipital lobe, (ii) oculomotor
that she/he might have a transient sensorimotor, auditory, visual, olfacto-
dysfunction or visual field deficits detected by confrontation, and
ry, gustatory, or language symptom. Each patient was aware of the timing
(iii) severe cognitive dysfunction reflected by verbal comprehension
of each stimulation trial but unaware of the location of stimulated sites.
index of b70. The study was approved by the Institutional ReviewBoard at Wayne State University, and written informed consent was
Extraoperative ECoG recording
obtained from the guardians of all participants.
Extraoperative video-ECoG recordings were obtained using a 192-
Subdural electrode placement
channel Nihon Kohden Neurofax 1100A Digital System (Nihon KohdenAmerica Inc, Foothill Ranch, CA, USA). The sampling frequency was set
Platinum grid and strip electrodes (10 mm intercontact distance,
at 1000 Hz with the amplifier band pass at 0.08–300 Hz (
4 mm diameter) were surgically implanted for extraoperative ECoG
The averaged voltage of ECoG signals derived from the fifth and
recording (. All electrode plates were stitched
sixth intracranial electrodes of the ECoG amplifier was used as the origi-
to adjacent plates and/or the edge of dura mater, to avoid movement of
nal reference. ECoG signals were then re-montaged to a common average
subdural electrodes after placement. Intraoperative pictures were taken
reference Channels contam-
with a digital camera before dural closure. Planar x-ray images (lateral
inated with large interictal epileptiform discharges or artifacts were ex-
and anteroposterior) were subsequently acquired with the subdural
cluded from the common average reference. No notch filter was used.
electrodes in place for electrode localization on the brain surface
All antiepileptic medications were discontinued on the day of subdural
electrode placement. Electrodes overlying seizure onset zones or struc-
fiducial markers were placed at anatomically well-defined locations
tural lesions were excluded from further analysis ().
on the patient's head for co-registration of the x-ray image with the
Surface EMG electrodes were placed on the left and right deltoid muscles,
T1-weighted spoiled gradient echo MR image. A three-dimensional
and electrooculography (EOG) electrodes were placed 2.5 cm below and
surface image was finally created with the location of electrodes directly
2.5 cm lateral to the left and right outer canthi.
defined on the brain surface ().
We confirmed the spatial accuracy of electrode display on the three-
dimensional brain surface image using intraoperative pictures(). By the
During video-ECoG recording, a series of visual stimuli were
age of 5 years, brain size becomes comparable to that of an adult
presented to each patient using a 22-inch Dell P2213 LCD monitor (a
(), while the youngest patient in the
refresh rate of 60 Hz; a pixel resolution of 1600 × 1050; Dell Inc,
present study was 9 year old. Automatic parcellation of cortical gyri
Round Rock, TX, USA) placed 60 cm away from the patient. The task
was performed using FreeSurfer software (), and
lasted approximately four minutes. Each patient completed the task
subdural electrodes were assigned anatomical labels (
while awake, unsedated, and comfortably seated on the bed in a
N. Matsuzaki et al. / NeuroImage 109 (2015) 249–259
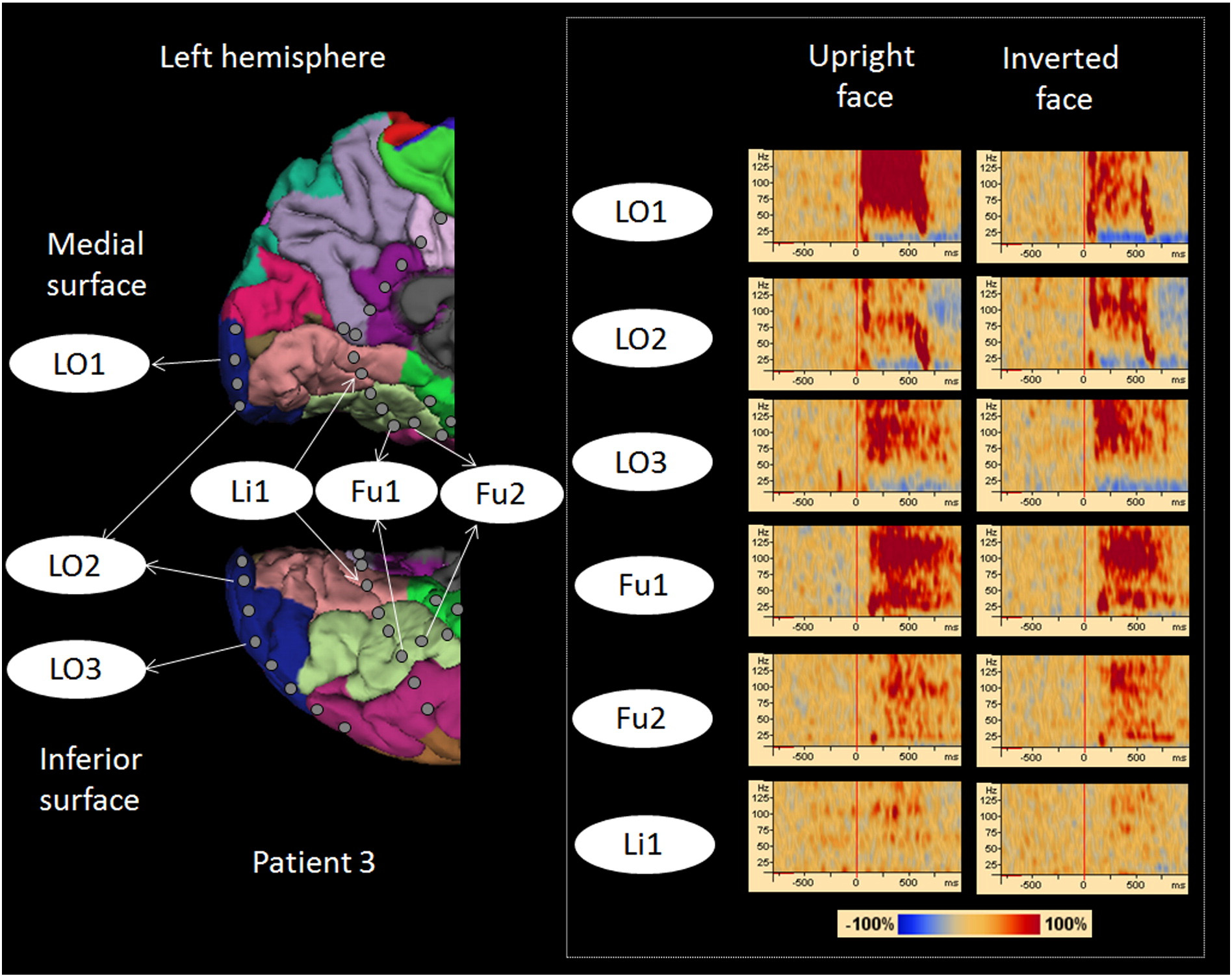
Fig. 1. Location of subdural electrodes over the occipital–temporal regions. (A) Patient 1. (B) Patient 2. (C) Patient 3. (D) Patient 4. (E) Patient 5. (F) Patient 6. Cortical gyri were parcellatedand color coded using FreeSurfer software ). White circles: sites of which stimulation resulted in phosphene. Red circles: sites of which stimulation resulted in othervisual percepts ("Objects looking funny" in Patient 1; "Funny looking" in Patient 2; "Background felt moving" during stimulation of lateral occipital sites in Patient 4; "I saw somethingbut cannot tell what it is" during stimulation of a lingual–fusiform pair in Patient 4; "Something blurry and transparent but not anything" during stimulation of three pairs in thelateral-occipital regions and "something changes" during stimulation of lingual sites in Patient 5). Gray circles: sites of which stimulation resulted in no visual symptom. Circle withX: Electrode affected by artifacts (excluded from analysis).
