Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Radial extracorporeal shock wave therapy (reswt) induces new bone formation in vivo: results of an animal study in rabbits
Copyright ! 2012 World Federation for Ultrasound in Medicine & Biology
Printed in the USA. All rights reserved
0301-5629/$ - see front matter
d Original Contribution
RADIAL EXTRACORPOREAL SHOCK WAVE THERAPY (RESWT) INDUCES NEW
BONE FORMATION IN VIVO: RESULTS OF AN ANIMAL STUDY IN RABBITS
HANS GOLLWITZER,TIMO GLOECKMICHAELA ROESSNER,RUPERT LANGER,y CARSTEN HORN,z
UDGER GERDESMEYER,x and PETER DIEHL
* Orthopedic Clinic, Technical University Munich, Munich, Germany; y Institute of Pathology and Pathological Anatomy,
Technical University Munich, Munich, Germany; z Orthop€adische Klinik, K€onig-Ludwig-Haus, Universit€at W€urzburg,
W€urzburg, Germany; x Sektion onkologische und rheumatologische Orthop€adie, im Universit€atsklinikum Schleswig Holstein,
Kiel, Germany; and jj Orthopedic Clinic, University Rostock, Rostock, Germany
(Received 11 June 2012; revised 28 August 2012; in final form 30 August 2012)
Abstract—The aim of this study was to investigate if radial extracorporeal shock wave therapy (rESWT) induces
new bone formation and to study the time course of ESWT-induced osteogenesis. A total of 4000 impulses of radial
shock waves (0.16 mJ/mm2) were applied to one hind leg of 13 New Zealand white rabbits with the contralateral
side used for control. Treatment was repeated after 7 days. Fluorochrome sequence labeling of new bone formation
was performed by subcutaneous injection of tetracycline, calcein green, alizarin red and calcein blue. Animals
were sacrificed 2 weeks (n 5 4), 4 weeks (n 5 4) and 6 weeks (n 5 5) after the first rESWT and bone sections
were analyzed by fluorescence microscopy. Deposits of fluorochromes were classified and analyzed for significance
with the Fisher exact test. rESWT significantly increased new bone formation at all time points over the 6-week
study period. Intensity of ossification reached a peak after 4 weeks and declined at the end of the study. New
bone formation was significantly higher and persisted longer at the ventral cortex, which was located in the direc-
tion to the shock wave device, compared with the dorsal cortex, emphasizing the dose-dependent process of ESWT-
induced osteogenesis. No traumata, such as hemorrhage, periosteal detachment or microfractures, were observed
by histologic and radiologic assessment. This is the first study demonstrating low-energy radial shock waves to
induce new bone formation in vivo. Based on our results, repetition of ESWT in 6-week intervals can be recommen-
ded. Application to bone regions at increased fracture risk (e.g., in osteoporosis) are possible clinical indications.
! 2012 World Federation for Ultrasound in Medicine & Biology.
Key Words: Lithotripsy, Shockwave, Osteogenesis, Bone growth, ESWL.
fracture healing have been reported ;
Extracorporeal shock wave therapy (ESWT) has been
Focused shock waves have demonstrated to induce
introduced to treat a variety of soft tissue pathologies
new bone formation in various animal models, both on
and high-quality randomized trials demonstrated effec-
normal, fractured and osteomized bone and bone defects
tiveness especially for enthesiopathies like plantar fascii-
tis or calcific tendonitis of the shoulder (
Disclosed mechanisms include the induction of
oxygen radicals and membrane hyperpolarization, fol-
). Furthermore, multiple studies indicated that
lowed by the expression of growth factors and stimulation
high-energy focused ESWT might also be appropriate
of osteoprogenitor cells (
to stimulate bone healing in delayed unions and
To activate bone healing in
nonunions ().
the clinical setting, ESWT is commonly performed with
Recently, activation of bone regeneration in a vascular
high-energy shock waves requiring some kind of anes-
bone necrosis ) and stimulation of
thesia and repeated interventions in intervals of 4–6
weeks (; ). However,
Address correspondence to: Hans Gollwitzer, Klinik f€ur
there are neither data available on the minimum energy
Orthop€adie und Sportorthop€adie der Technischen Universit€at M€unchen,
required for bone stimulation, nor data on the dynamic
Ismaninger Str. 21, 81675 M€unchen, Germany. E-mail:
and persistence of ESWT-induced osteogenesis. Current
FLA 5.1.0 DTD ! UMB9316_proof ! 26 September 2012 ! 4:03 pm ! ce 49
Ultrasound in Medicine and Biology
Volume -, Number -, 2012
treatment recommendations are mainly based on empir-
Table 1. Treatment interventions, fluorochrome
ical rather than controlled experimental data.
application and end points
Radial ESWT (rESWT) is a relatively new and cost-
effective method of shock wave application. Radial
shock waves are generated ballistically by accelerating
tetracycline (25 mg/kg s.c.)
rESWT (4000 impulses,
a bullet to hit an applicator, which finally transforms
0.16 mJ/mm2, 4 bar, 8 Hz)
the kinetic energy into radially expanding pressure
rESWT (4000 impulses,
waves (). Compared with the
0.16 mJ/mm2, 4 bar, 8 Hz)
calcein green (20 mg/kg s.c.)
commonly used focused shock waves, rESWT is charac-
terized by a larger treatment area, which simplifies appli-
alizarin red (30 mg/kg s.c.)
cation by reflecting pathology zone rather than a point
calcein blue (30 mg/kg, s.c).