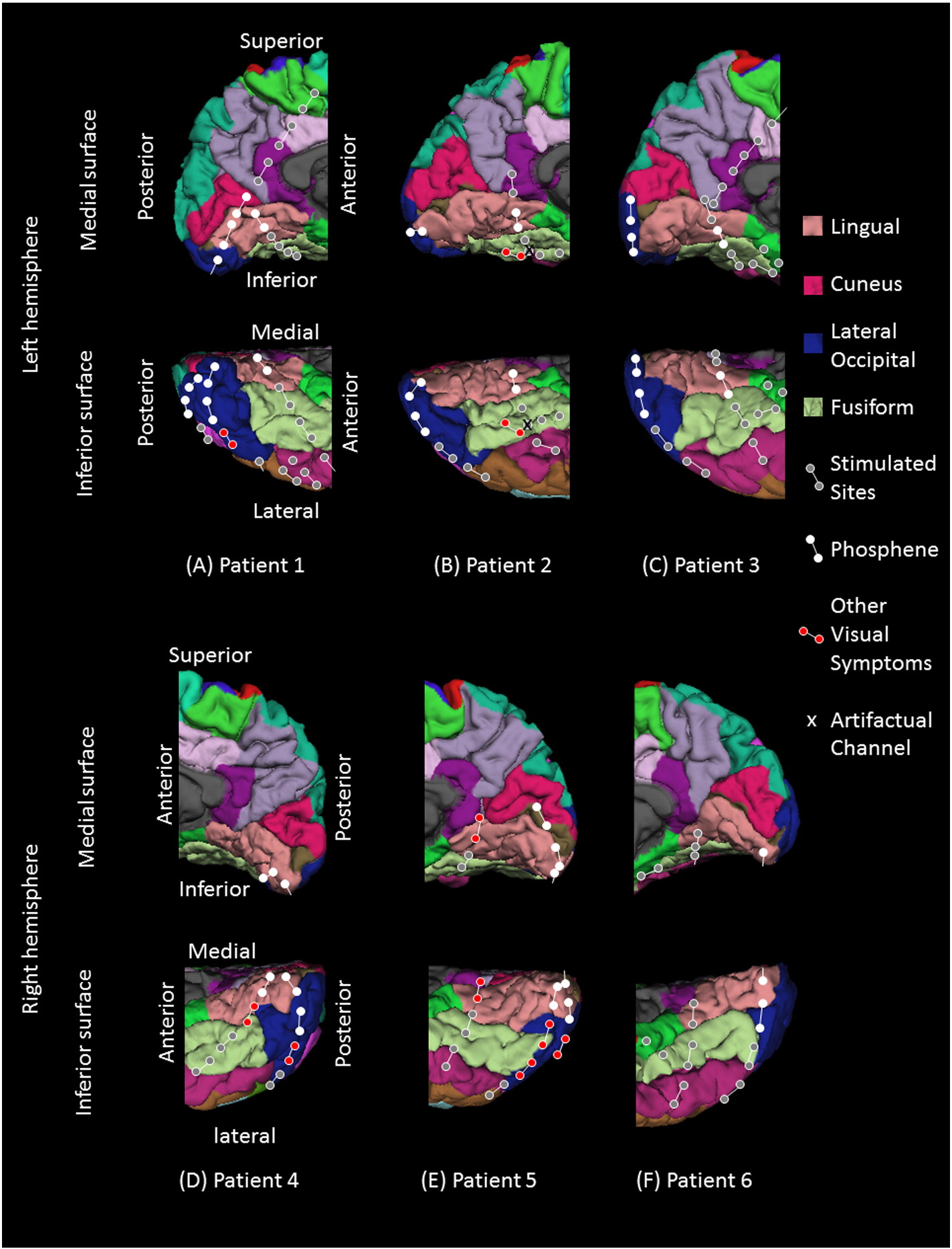
dimly-lit room. Stimuli included a star-shaped drawing, upright faces,
house stimuli were presented 40 times exclusively in the upright
inverted faces, upright houses, a circular checkerboard reversal pattern,
orientation. A black-and-white circular checkerboard reversal pattern
and an annular checkerboard reversal pattern (). Stimuli were
(radius: 9.6°) and an annular checkerboard reversal pattern (inner
binocularly presented in grayscale on a black background at the center
radius: 9.6°; outer radius: 23°), each presented with a pulse duration
of the monitor. They appeared in a pseudorandom order for a duration
of 133 ms and a train duration of 533 ms, were each presented four
of 533 ms each, with an inter-stimulus interval ranging from 2.0 to
times. In other words, a total of 16 pulses of circular and annular check-
2.5 s. The order of presentation of stimuli was counterbalanced across
erboard stimuli were presented to each patient to localize the lower-
image categories. Each patient was instructed to fixate on the center
order visual cortex for the central and peripheral fields, respectively.
of the screen (indicated by the fixation cross of 1° height and widthduring the inter-stimulus interval), and to overtly say ‘star' only when
Measurement of event-related gamma activity
the target star-shaped drawing (size: 9 × 9°; color: white) was present-ed. No other action was required. Stimuli also included 40 faces and
The general principle of our time-frequency analysis was previously
40 houses ). Each of the
face and house stimuli was cropped into an oval-like shape (about 11°
). In short, we determined the spatial-temporal dynamics of
width and 16° height). Face stimuli were presented 40 times in the
high-gamma amplitudes at 80–150 Hz following the onset of image
upright orientation and 40 times in the inverted orientation, whereas
presentation, and we believe this is a reasonable summary measure of
N. Matsuzaki et al. / NeuroImage 109 (2015) 249–259
Fig. 2. Examples of visual stimuli. (A) Upright face. (B) Inverted face. (C) House. (D) Circular checkerboard pattern. (E) Annular checkerboard pattern. (F) Star shape.
in-situ cortical activity. A study of macaque monkeys using single-unit
upright and inverted face stimuli as well as between lower-order visual
recordings suggested that task-related augmentation of high-gamma
regions and other sites. We hypothesized that upright face-preferential
activity was tightly correlated to an increased firing rate (
high-gamma augmentation occurred at this time because a behavioral
Our previous ECoG study of the effect of photic stimulation on
study suggested preferential saccades toward an upright face occurred
the occipital cortex revealed that the earliest and largest amplitude
as early as 100 ms This analysis was done using a
augmentation involved the 80–150 Hz frequency band (
linear mixed-model analysis, with ‘stimulus category' (e.g., upright vs
). The peak frequency of high-gamma modulations during
inverted face), ‘phosphene' (presence or absence), and ‘interaction be-
various forms of sensorimotor and cognitive tasks also involved this
tween stimulus category and phosphene' as fixed effects, and with ‘indi-
frequency band ().
vidual electrodes' as a random effect. Subsequently, estimates of fixed
Each ECoG trial was transformed into the time–frequency domain
effects were plotted from 10 until 300 ms in order to determine when up-
using a complex demodulation technique (
right face-preferential high-gamma augmentation in the lower-order vi-
incorporated in BESA® EEG V.5.1.8 software (BESA GmbH, Gräfelfing,
sual cortex was maximized. The level of significance was set at p = 0.05.
German; ). The time-frequency transform wasobtained by multiplication of the time-domain signal with a complex
Relationship between high-gamma activities elicited by upright face and
exponential, followed by a low-pass filter. The low-pass filter used
checkerboard stimuli
here was a finite impulse response filter of Gaussian shape, makingthe complex demodulation effectively equivalent to a Gabor transform.
We determined whether high-gamma augmentation for upright
The filter had a full width at half maximum of 2 × 15.8 ms in the tempo-
faces coincided with central- or peripheral-field lower-order visual
ral domain and 2 × 7.1 Hz in the frequency domain. The corresponding
processing. The analysis was done using a linear mixed-model analysis
time-frequency resolution was ±15.8 ms and ±7.1 Hz (defined as the
as described above. Fixed factors here included circular and annular
50% power drop of the finite impulse response filter). A given ECoG
stimuli-related high-gamma augmentation (Based upon previ-
signal was assigned an amplitude (a measure proportional to the square
ous observations
root of power) as a function of time and frequency at each trial. Time-
we expected that central-field checkerboard stimuli
frequency transformation was performed for frequencies between 80
would elicit the largest gamma augmentation in sites proximal to the
and 150 Hz and latencies between −800 and +1000 ms relative to
occipital pole, whereas peripheral-field stimuli would elicit the largest
the image presentation, in steps of 5 Hz and 10 ms. At each time-
augmentation in the medial occipital sites along the calcarine fissure.
frequency bin, we analyzed the percent change in amplitude (averaged
Thereby, we expected that upright face-preferential high-gamma
across trials) relative to the mean amplitude in a reference period at
augmentation would be more highly correlated with high-gamma
−800 to −600 ms relative to the onset of image presentation. Such a
augmentation induced by central than peripheral stimuli.
change in amplitude has been termed "temporal spectral evolution"(TSE)
Statistical assessment of high-gamma activity preferentially elicited by
Patient profiles
upright face stimuli
We studied six children with focal epilepsy (age range: 9–17 years;
Using the statistical software incorporated in IBM SPSS Statistics 22
average age: 13.5 years; 3 females, ) who satisfied the inclusion
(IBM Corp., Chicago, IL, USA), we subsequently determined whether
and exclusion criteria described above. All six children were right-
the percent change of high-gamma activity at 90 ms differed between
handed and had normal or corrected-to-normal visual acuity. Across
N. Matsuzaki et al. / NeuroImage 109 (2015) 249–259
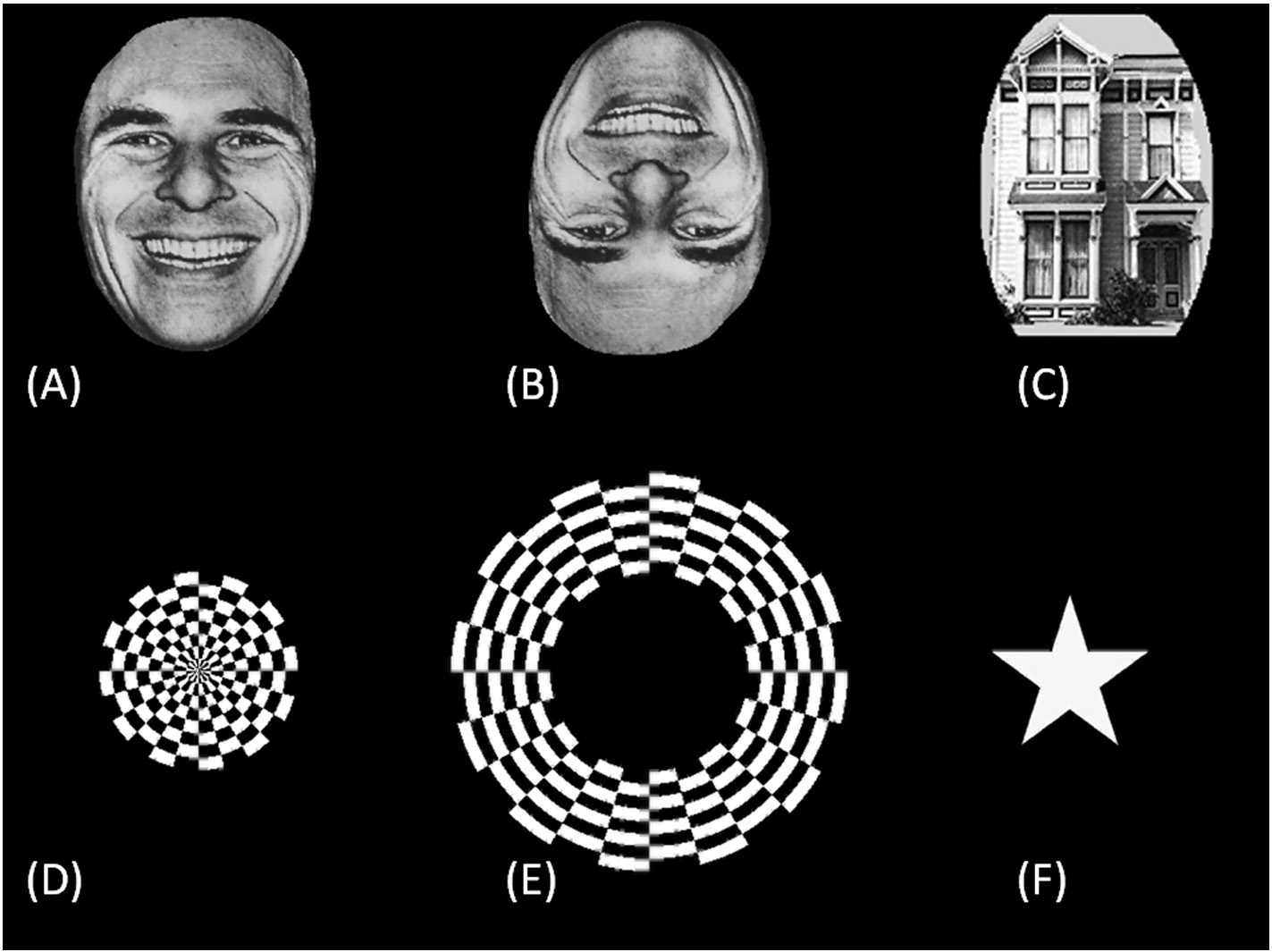
Fig. 3. High-gamma augmentation elicited by checkerboard pattern stimuli. (A) Patient 1. (B) Patient 2. (C) Patient 3. (D) Patient 4. (E) Patient 5. (F) Patient 6. White and red semicirclesreflect the averaged percent change in high-gamma activity during circular (central) and annular (peripheral) checkerboard pattern stimulations, respectively. The degree of high-gammaaugmentation elicited by central-field stimuli was larger in the region proximal to the occipital pole, while that by peripheral-field stimuli was larger in the more anterior medial surface ofthe occipital lobe. Gray circles: electrode sites outside the regions of interest. Gray circle with X: Electrode affected by artifacts (excluded from analysis).