). Furthermore, radial shock
Group III (n 5 5)
waves miss the typical steepening of focused shock-
waves and, therefore, are physically more correctly clas-
rESWT 5 radial extracorporeal shock wave therapy.
sified as pressure waves. rESWT is considered critically
with bone pathologies because of to its unfocused distri-
with tetracycline was started prior to treatment to label
bution and lower energy level, both resulting in reduced
the baseline value, followed by injection of calcein green,
tissue penetration.
alizarin red and calcein blue after completion of both
The present study was conducted to investigate the
shock wave sessions ).
effect of rESWT on bone formation and to study the
time course of ESWT-induced osteogenesis, which is
mandatory to establish the most effective treatment
Analysis of new bone formation
protocol for bone stimulation.
Animals were sacrificed at 2 weeks (n 5 4), 4 weeks
(n 5 4) and 6 weeks (n 5 5) after the first rESWT
(with an overdose of pentobarbital. Rabbit
femurs with adjacent soft tissues were removed carefully
Shock wave treatment
and contact radiographs were taken. Fixation was carried
The present study was approved by the animal use
out in 100% (v/v) methanol for one week, followed by
and care committee of the regional government (Regier-
dehydration in ethanol 100% (v/v) for 5 days, and defat-
ung von Oberbayern). A total of 13 female New Zealand
ting in xylol for 24 h. Bone samples were embedded in
white rabbits (3.5–4.5 kg) were included in the animal
PMMA. Thereafter, sagittal sections with a thickness of
model. Radial shock waves were applied with a Swiss
approximately 75 mm were cut and investigated with
Dolorclast shock wave device (EMS Electro Medical
broad-band fluorescence microscopy. Visualization of
Systems, Nyon, Switzerland) to one randomized femur
tetracycline, calcein green and alizarin red was achieved
of each animal, while the contralateral side served as in-
with Filter 09 (Carl Zeiss MicroImaging GmbH, Jena,
traindividual control. Prior to each treatment, the animals
Germany). Filter 02 (Carl Zeiss MicroImaging GmbH)
were anesthetized with medetomidine, ketamine and
was used to investigate alizarin red and calcein blue
metamizole, and the left hind-leg was shaved. The appli-
bands of new bone formation. The fluorescing bands
cation site was localized at the ventral thigh, precisely
were analyzed, and type of fluorochrome, intensity,
superior to the patella with the rabbit in supine position
extension and localization (endosteal/periosteal; ventral/
and the knee joint in 45 degree flexion. rESWT was
dorsal cortex) were documented.
applied with an ultrasound transmission gel used as
The magnitude and distribution of newly formed
contact medium with the following parameters: impulse
bone was evaluated by blinded review according to the
count 4000 per intervention, impulse rate 8/s, pressure
classification provided in The total accumulated
4 bar, and energy flux density 0.16 mJ/mm2. The treat-
ossification bands (independent of the type of fluoro-
ment was repeated with similar preparation 7 days after
chrome) were classified with rating system A, which
the first intervention. A flowchart of the study protocol
was modified after Maier et al. (). For
is provided in .
the assessment of osteogenetic activity at the different
time points (analyses of the single fluorochrome bands),
Polychrome sequence labeling of newly formed bone
the rating system was modified to a total of five different
To allow microscopic work-up of new bone forma-
intensities (rating system B). Microscopic work-up
tion, polychrome sequence labeling was performed with
further included a qualitative histologic analysis for mi-
different clearly contrasting fluorescent dyes adminis-
crotraumata such as fractures, hematomas and periosteal
tered subcutaneously once per day. Intravital staining
FLA 5.1.0 DTD ! UMB9316_proof ! 26 September 2012 ! 4:03 pm ! ce 49
Radial shock wave therapy for bone stimulation d H. GOLLWITZER et al.
Table 2. Classification of new bone formation
(whereas examination of the untreated contralat-
eral femurs demonstrated only sporadically weak signs of
Rating system A: total accumulated new bone formation
new bone formation that persisted at this very minor
Intensity of new bone formation
grade over the entire study period (and
Significant induction of bone formation by rESWT
No signs of new bone formation
Sporadic new endosteal and/or periosteal bone formation,
could be demonstrated already in the first week after
without covering the entire bone surface
shock wave application and persisted at least until week
New endosteal and/or periosteal bone formation, covering
6, which was documented by the newly formed fluores-
the entire bone surface
cent bands with dyes administered in the late phase of
Rating system B: new bone formation at specific time points
the experiment (alizarin red and calcein blue,
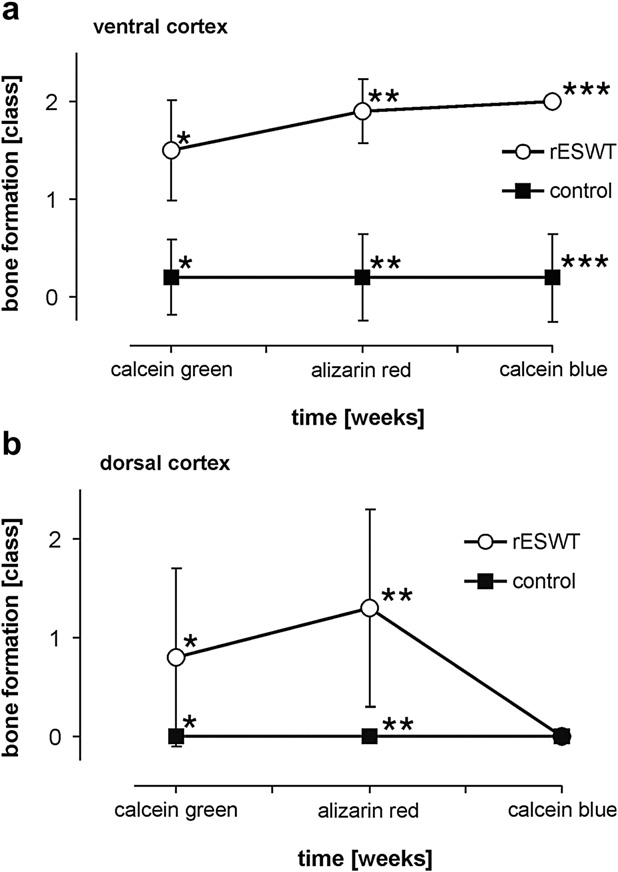
and ). New bone formation reached a peak after 4 weeks
Intensity of new bone formation
and declined to lesser intensity 6 weeks after shock wave
No signs of new bone formation or only weak,
application (Nevertheless, osteogenesis after
inhomogeneous fluorescent band
rESWT was significantly increased compared with the
Homogeneous band of new bone formation at one cortex
only, with low intensity/smooth borders
untreated control at all time points (p , 0.05).