patients, a total of 28, 3, 36, and 26 electrodes, free of artifacts, were lo-
Electrical stimulation data
cated in the lingual gyrus, cuneus gyrus, lateral occipital region, and fu-siform gyrus posterior to the midbrain, respectively ). All of these
Across patients, stimulation of 46 sites elicited phosphenes. These
93 electrodes were included for further analysis.
were classified as lower-order visual sites (21 in the lingual, 3 in thecuneus, 21 in the lateral-occipital, and 1 in the fusiform gyrus). The
only fusiform site classified as a lower-order visual area was located
Patient profile.
very near to the lingual gyrus ). Visual assessment suggested
that the lower-order visual sites defined by electrical stimulation
in the present study lay within Brodmann Areas 17 and 18
Stimulation of 15 sites elicited perception of some forms of visual dis-
tortion (2 in the lingual, 0 in the cuneus, 8 in the lateral occipital, and 5 in
the fusiform gyrus) (The percepts included: "Objects looking
", "Background felt moving", "I saw something but cannot tell
what it is", and "Something blurry and transparent but not anything".
LCM: Lacosamide. LEV: Levetiracetam. LMG: Lamotrigine. OXC: Oxcarbazepine. PHT:
The remaining 32 sites were not proven to be eloquent by electrical
phenytoin. VPA: Valproic acid. ZNS: Zonisamide.
a Seizures were not captured, and a structural lesion in the left parietal lobe was removed.
stimulation (4 in the lingual, 0 in the cuneus, 7 in the lateral occipital,
N. Matsuzaki et al. / NeuroImage 109 (2015) 249–259
and 21 in the fusiform gyrus). The 2 by 4 Fisher Exact Probability Test
(F = 5.1; p = 0.027); no significant effect of stimulus category was
suggested that the chance of stimulation-induced visual symptoms
found (F = 1.0; p = 0.76). The fixed effect of phosphene on high-
was greater in the lingual, cuneus, and lateral occipital regions,
gamma augmentation was 0.98 (95% confidence interval [95%CI]: 0.61
compared to in the fusiform gyrus (p b 0.001).
to 1.35), whereas that of interaction between phosphene and uprightface was 0.23 (95%CI: 0.027 to 0.44). In other words, lower-order visual
High-gamma augmentation elicited by checkerboard reversal
sites, compared to the remaining sites, showed 98% larger high-gamma
augmentation at 90 ms, and upright face stimuli, compared to invertedones, showed 23% larger high-gamma augmentation only within the
Both circular (central-field) and annular (peripheral-field) checker-
lower-order visual sites at the same moment. The plots of estimated
board stimuli elicited high-gamma augmentation in the occipital lobe
fixed effects suggest that the effect of lower-order visual sites on high-
with the maximum peak amplitude in the lower-order visual sites de-
gamma augmentation became larger than zero between 40 and
fined by electrical stimulation ). A clear gradient of high-gamma
150 ms with a peak at 90 ms. Likewise, the effect of interaction between
amplitudes across sites in the anterior–posterior axis was noted;
visual processing sites (lower-order versus others) and face orientation
central-field stimuli were associated with largest high-gamma augmen-
became larger than zero between 40 and 90 ms with the peak occurring
tation in the polar region, while peripheral-field stimuli with largest
at 80–90 ms namely, upright-face-preferential high-gamma
high-gamma augmentation in the anterior medial region ).
augmentation in lower-order visual regions occurred within 100 msof stimulus presentation and lasted for 50 ms. shows relative
High-gamma augmentation preferentially elicited by upright, compared to
high-gamma augmentation elicited by upright vs inverted faces at 40–
inverted face stimuli
90 ms at each electrode site, and we found that upright face-preferential high-gamma augmentation intensely involved the lower-
Examples of high-gamma augmentation elicited by upright and
order visual sites especially in the occipital pole.
inverted faces are presented in The temporal dynamics of suchhigh-gamma activities in the lower-order visual sites as well as fusiform
Upright face-preferential high-gamma augmentation exerted by lower-
sites are presented in A linear mixed-model analysis employed to
order visual sites dedicated to the central rather than the peripheral field
upright and inverted face-related high-gamma augmentation at 90 msrevealed a significant effect of phosphene (F = 37.4; p b 0.001) and a
The plots of estimated fixed effects on linear mixed-model anal-
significant interaction between phosphene and stimulus category
ysis employed to upright and inverted face-related high-gamma
Fig. 4. High-gamma augmentation elicited by upright- and inverted-face stimuli. Time-frequency plots are presented in the right side. Both types of face stimuli elicited early high-gammaaugmentation in lateral-occipital sites (Channels LO1, LO2, and LO3) and delayed augmentation in fusiform sites (Channels Fu1 and Fu2). High-gamma augmentation at a lingual site (Li1)was, if any, minimal. Upright face stimuli, compared to inverted ones, elicited larger high-gamma augmentation at Channel LO1.