Homogeneous band of new bone formation at one cortex
Differentiation of rESWT-induced osteogenesis at
only, with high intensity/sharp delineation
the ventral and dorsal femoral cortex was carried out
Homogeneous bands of new bone formation at both cortices,
with low intensity/smooth borders
because shock waves were applied to the ventral thigh
Homogeneous bands of new bone formation at both cortices,
and a distance-related decline of shock wave energy in
with high intensity/sharp delineation
bone was expected. Compared with the untreated control
side, osteoneogenesis was significantly increased at the
ventral cortex at all time points and at the dorsal
Radiologic work-up was performed with contact
cortex for approximately 4 weeks after rESWT b).
radiographs (3 mA, 35 kV, 60 s) before and microradio-
Thereafter, new bone formation declined at the dorsal
graphs (3 mA, 15 kV, 45 s) after sectioning of the ex-
cortex to values indifferent of the untreated control
planted femurs. Assessment included new periosteal
(b). When both cortices were compared, rESWT-
and endosteal bone formation, callus formation, cortical
induced bone formation reached significantly higher
and trabecular fractures, and periosteal detachment. The
levels at the ventral cortex compared with the dorsal
lungs of all animals were also harvested and examined
cortex in the early phase (calcein green, p 5 0.031) and
both macroscopically and histologically for signs of em-
in the late phase of the experiment (calcein blue, p ,
bolism or dislocated bone trabeculae within pulmonary
0.008) but not during the peak of osteogenesis at 4 weeks
vessels, which had been previously described after the
(alizarin red, p 5 0.206). No significant differences were
application of high-energy ESWT ).
observed with regard to endosteal and periosteal bone
Statistical analysis of new bone formation in
formation (p . 0.05) and no significant signs of new
treated and untreated femora was performed with the
bone formation were observed in trabecular bone.
Fisher exact test, with p , 0.05 considered statistically
Contact radiographs and or microradiographs were
negative for calcified bone remodeling, bone resorption,
osteolysis or callus formation. Furthermore, no trabecular
or cortical fractures were detected. Qualitative histology
did not show intraosseous bleeding, periosteal detach-
The present study was conducted to investigate the
ment or microfractures. Furthermore, neither signs of
effect of low-energy radial shock waves on osteogenesis
pulmonary embolisms nor displaced bone fragments
and to study the dynamics of ESWT-induced new bone
were observed in the lung sections. No side effects of
formation. Thus, radial shock waves were applied to the
rESWT were found but some hematoma at the application
distal femur of New Zealand white rabbits and fluorescent
sequence labeling of newly formed bone was realized
with different fluorescent dyes. Integration of the fluores-
cent dyes into bands of newly deposited bone was shown
by fluorescence microscopy and was significantly
In an effort to achieve bone healing in a noninvasive
increased after rESWT ). The different
way, several experimental and clinical studies investi-
colored fluorescent dyes allowed a description of the
gated ESWT for bone stimulation and indicated
time course of new bone formation. Sharp and
improved bone union and increased bone turnover after
homogeneous bands of integrated fluorochromes were
the application of focused high-energy shock waves
observed in all bone specimens treated with rESWT
FLA 5.1.0 DTD ! UMB9316_proof ! 26 September 2012 ! 4:03 pm ! ce 49
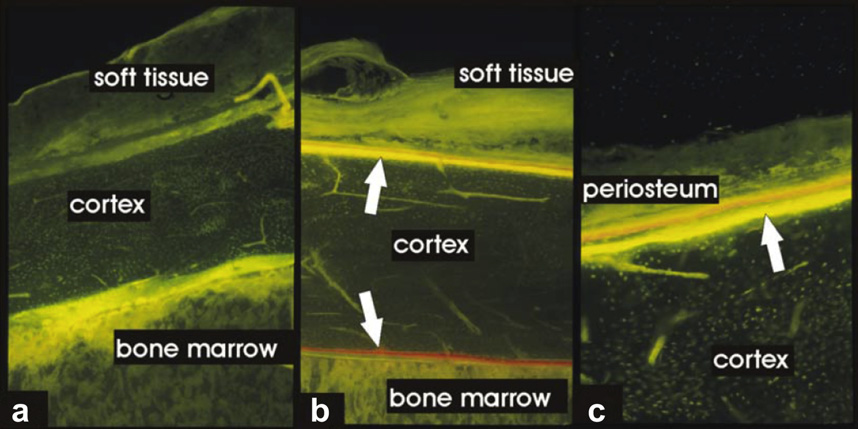
Ultrasound in Medicine and Biology
Volume -, Number -, 2012
Fig. 1. Fluorochrome sequence labeling of new bone formation at the ventral cortex of rabbit femurs, investigated 4 weeks
after the first radial extracorporeal shock wave therapy (rESWT) application: (a) untreated bone (magnification 350,
Zeiss Filter 09); (b) rESWT treated bone (magnification 350) and (c) rESWT treated bone (magnification 3100). Arrows
indicate bands of both periosteal and endosteal new bone formation.