N. Matsuzaki et al. / NeuroImage 109 (2015) 249–259
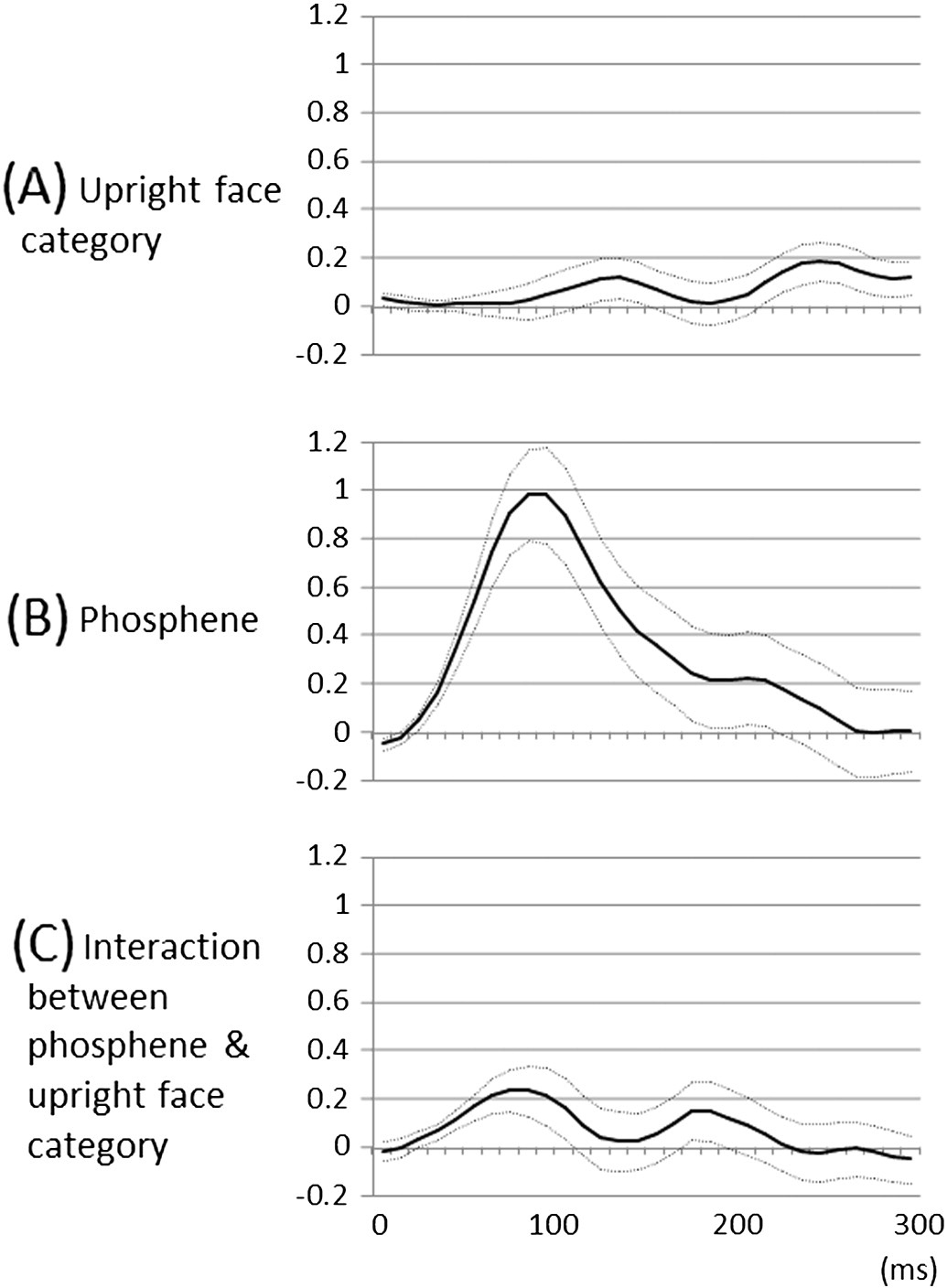
Fig. 6. Fixed effects of ‘stimulus category' and ‘underlying lower-order visual function' onhigh-gamma augmentation over the time course. (A) The linear mixed-model analysissuggested that the effect of upright face category on high-gamma augmentation became
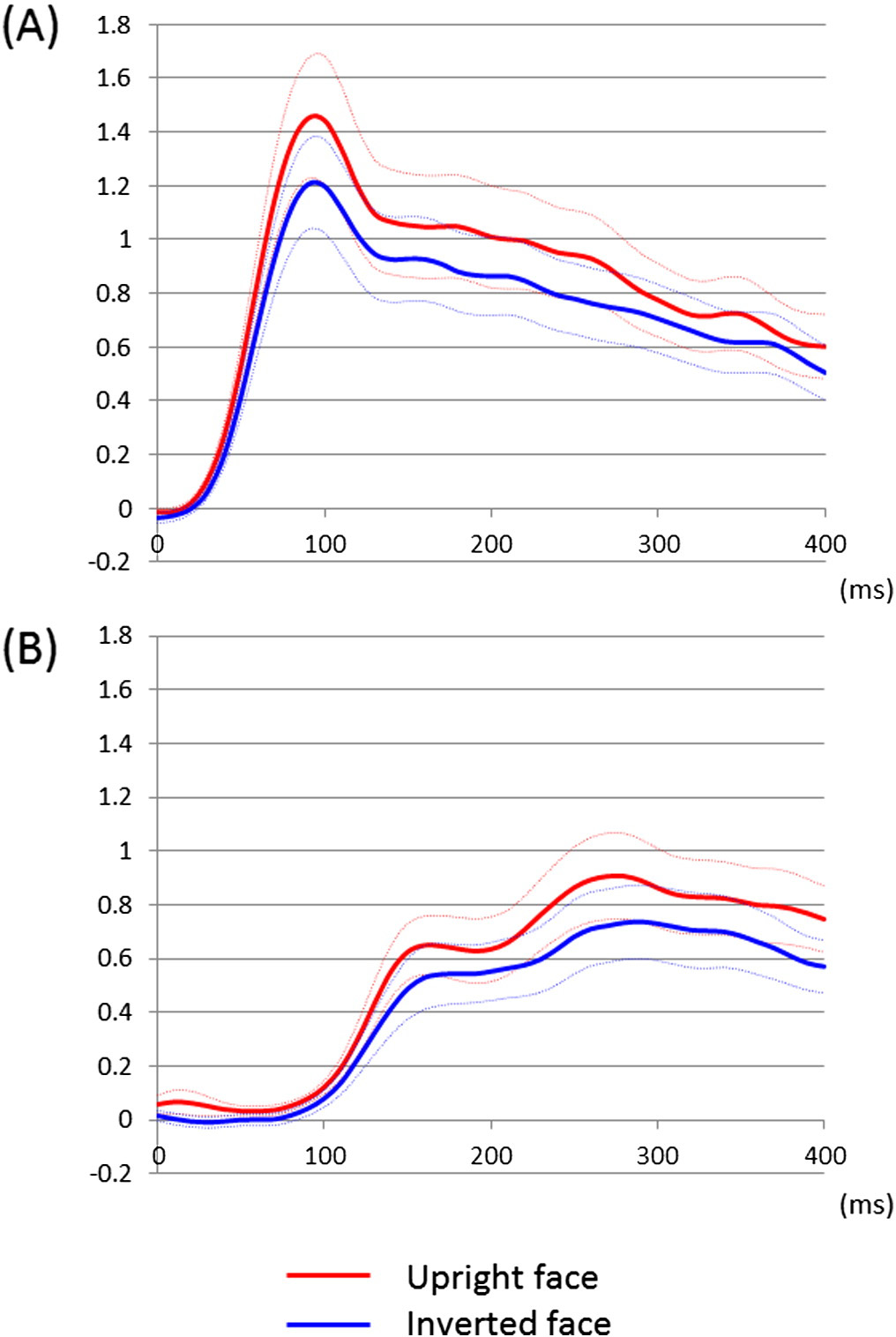
Fig. 5. Temporal dynamics of high-gamma augmentation elicited by upright- and
larger than zero at 240–260 ms, compared to that of inverted face. During this time period,
inverted-face stimuli. (A) The mean high-gamma amplitudes across 46 lower-order visual
upright face category was associated with larger high-gamma augmentation up to 18%
sites are plotted over the time (red line: upright face; blue line: inverted face; dotted lines:
compared to inverted face category, regardless of the underlying lower-order visual
mean ± standard error). Upright face stimuli, compared to inverted ones, elicited at least
function. Solid line: estimated mean. Dotted lines: mean ± standard error. (B) Likewise,
20% larger high-gamma augmentation in lower-order visual sites at 70 to 110 ms. (B) The
the effect of lower-order visual sites defined by stimulation-induced phosphene was
mean high-gamma amplitudes across 25 fusiform sites not associated with stimulation-
larger than zero at 40–150 ms, compared to that of the remaining sites. During this period,
induced phosphene are plotted over the time. High-gamma augmentation in these sites
lower-order visual sites were associated with larger high-gamma augmentation up to 98%
was minimal at b100 ms. Upright face stimuli, compared to inverted ones, elicited at
compared to the remaining sites. (C) The effect of interaction between lower-order visual
least 15% larger high-gamma augmentation in such fusiform sites at 230 to 290 ms.
sites and upright face category became larger than zero at 40–90 ms. During this period,upright face stimuli elicited up to 23% larger high-gamma augmentation compared toinverted ones only within the lower-order visual sites.
augmentation are presented in In short, ‘upright face stimuli-preferential high-gamma augmentation' was correlated to ‘high-gamma augmentation elicited by central-field checkerboard stimuli'
lower-order visual sites could have been simply attributed to the
better than to ‘such augmentation elicited by peripheral-field stimuli'.
location of electrode sites relative to the calcarine fissure. In general,
The effect of interaction between central-field and upright face-
the occipital cortex below the calcarine fissure processes visual informa-
preferential high-gamma augmentations was larger than zero between
tion in the upper field, while that above the calcarine fissure does so for
50 and 300 ms (, whereas the effect of interaction between
the lower field We
peripheral-field and upright face-preferential high-gamma augmenta-
found that 8 and 38 out of the 46 lower-order visual sites were located
tions was larger than zero only between 70 and 90 ms E).
above and below the calcarine fissures, respectively, in the present
At 90 ms, each 100% increase in central-field-related high-gamma
augmentation was associated with an 18% upright face-preferential
We applied a linear mixed-model analysis to determine whether the
high-gamma augmentation in a given site, whereas each 100% increase
percent change of high-gamma activity at 90 ms were associated with
in peripheral-field-related high-gamma augmentation was associated
following fixed factors: ‘stimulus category' (i.e.: upright vs inverted
with only a 6% upright face-preferential high-gamma augmentation.
face), ‘upper/lower visual fields' (i.e.: above vs below the calcarinefissure), and ‘interaction between stimulus category and visual fields'.
A significant effect of stimulus category (F = 5.9; p = 0.02) was
Relationship between upper/lower visual fields and upright
found, but neither significant effect of visual fields (F = 2.8; p = 0.1)
face-preferential high-gamma augmentation
nor interaction between stimulus category and visual fields was found(F = 0.2; p = 0.6). We likewise explored if the percent change of
As a post-hoc analysis, we determined if upright face-preferential
high-gamma activity at 40–80 ms differed between the lower-order
high-gamma augmentation (relative to inverted face stimuli) in
visual sites above and below the calcarine fissures. This linear mixed-
N. Matsuzaki et al. / NeuroImage 109 (2015) 249–259
Fig. 7. Upright- and inverted-face-related high-gamma augmentation, at 40–90 ms, at individual electrode sites. Red filled circles: sites showing upright-face-related high-gammaaugmentation larger than inverted-face-related one. Blue filled circles: sites showing inverted-face-related high-gamma augmentation larger than upright-face-related one. Whiterings: lower-order visual sites defined by electrical stimulation.
model analysis failed to find a significant effect of visual fields or
40 and 90 ms post-stimulus presentation. Our ECoG observation
interaction between stimulus category and visual fields (p N 0.1).
supports the hypotheses that the lower-order visual cortex is involvedin preferential processing of upright face stimuli at b100 ms, and
High-gamma augmentation elicited by upright face stimuli compared to
provides a plausible explanation for the behavioral observation that
saccadic responses toward an upright face take place as early as100 ms (. We also demonstrated that upright
The results are summarized in Supplementary Figure S1 and S2
face-preferential cortical activation was better correlated with lower-
(online material). In short, a linear mixed-model analysis suggested a
order visual function dedicated to the central field rather than that to
significant positive effect of phosphene on high-gamma augmentation
the periphery. This finding is consistent with an fMRI observation that
as well as a significant interaction between visual processing level of
the BOLD response during face recognition is concentrated in the
sites (lower-order versus other) and stimulus category; house stimuli,
occipital–temporal visual pathway for the central visual field (
compared to upright faces, showed greater high-gamma augmentation
within the lower-order visual sites. As best illustrated in Supplementary
The ECoG effect of face inversion, taking place at a latency of 40–
Figures S2C and S2E (online material), preferential high-gamma
90 ms, is difficult to explain by the effect of feedback from higher-
augmentation in response to house stimuli, compared with upright
order visual areas. Our observation of such rapid category-specific
face stimuli, was correlated to that elicited by peripheral-field checker-
neural activation in the lower-order visual regions can be incorporated
board stimuli better than that by central field.
into the conceptual framework for understanding feed-forward hierar-chical processing in the visual system. We believe that such rapid
upright face-preferential high-gamma augmentation may reflect theinitial visual cues facilitating face detection rather than face recognition
In this study, we identified a neural correlate of the face inversion
per se. Although the origins of this effect are not yet known, it may
effect within lower-order visual regions at a temporal range between
reflect a sensitivity at the earliest stages of cortical visual processing to
N. Matsuzaki et al. / NeuroImage 109 (2015) 249–259
Fig. 8. Fixed effects of ‘stimulus category' and ‘cortical retinotopy' on high-gamma augmentation over the time course. (A) The effect of upright face category on high-gamma augmenta-tion, compared to that of inverted face. (B) The effect of high-gamma augmentation elicited by central-field checkerboard stimulation. (C) The effect of interaction ‘upright face category'and ‘high-gamma augmentation elicited by central-field checkerboard stimulation'. At 90 ms, each 100% increase in central-field-related high-gamma augmentation was associated withan 18% upright face-preferential high-gamma augmentation in a given site. (D) The effect of high-gamma augmentation elicited by peripheral-field checkerboard stimulation. (E) Theeffect of interaction ‘upright face category' and ‘high-gamma augmentation elicited by peripheral-field checkerboard stimulation'. At 90 ms, each 100% increase in peripheral-field-relatedhigh-gamma augmentation was associated with a 6% upright face-preferential high-gamma augmentation in a given site.
the characteristic arrangement of low-level features signaling the
augmentation within lower-order visual areas upon face detection
presence of one of the most prevalent and salient stimuli that people
or recognition, possibly by correlating category-preferential high-
encounter: ‘the upright human face'. Indeed, it has been previously
gamma augmentation and the effect of direct stimulation on face
proposed that low-level amplitude spectrum patterns commonly seen
detection or recognition tasks. Unlike TMS, electrical stimulation via
in upright face stimuli would lead to rapid saccades toward upright
subdural electrodes can be delivered to a small region in the medial-
inferior surfaces of the occipital lobes ().