). Whereas a positive effect of ESWT on heal-
larger treatment areas, has not been investigated so far.
ing of nonunions has been described in most published
The present study is the first investigation on the
studies, proof of effectiveness by means of a experimental
dynamics of ESWT-induced bone formation and the os-
study is still lacking (Further-
teogenetic potential of radial shock waves.
more, recommendations on treatment parameters such
as energy flux density, impulse rate, number of treatment
Principles of shock wave therapy
interventions and treatment free intervals vary consider-
Shock waves can be generated by electrohydraulic,
ably and are mainly based on empirical data of uncon-
electromagnetic or piezoelectric methods or (like radial
trolled trials Basic research has
shock waves in the present study) by pneumatic acceler-
provided a better understanding on the mechanisms of
ation of an applicator bullet within the hand piece
ESWT and its interaction with bone. However, data on
(Whereas ‘‘conven-
the dynamics of ESWT-induced osteogenesis are rare in
tional'' shock waves known from lithotripsy are focused
spite of the high clinical relevance to determine the
to a zone of highest energy in front of the applicator,
most appropriate treatment protocols. Furthermore, new
radial shock waves are unfocused and distributed in
bone formation after the application of radial, unfocused
a radial manner. Consequently, radial shock waves reach
ESWT, which might be advantageous by addressing
lower energy flux densities but address greater treatment
areas (Shock waves are
single high amplitude sound waves that propagate in
tissue with a sudden rise from ambient pressure to its
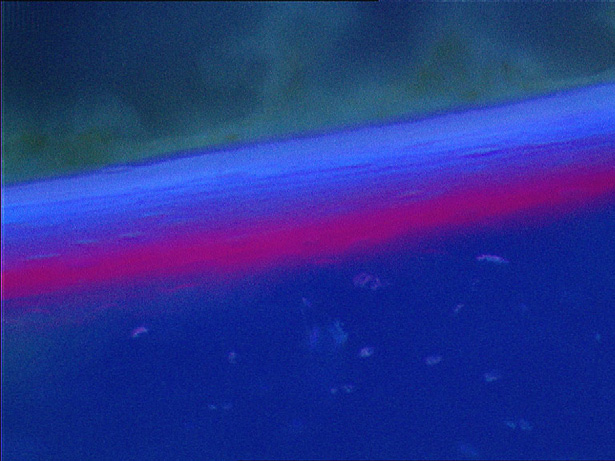
Fig. 2. Endosteal fluorochrome deposition of alizarin red and
Fig. 3. Accumulated new bone formed at the ventral femoral
calcein blue documented persisting new bone formation 6 weeks
cortex 2 to 6 weeks after the first radial extracorporeal shock
after first radial extracorporeal shock wave therapy (rESWT)
wave therapy (rESWT) (rating system A). Stars indicate statis-
(magnification 3100, Zeiss filter 02).
tically significant differences (*p 5 0.029; **p 5 0.008).
FLA 5.1.0 DTD ! UMB9316_proof ! 26 September 2012 ! 4:03 pm ! ce 49
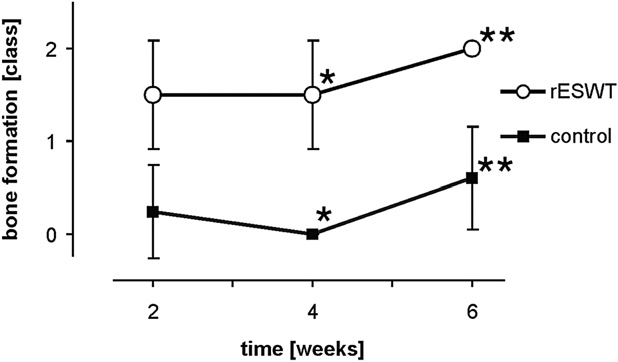
Radial shock wave therapy for bone stimulation d H. GOLLWITZER et al.
Fig. 4. Assessment of new bone formation 2 to 6 weeks after the
first radial extracorporeal shock wave therapy (rESWT) (1 to
5 weeks after second rESWT) with evaluation of the single fluo-
rochromes applied (means and standard deviations, rating
system B). Stars indicate statistically significant differences
(*p , 0.0005; **p , 0.0005; ***p 5 0.008). Significant stim-
ulation of bone formation was demonstrated already 2 weeks
(calcein green) after the first rESWT with a peak of osteogenesis
at 4 weeks (alizarin red) and a consecutive decline until the
study end at week 6 (calcein blue).
maximum pressure at the wave front, followed by a lower
tensile amplitude Radial
shock waves are missing the typical steepening effect
of focused shock waves and, therefore, physically
resemble simple pressure waves. The most important
mechanical effects of shock waves are reflection with
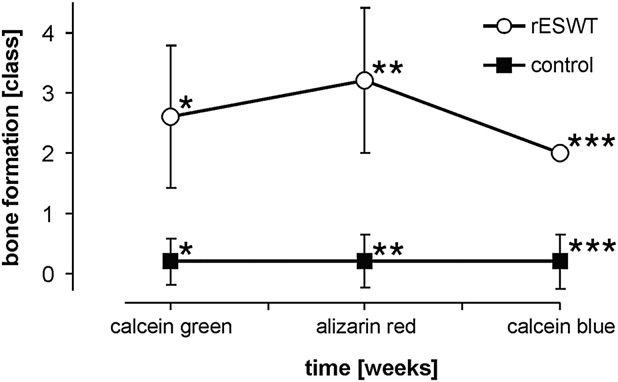
Fig. 5. New bone formation (a) at the ventral femoral cortex, and
pressure and tension forces at borders of different imped-
(b) at the dorsal femoral cortex at different time points 2 to 6
weeks after the first radial extracorporeal shock wave therapy
ances as well as the generation of cavitation bubbles in
(rESWT) represented by the corresponding bands of single fluo-
liquids, which induce shear forces by high velocity liquid
rochromes (rating system B). Stars indicate statistically signifi-
streams (‘‘jet-streams'') (
cant differences: (a) ventral femoral cortex: *p , 0.0005;
**p , 0.0005; ***p 5 0.008. (b) dorsal femoral cortex:
*p , 0.0005; **p , 0.0005. Ossification declined at the dorsal
cortex compared with the ventral cortex that was closely oriented
Mechanism of ESWT-induced new bone formation
to the shock wave device emphasizing the energy-dependent
Various studies have investigated the effect of
manner of new bone formation (p 5 0.008, calcein blue).
focused shock waves on normal, osteotomized and frac-
tured bone in different animal models and cell culture
factors like TGF-b1, VEGF-A and mitogen-activated
protein kinases (MAPK)
). Consequently, increased proliferation and
Whereas the effectiveness of ESWT to stimulate
differentiation of mesenchymal stem cells to osteoblasts
bone healing after fracture is discussed controversially,
was observed. G-proteins of the cell membrane, which
the positive osteogenic effect on normal bone and bone
respond to mechanical stresses, were supposed to play
defects has been proven. Wang and coworkers intensively
a role in translating the kinetic energy of shock waves
studied shock wave induced reactions in bone on the
to Ras activation. Furthermore, shock waves were shown
molecular level and were able to reveal some of the basic
to produce oxygen radicals, which are also supposed to
play a key role in connecting the mechanical shock
Thereby, two major mechanisms have been de-
wave energies and the resulting biological effects
tected to be involved in the translation of mechanical
shock wave energy to biologic responses: membrane
Wang et al. further showed that oxygen radical produc-
hyperpolarization and the formation of free radicals.
tion was followed by a stimulation of a cascade of kinases
Wang et al. and Chen et al. demonstrated shock waves
and growth factors like VEGF, TGF-b1, BMP-1, BMP-2,
to induce hyperpolarization of cell membranes, followed
BMP-7 etc., followed by an increased growth and differ-
by Ras activation and a local increase of stimulating
entiation of mesenchymal cells toward osteoprogenitor
FLA 5.1.0 DTD ! UMB9316_proof ! 26 September 2012 ! 4:03 pm ! ce 49
Ultrasound in Medicine and Biology
Volume -, Number -, 2012
controversially (; ;
). In our qualitative analysis, we neither
observed any histologically detectable traumata (like
Dose-dependent effects
fractures, hematomas or periosteal detachments) nor frac-
Dose-dependent stimulation of bone cells in vitro
tures or callus formation detectable by microradiography.
was observed by Kusnierczak et al. after shock wave
Our data are in accordance with results published by
application, with minimum threshold energy necessary
others demonstrating that cortical fractures and periosteal
to effect bone cell growth ).
detachment are no prerequisites for new bone formation
However, bone cell stimulation was contributed to the
total amount of energy applied, rather than single param-
new bone formation was limited to endosteum and perios-
eter like energy flux density or number of administered
teum in our investigation, whereas other studies also
impulses. Furthermore, cell damage by excessive energy
demonstrated trabecular new bone formation related to
flux densities was described. Wang et al. and Chen et al.
trabecular microfractures ). We
confirmed those findings in vivo proving a dose-
conclude that iatrogenic fractures are not mandatory for
dependent effect of ESWT on bone mass and bone
periosteal and endosteal new bone formation; however,
strength in acute fracture healing in rabbits
it remains to be clarified if microfractures provide an
) and in bone defect models in rats
additional stimulus for new bone formation in cancellous
). Furthermore, suppres-
sion of osteogenetic influence was observed with the
application of excessive energy levels. Maier et al. also
Dynamic of ESWT-induced bone formation
provided data about deleterious effects of very high
In the treatment of bone pathologies, ESWT is
energy flux densities ($0.9 mJ/mm2), demonstrating
usually repeated to complete three to six interventions
soft tissue edema, cortical fractures, periosteal detach-
with treatment free intervals ranging from 4–8 weeks
ment, intraosseous bleeding and even displacement of
(However, these recommenda-
bone fragments to pulmonary vessels with the risk of
tions are based on empirical clinical observations and
pulmonary embolism (, ).
not on controlled experimental data. In our study, osteo-
Apart from the studies with bone defects, other authors
genesis was induced significantly by rESWT already
described osteostimulative effects with lower energies.
within the first week after shock wave treatment. A
Tischer et al. detected signs of new bone formation in
peak of new bone formation was observed 4 weeks after
areas located well outside the focus zone
the first rESWT with a consecutive decline of osteogene-
sis at week 6. The decline of new bone formation was
Our study is the first proving a significant induction
most prominent at the dorsal femoral cortex, whereas
of new bone formation by rESWT, thereby applying low
increased bone formation persisted at the ventral cortex
energy flux densities (0.16 mJ/mm2) but relatively high
for at least 5 weeks after the last shock wave application.
impulse numbers (2 3 4000 impulses). Once induced,
We, therefore, anticipate that both the intensity of new
new bone formation persisted for at least 5 weeks after
bone formation as well as its persistence over time is
the last shock wave application. Bone growth was also
dose-dependent. Our results suggest repeating shock
activated at the dorsal femoral cortex in spite of the rela-
wave treatment after approximately 5–6 weeks, since
tively low energy flux density, proving penetration of
a significant decline of new bone formation was observed
radial shock waves through soft tissue and bone.
after that period.