Causal significance of upright face-preferential high-gamma augmentation
Given the limited sample possible in ECoG studies, we are not able
in face recognition
to make strong interpretations regarding the developmental effects inour measurements. Moreover, based on this study it is not possible to
While category-preferential high-gamma augmentation is often
infer whether our ECoG findings derived from older children would
inferred to be a biomarker reflecting cortical processing for recognition
be generalizable to infant or adult populations. In the present study,
of a given category image, we must emphasize that the causal signifi-
regardless of age, all study patients equally appeared engaged to the
cance of upright face-preferential high-gamma augmentation noted in
task. Our previous ECoG study of 77 patients failed to find a significant
this study still remains to be determined. We presented upright face
difference in the spectral frequency of language-related high-gamma
stimuli with the eyes in the center-to-upper field and the mouth in
activities across ages from 4 to 56 ). Neuroimaging
the lower field, as many of the previous studies did (
studies have demonstrated a protracted development of higher-order
face-selective regions through late childhood and into adolescence,
Therefore, upright face-preferential high-gamma aug-
with a trajectory of increasing face-selectivity with increasing age
mentation in the lower-order visual areas could be simply attributed
that plateaus by early adulthood (
to a richer physical properties of eyes-eyebrows compared to a mouth.
If this developmental pattern generalizes to face-specific effects
We applied a post-hoc analysis to test this possibility, but failed to
elsewhere in the brain, we might expect that adults would show the
explain upright face-preferential high-gamma augmentation by
same or greater high-gamma augmentation for upright over inverted
predominant ECoG sampling from the sites below the calcarine fissure.
faces in lower-order visual cortex than we observed in our patients,
Further studies on a larger number of participants are necessary to
whereas younger children and infants might be expected to show
determine the causal role of upright-face-preferential high-gamma
less. However, further studies of a larger number of patients across
N. Matsuzaki et al. / NeuroImage 109 (2015) 249–259
variable age groups would be necessary to conclusively determine how
Appendix A. Supplementary data
the high-gamma augmentation effects described here may generalizeto other stages of development.
Supplementary data to this article can be found online at
Other methodological considerations
Our ECoG studies inevitably suffer from sampling limitations, and
we were unable to exclude variability in spatial sampling across partic-
Alkonyi, B., Juhász, C., Muzik, O., Asano, E., Saporta, A., Shah, A., Chugani, H.T., 2009.
ipants. The location of subdural electrode placement was purely deter-
mined by the clinical needs (; several subdural
Amunts, K., Malikovic, A., Mohlberg, H., Schormann, T., Zilles, K., 2000.
strip electrodes were placed in the occipital and temporal regions in
order to determine the boundary between the epileptogenic zone and
eloquent visual areas. Placement of larger grid electrodes in the occipital
Asano, E., Juhász, C., Shah, A., Sood, S., Chugani, H.T., 2009a.
lobe was not indicated in any of our patient cohort. Nonetheless, all pa-
tients showed similar spatial patterns in high-gamma augmentation
Asano, E., Nishida, M., Fukuda, M., Rothermel, R., Juhász, C., Sood, S., 2009b.
elicited by central- and peripheral-field checkerboard pattern stimuli
(). Large high-gamma augmentation elicited by central-field
Ball, T., Kern, M., Mutschler, I., Aertsen, A., Schulze-Bonhage, A., 2009.
stimuli was noted in the occipital pole, whereas that by peripheral-
field stimuli was noted in the more anterior-medial occipital region.
Birmingham, E., Meixner, T., Iarocci, G., Kanan, C., Smilek, D., Tanaka, J.W., 2013.
Electrodes located in the fusiform gyri, traditionally considered to be a
part of higher-order visual areas
Brown, E.C., Rothermel, R., Nishida, M., Juhász, C., Muzik, O., Hoechstetter, K., Sood, S.,
did not show large high-gamma
Chugani, H.T., Asano, E., 2008.
augmentation elicited by such a simple checkerboard stimuli.
Another limitation of ECoG studies is the challenge of recruiting
Crouzet, S.M., Thorpe, S.J., 2011.
participants. The present ECoG study, like many others using this tech-
nique, is based on data from a limited number of participants. Although
Crouzet, S.M., Kirchner, H., Thorpe, S.J., 2010.
these sample sizes constrain our ability to draw conclusions, it is worth
Dalal, S.S., Edwards, E., Kirsch, H.E., Barbaro, N.M., Knight, R.T., Nagarajan, S.S., 2008.
noting that measurement of ECoG high-gamma augmentation repre-
sents up to a 100-fold improvement in signal-to-noise ratio over scalp
Dekaban, A.S., Sadowsky, D., 1978.
EEG recording Thus, many ECoG studies of less than
10 patients, using a repeated measurement approach, benefited from
sufficient power to observe statistically significant effects on visually-
Desikan, R.S., Ségonne, F., Fischl, B., Quinn, B.T., Dickerson, B.C., Blacker, D., Buckner, R.L.,
Dale, A.M., Maguire, R.P., Hyman, B.T., Albert, M.S., Killiany, R.J., 2006.
related high-gamma measures ).
We localized lower-order visual sites using a bipolar stimulation
method (). Because patient partic-
Ekman, P., Friesen, W., 1976.
ipation time was limited, we were unable to determine whether either
Engell, A.D., McCarthy, G., 2010.
or both of the electrodes within each pair were responsible for genera-
tion of phosphene; thus, we are unable to exclude the possibility that a
Farah, M.J., Wilson, K.D., Drain, M., Tanaka, J.N., 1998.
small subset of non-eloquent sites may have been mistakenly chosen as
a lower-order visual site in the present study. Nonetheless, all patients
Fletcher-Watson, S., Findlay, J.M., Leekam, S.R., Benson, V., 2008.
shared a similar spatial pattern in lower-order visual areas defined by
electrical stimulation. Phosphenes were most frequently elicited by
Fukuda, M., Nishida, M., Juhász, C., Muzik, O., Sood, S., Chugani, H.T., Asano, E., 2008.
stimulation of electrode pairs located in either lingual or lateral occipital
sites. We still don't know whether eliciting phosphenes via electrical
Gauthier, I., Tarr, M.J., Anderson, A.W., Skudlarski, P., Gore, J.C., 1999.
stimulation is an equally sensitive method for localization of lower-
order visual sites across occipital regions. We noted that electrical
Golarai, G., Liberman, A., Yoon, J.M.D., Grill-Spector, K., 2010. Differential development of
stimulation of the occipital pole often resulted in perception of a small
the ventral visual cortex extends through adolescence. Front. Hum. Neurosci. http://
phosphene (e.g., the size of a ladybug) in the central visual field, where-
Haxby, J.V., Ungerleider, L.G., Clark, V.P., Schouten, J.L., Hoffman, E.A., Martin, A., 1999.
as stimulation of more anterior-medial occipital areas resulted in
perception of a larger phosphene (e.g., the size of a tennis ball) in the
more peripheral visual field. Very anterior-medial lingual–cuneus
Hochberg, J., Galper, R.R., 1967.
regions were often associated with stimulation-induced phosphenes
Hoechstetter, K., Bornfleth, H., Weckesser, D., Ille, N., Berg, P., Scherg, M., 2004.
in a far peripheral field but not with checkerboard stimuli-related
high-gamma augmentation. We suspect that such regions could not
Horton, J.C., Hoyt, W.F., 1991.
be effectively activated by annular checkerboard stimuli presented in
a regular LCD monitor.