However, induction of new bone formation was signifi-
Interestingly, fluorescent microscopy also demon-
cantly greater and lasted longer at the ventral cortex
strated inhomogeneous and weak bands of tetracycline
that had been directed toward the shock wave device,
in the rESWT treated bone, whereas no tetracycline depo-
compared with the dorsal femoral cortex. These observa-
sition was observed in the control group. Thus, we antic-
tions can be explained by a distance-related decline of
shock wave energy while penetrating the thigh and
immediately after ESWT followed by integration of re-
confirm the dose-dependency of shock-wave induced os-
maining circulating tetracycline that had been injected
teogenesis. Consequently, the application of shock waves
prior to shock wave treatment.
from different sides of the treated bone is recommended
Abundant experimental and clinical evidence exist
in the clinical setting to provide relevant energy levels
that mechanical stimuli can both positively and nega-
to all cortices.
tively influence fracture healing, bone regeneration and
The significance of microtraumata like periosteal
detachment and cortical and trabecular microfractures
Apart from focused shock waves,
for the induction of osteogenesis has been discussed
especially, cyclic loading and vibrational stimulation
FLA 5.1.0 DTD ! UMB9316_proof ! 26 September 2012 ! 4:03 pm ! ce 49
Radial shock wave therapy for bone stimulation d H. GOLLWITZER et al.
have been abundantly investigated with positive effects
Diehl P, Gerdesmeyer L, Gollwitzer H, Sauer W, Tischer T. Calcific
on bone regeneration in fractured and normal bone
tendinitis of the shoulder. Orthopade 2011;40:733–746.
Elster EA, Stojadinovic A, Forsberg J, Shawen S, Andersen RC,
Schaden W. Extracorporeal shock wave therapy for nonunion of
has to be discussed in this context, since rESWT produces
the tibia. J Orthop Trauma 2010;24:133–141.
repeated mechanical stimuli by controlled compression
Fritton JC, Myers ER, Wright TM, van der Meulen MC. Loading
induces site-specific increases in mineral content assessed by micro-
and distension perpendicular to the treated bone, which
computed tomography of the mouse tibia. Bone 2005;36:
can be easily applied in the clinical setting. Future studies
have to show if rESWT is beneficial in the treatment and
Gardner MJ, van der Meulen MC, Demetrakopoulos D, Wright TM,
Myers ER, Bostrom MP. In vivo cyclic axial compression affects
prevention of bone pathologies like osteoporosis and
bone healing in the mouse tibia. J Orthop Res 2006;24:
fracture nonunions.
We conclude that the osteogenetic effect of ESWT is
Gerdesmeyer L, Maier M, Haake M, Schmitz C. [Physical-technical
principles of extracorporeal shockwave therapy (ESWT)]. Ortho-
a complex, dose-dependent biologic response persisting
for several weeks after stimulation. rESWT has proven
Gerdesmeyer L, Wagenpfeil S, Haake M, Maier M, Loew M, Wortler K,
effective to induce new bone formation in normal bone
Lampe R, Seil R, Handle G, Gassel S, Rompe JD. Extracorporeal
shock wave therapy for the treatment of chronic calcifying tendon-
and might be advantageous because of the application
itis of the rotator cuff: A randomized controlled trial. JAMA 2003;
to larger treatment areas. However, limitations might be
found in pathologies far below the skin level because of
Gerdesmeyer L, Gollwitzer H, Diehl P, Wagner K. Radiale extrakorpor-
rale Stoßwellentherapie (rESWT) in der Orthop€adie. J Miner Stoff-
a distance related decline of shock wave energy. Never-
theless, rESWT might offer new perspectives in the
Gerdesmeyer L, Frey C, Vester J, Maier M, Weil L Jr, Weil L Sr,
therapy of bone pathologies as larger tissue areas can
Russlies M, Stienstra J, Scurran B, Fedder K, Diehl P, Lohrer H,
Henne M, Gollwitzer H. Radial extracorporeal shock wave therapy
be effectively treated.
is safe and effective in the treatment of chronic recalcitrant plantar
fasciitis: Results of a confirmatory randomized placebo-controlled
Acknowledgments—The authors do not have professional or financial
multicenter study. Am J Sports Med 2008;36:2100–2109.
affiliations that might have biased the present study. The work presented
Gollwitzer H, Brandner H, Gloeck T. [Extracorporeal shock wave
in this manuscript was supported by a fund of the Kommission f€ur Kli-
therapy for bony non-union: Evidence-based therapy–Review of
nische Forschung (KKF) of the Technical University Munich.
the literature and personal results]. Trauma Berufskrankh 2006;8:
Gollwitzer H, Diehl P, von Korff A, Rahlfs VW, Gerdesmeyer L. Extra-
corporeal shock wave therapy for chronic painful heel syndrome: A
prospective, double blind, randomized trial assessing the efficacy of
Alvarez RG, Cincere B, Channappa C, Langerman R, Schulte R,
a new electromagnetic shock wave device. J Foot Ankle Surg 2007;
Jaakkola J, Melancon K, Shereff M, Cross GL. Extracorporeal shock
wave treatment of non- or delayed union of proximal metatarsal
Haupt G, Haupt A, Ekkernkamp A, Gerety B, Chvapil M. Influence of
fractures. Foot Ankle Int 2011;32:746–754.
shock waves on fracture healing. Urology 1992;39:529–532.
Augat P, Merk J, Ignatius A, Margevicius K, Bauer G, Rosenbaum D,
Kusnierczak D, Brocai DR, Vettel U, Loew M. [Effect of extracorporeal
Claes L. Early, full weight bearing with flexible fixation delays frac-
shockwave administration on biological behavior of bone cells
ture healing. Clin Orthop Relat Res 1996;328:194–202.
in vitro]. Z Orthop Ihre Grenzgeb 2000;138:29–33.
Carter DR, Blenman PR, Beaupre GS. Correlations between mechanical
Maier M, Milz S, Tischer T, Munzing W, Manthey N, Stabler A,
stress history and tissue differentiation in initial fracture healing.
Holzknecht N, Weiler C, Nerlich A, Refior HJ, Schmitz C. Influence
J Orthop Res 1988;6:736–748.
of extracorporeal shock-wave application on normal bone in an
Chen YJ, Kuo YR, Yang KD, Wang CJ, Huang HC, Wang FS. Shock
animal model in vivo. Scintigraphy, MRI and histopathology.
wave application enhances pertussis toxin protein-sensitive bone
J Bone Joint Surg Br 2002;84:592–599.
formation of segmental femoral defect in rats. J Bone Miner Res
Maier M, Freed JA, Milz S, Pellengahr C, Schmitz C. [Detection of bone
fragments in pulmonary vessels following extracorporeal shock
Chen YJ, Wurtz T, Wang CJ, Kuo YR, Yang KD, Huang HC, Wang FS.
wave application to the distal femur in an in vivo animal model].
Recruitment of mesenchymal stem cells and expression of TGF-beta
Z Orthop Ihre Grenzgeb 2003a;141:223–226.
1 and VEGF in the early stage of shock wave-promoted bone regen-
Maier M, Averbeck B, Milz S, Refior HJ, Schmitz C. Substance P and
eration of segmental defect in rats. J Orthop Res 2004a;22:526–534.
prostaglandin E2 release after shock wave application to the rabbit
Chen YJ, Kuo YR, Yang KD, Wang CJ, Sheen Chen SM, Huang HC,
femur. Clin Orthop Relat Res 2003b;406:237–245.
Yang YJ, Yi-Chih S, Wang FS. Activation of extracellular signal-
Maier M, Hausdorf J, Tischer T, Milz S, Weiler C, Refior HJ, Schmitz C.
regulated kinase (ERK) and p38 kinase in shock wave-promoted
[New bone formation by extracorporeal shock waves. Dependence
bone formation of segmental defect in rats. Bone 2004b;34:
of induction on energy flux density]. Orthopade 2004;33:
Claes LE, Heigele CA. Magnitudes of local stress and strain along bony
Moretti B, Notarnicola A, Garofalo R, Moretti L, Patella S,
surfaces predict the course and type of fracture healing. J Biomech
Marlinghaus E, Patella V. Shock waves in the treatment of stress
fractures. Ultrasound Med Biol 2009;35:1042–1049.
Delacretaz G, Rink K, Pittomvils G, Lafaut JP, Vandeursen H, Boving R.
Tischer T, Milz S, Anetzberger H, Muller PE, Wirtz DC, Schmitz C,
Importance of the implosion of ESWL-induced cavitation bubbles.
Ueberle F, Maier M. [Extracorporeal shock waves induce
Ultrasound Med Biol 1995;21:97–103.
ventral-periosteal new bone formation out of the focus zone–
Delius M, Draenert K, Al Diek Y, Draenert Y. Biological effects of shock
results of an in vivo animal trial]. Z Orthop Ihre Grenzgeb 2002;
waves: In vivo effect of high energy pulses on rabbit bone. Ultra-
sound Med Biol 1995;21:1219–1225.
van der Jagt OP, Piscaer TM, Schaden W, Li J, Kops N, Jahr H, van der
Delius M, Ueberle F, Eisenmenger W. Extracorporeal shock waves act
Linden JC, Waarsing JH, Verhaar JA, de Jong M, Weinans H. Unfo-
by shock wave-gas bubble interaction. Ultrasound Med Biol 1998;
cused extracorporeal shock waves induce anabolic effects in rat
bone. J Bone Joint Surg Am 2011;93:38–48.
FLA 5.1.0 DTD ! UMB9316_proof ! 26 September 2012 ! 4:03 pm ! ce 49
Ultrasound in Medicine and Biology
Volume -, Number -, 2012
Wang CJ, Yang KD, Wang FS, Hsu CC, Chen HH. Shock wave treat-
marrow stromal cells towards osteoprogenitors associated with
ment shows dose-dependent enhancement of bone mass and bone
induction of TGF-beta1. J Bone Joint Surg Br 2002a;84:457–461.
strength after fracture of the femur. Bone 2004;34:225–230.
Wang FS, Wang CJ, Sheen-Chen SM, Kuo YR, Chen RF, Yang KD.
Wang CJ, Wang FS, Huang CC, Yang KD, Weng LH, Huang HY. Treat-
Superoxide mediates shock wave induction of ERK-dependent oste-
ment for osteonecrosis of the femoral head: Comparison of extracor-
ogenic transcription factor (CBFA1) and mesenchymal cell differen-
poreal shock waves with core decompression and bone-grafting.
tiation toward osteoprogenitors. J Biol Chem 2002b;277:
J Bone Joint Surg Am 2005;87:2380–2387.