Jacobs, J., Levan, P., Châtillon, C.E., Olivier, A., Dubeau, F., Gotman, J., 2009.
Kanwisher, N., McDermott, J., Chun, M.M., 1997.
This work was supported by NIH grant NS64033 (to E. Asano) as well
Kim, S.E., Kim, W.S., Kim, B.G., Chung, D., Jeong, J., Lee, J.S., Tae, W.S., Hong, S.B., Lee, H.W.,
as the intramural grant from Children's Hospital of Michigan Foundation
(to E. Asano). We are grateful to Harry T. Chugani, MD, Sandeep Sood,
MD, Csaba Juhász, MD, PhD, Robert Rothermel, PhD, Yutaka Nonoda,
Kirchner, H., Thorpe, S.J., 2006.
MD, Nicole Goellnitz, RN, MSN, CPNP, and Carol Pawlak, REEG/EPT
Kojima, K., Brown, E.C., Matsuzaki, N., Asano, E., 2013a.
at Children's Hospital of Michigan, Wayne State University for the
collaboration and assistance in performing the studies described above.
N. Matsuzaki et al. / NeuroImage 109 (2015) 249–259
Kojima, K., Brown, E.C., Matsuzaki, N., Rothermel, R., Fuerst, D., Shah, A., Mittal, S., Sood, S.,
Puce, A., Allison, T., Gore, J.C., McCarthy, G., 1995.
Asano, E., 2013b.
Quinn, P.C., Eimas, P.D., 1998.
Kojima, K., Brown, E.C., Rothermel, R., Carlson, A., Fuerst, D., Matsuzaki, N., Shah, A.,
Quinn, P.C., Doran, M.M., Reiss, J.E., Hoffman, J.E., 2009.
Atkinson, M., Basha, M., Mittal, S., Sood, S., Asano, E., 2013c.
Ray, S., Crone, N.E., Niebur, E., Franaszczuk, P.J., Hsiao, S.S., 2008.
Korzeniewska, A., Franaszczuk, P.J., Crainiceanu, C.M., Kuś, R., Crone, N.E., 2011.
Rossion, B., Caharel, S., 2011.
Kumar, G., Juhász, C., Sood, S., Asano, E., 2012.
Salmelin, R., Hari, R., 1994.
Lachaux, J.P., George, N., Tallon-Baudry, C., Martinerie, J., Hugueville, L., Minotti, L.,
Scherf, K.S., Behrmann, M., Humphreys, K., Luna, B., 2007.
Kahane, P., Renault, B., 2005.
Levy, I., Hasson, U., Avidan, G., Hendler, T., Malach, R., 2001.
Schneider, W., Noll, D.C., Cohen, J.D., 1993.
Matsuzaki, N., Nagasawa, T., Juhász, C., Sood, S., Asano, E., 2012.
Summerfield, C., Egner, T., Mangels, J., Hirsch, J., 2006.
Uematsu, M., Matsuzaki, N., Brown, E.C., Kojima, K., Asano, E., 2013.
Mendola, J.D., Buckthought, A., 2013.
Miller, K.J., Makeig, S., Hebb, A.O., Rao, R.P., denNijs, M., Ojemann, J.G., 2007.
Vaidya, A.R., Jin, C., Fellows, L.K., 2014.
Valenza, E., Simion, F., Cassia, V.M., Umiltà, C., 1996.
Miller, K.J., Hermes, D., Schalk, G., Ramsey, N.F., Jagadeesh, B., den Nijs, M., Ojemann, J.G.,
Vidal, J.R., Ossandón, T., Jerbi, K., Dalal, S.S., Minotti, L., Ryvlin, P., Kahane, P., Lachaux, J.P.,
Rao, R.P., 2009.
2010. Category-specific visual responses: an intracranial study comparing gamma,
beta, alpha, and ERP response selectivity. Front. Hum. Neurosci. 2010. http://dx.doi.
Murphey, D.K., Maunsell, J.H., Beauchamp, M.S., Yoshor, D., 2009.
Wellmer, J., von Oertzen, J., Schaller, C., Urbach, H., König, R., Widman, G., Van Roost, D.,
Elger, C.E., 2002.
Muzik, O., Chugani, D.C., Zou, G., Hua, J., Lu, Y., Lu, S., Asano, E., Chugani, H.T., 2007.
Multimodality data integration in epilepsy. Int. J. Biomed. Imaging http://dx.doi.org/
Woods, D.L., Yund, E.W., Kang, X.J., 2003. Unified functional/anatomical maps of human
auditory cortex. Archives of Neurobehavioral Experiments and Stimuli 46 (Link to:
Nagasawa, T., Matsuzaki, N., Juhász, C., Hanazawa, A., Shah, A., Mittal, S., Sood, S., Asano, E.,
Wu, H.C., Nagasawa, T., Brown, E.C., Juhasz, C., Rothermel, R., Hoechstetter, K., Shah, A.,
Mittal, S., Fuerst, D., Sood, S., Asano, E., 2011.
Papp, N., Ktonas, P., 1977.
Yin, R.K., 1969.
Parvizi, J., Jacques, C., Foster, B.L., Witthoft, N., Rangarajan, V., Weiner, K.S., Grill-Spector,
Yoshor, D., Bosking, W.H., Ghose, G.M., Maunsell, J.H., 2007.
Yovel, G., Kanwisher, N., 2004.
Pieters, T.A., Conner, C.R., Tandon, N., 2013.
Zijlmans, M., van Eijsden, P., Ferrier, C.H., Kho, K.H., van Rijen, P.C., Leijten, F.S., 2009.
Pitcher, D., Walsh, V., Yovel, G., Duchaine, B., 2007.
Source: http://ofenlab.wayne.edu/noa_ofen_publications/ni2015matsuzaki-schwarzlose-nishida-ofen-asano.pdf
September 2005Volume 23 Sunday Eucharist8am & 10amSaturday Vigil 5pmMonday 7.15am Cluny ConventTuesday 7amWednesday 9amThursday 7amFriday 12.05pmSaturday 8am Adoration ofBlessed SacramentFriday 11amBenedictionFriday 12 noonReconciliationFriday 11.30amSaturday 9.30am
twi11006_fm.fm Page 461 Wednesday, Apr twi11006_fm.fm Page il 18, 2007 11:07 AM Prediabetes: a position statement from the Australian Diabetes Society and Australian Diabetes Educators Association Stephen M Twigg, Maarten C Kamp, Timothy M Davis, Elizabeth K Neylon and Jeffrey R Flack onditions in which blood glucose levels are elevated but not in the range of diabetes mellitus occur commonly. The term