Wang CJ, Liu HC, Fu TH. The effects of extracorporeal shockwave on
Wang FS, Yang KD, Kuo YR, Wang CJ, Sheen-Chen SM, Huang HC,
acute high-energy long bone fractures of the lower extremity. Arch
Chen YJ. Temporal and spatial expression of bone morphogenetic
Orthop Trauma Surg 2006;127:137–142.
proteins in extracorporeal shock wave-promoted healing of
Wang FS, Wang CJ, Huang HJ, Chung H, Chen RF, Yang KD. Physical
segmental defect. Bone 2003;32:387–396.
shock wave mediates membrane hyperpolarization and Ras activa-
Wang FS, Wang CJ, Chen YJ, Chang PR, Huang YT, Huang HC,
tion for osteogenesis in human bone marrow stromal cells. Biochem
Sun YC, Yang YJ, Yang KD. Ras modulation of superoxide activates
Biophys Res Commun 2001;287:648–655.
ERK-dependent angiogenic transcription (HIF-1a) and VEGF-A
Wang FS, Yang KD, Chen RF, Wang CJ, Sheen-Chen SM. Extracorpo-
expression in shock wave-stimulated osteoblasts. J Biol Chem
real shock wave promotes growth and differentiation of bone-
FLA 5.1.0 DTD ! UMB9316_proof ! 26 September 2012 ! 4:03 pm ! ce 49
Our reference: UMB 9316
AUTHOR QUERY FORM
Please e-mail or fax your responses and any corrections to:
Article Number: 9316
Fax: 1-845-883-5682
Please check your proof carefully and mark all corrections at the appropriate place in the proof (e.g., by using on-screenannotation in the PDF file) or compile them in a separate list. Note: if you opt to annotate the file with software other thanAdobe Reader then please also highlight the appropriate place in the PDF file. To ensure fast publication of your paper pleasereturn your corrections within 48 hours.
For correction or revision of any artwork, please consult .
Any queries or remarks that have arisen during the processing of your manuscript are listed below and highlighted by flags inthe proof.
Query / Remark: Click on the Q link to find the query's location in text
Please insert your reply or correction at the corresponding line in the proof
Per Journal style, included first names. Please verify.
Please verify spelling and use of osteomized.
Please expand PMMA.
Please expand TGF-b1 and VEGF-A.
Please expand BMP.
Edited to "iatrogenic fractures." Please review.
Please confirm that given names and surnames have been identified correctly.
Please check this box if you have no
corrections to make to the PDF file
Thank you for your assistance.
Source: https://www.orthopaediezentrum-muenchenost.de/files/RADIAL_EXTRACORPOREAL_SHOCK_WAVE_THERAPY_2012.pdf
The Role and Use of PEA in Depression & Neurobehavioral Disorders by DR RICHARD CLARK KAUFMAN The Phenylethylamine Hypothesis of Depression According to the "Phenylethylamine Hypothesis of Depression" proposed in 1974, the endogenous trace amine Beta- Phenylethylamine (PEA) sustains psychological energy just as thyroid hormone sustains physical energy And a deficit of PEA produces depressions. The Phenylethylamine hypothesis goes on to state that PEA is a neuromodulator of mood, attention, pleasure-seeking behavior, and libido. The phenylethylamine hypothesis led to simple safe and effective way of treating depression and other affective disorders by based on years of research conducted by Dr. Hector Sabelli and colleagues. Take an oral replacement of PEA as replacement to correct an underlying deficiency or defect in neural transmitter functioning. The majorities of depressed individuals show a significant reduction in their symptoms or have complete recovery without any adverse reactions. Plus, there're is significant increases in cognitive performance functions, attention, awareness, and feelings of pleasure, libido, normal social behavior and sense of wellbeing. PEA. More than Endogenous Amphetamine in our Brain The Phenylethylamine Hypothesis of Depression stems from the observation that amphetamines increased energy and relieved depressive symptoms of depressive patients. Amphetamine is essentially phenylethylamine with an added methyl group. Studies show that PEA induces behavioral and electrophysiological effects similar to those of amphetamine. Unlike amphetamine, PEA is endogenous to the brain and does not develop tolerance or dependency, or produce any side effects. The stimulant effects of amphetamines and PEA are attributed to the release of catecholamines (noradrenalin, dopamine). This is the basis for the catecholamine hypothesis of depression. However current research shows that PEA is significantly more effective than amphetamine in relieving depression and has therapeutic value in a wide range of neurological and behavioral disorders, Endogenous Mesencephalic Enhancer and Transmitter Signal Amplifier Starting around 1995, Dr Joesph Knoll and his colleagues began presenting their evidence of PEA as an endogenous "mesencephalic enhancer". There are enhancer-sensitive neurons in the brain work in a split-second on a high activity level due to endogenous enhancer substances. The mesencephalic enhancer PEA enhancers of the impulse propagation mediated release of catecholamines (dopamine, epinephrine) and serotonin in the brain.
Report on research visit to University of California, Irvine (UCI) under HEQEP CP 3137 Dr. Md Yusuf Sarwar Uddin Deputy SPM CP 3137 Assistant Professor Department of CSE, BUET, Dhaka-1000. I made a research visit to University of California, Irvine (UCI) United States fromSeptember 20, 2014 to January 31, 2015 to conduct a research related to project"Capacity building for post-graduate research on remote health monitoring inBangladesh". I worked with Prof Nalini Venkatasubramanium at DistributedMiddleware Services (DMS) lab of Information and Computer Sciences departmentof UCI. The visit was quite great and a set of interesting problems was studiedduring the visit.





