Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Pnec.co.ir


This article appeared in a journal published by Elsevier. The attached
copy is furnished to the author for internal non-commercial research
and education use, including for instruction at the authors institution
and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or
licensing copies, or posting to personal, institutional or third party
websites are prohibited.
In most cases authors are permitted to post their version of the
article (e.g. in Word or Tex form) to their personal website or
institutional repository. Authors requiring further information
regarding Elsevier's archiving and manuscript policies are
encouraged to visit:


Author's personal copy
Ultrasonics Sonochemistry 18 (2011) 1165–1171
Contents lists available at ScienceDirect
Effect of local dual frequency sonication on drug distribution from polymericnanomicelles
Hadi Hasanzadeh a,1, Manijhe Mokhtari-Dizaji a,⇑, S. Zahra Bathaie b, Zuhair M. Hassan c
a Department of Medical Physics, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iranb Department of Clinical Biochemistry, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iranc Department of Immunology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
To overcome the side effects caused by systemic administration of doxorubicin, nanosized polymeric
Received 17 January 2011
micelles were used in combination with dual frequency ultrasonic irradiation. These micelles release
Received in revised form 12 March 2011
the drug due to acoustic cavitation, which is enhanced in dual frequency ultrasonic fields. To form the
Accepted 17 March 2011
drug-loaded micelles, Pluronic P-105 copolymer was used, and doxorubicin was physically loaded into
Available online 1 April 2011
stabilized micelles with an average size of 14 nm. In this study, adult female Balb/C mice were trans-planted with spontaneous breast adenocarcinoma tumors and were injected with a dose of 1.3 mg/kg
doxorubicin in one of three forms: free doxorubicin, micellar doxorubicin without sonication and micel-
lar doxorubicin with sonication. To increase cavitation yield, the tumor region was sonicated for 2.5 min
at simultaneous frequencies of 3 MHz (ISATA = 2 W/cm2) and 28 kHz (ISATA = 0.04 W/cm2). The animals
were sacrificed 24 h after injection, and their tumor, heart, spleen, liver, kidneys and plasma were sepa-
Breast adenocarcinoma
rated and homogenized. The drug content in the tissues was determined using tissue fluorimetry(350 nm excitation and 560 nm emission), and standard drug dose curves were obtained for each tissue.
The results show that in the group that received micellar doxorubicin with sonication, the drug concen-tration in the tumor tissue was significantly higher than in the free doxorubicin injection group (8.69times) and the micellar doxorubicin without sonication group (2.60 times). The drug concentration inother tissues was significantly lower in the micellar doxorubicin with sonication group relative to the freedoxorubicin (3.35 times) and the micellar drug without sonication (2.48 times) groups (p < 0.05). We con-clude that dual frequency sonication improves drug release from micelles and increases the drug uptakeby tumors due to sonoporation. The proposed drug delivery system creates an improved treatment capa-bility while reducing systemic side effects caused by drug uptake in other tissues.
Ó 2011 Elsevier B.V. All rights reserved.
desired site of action and increasing its uptake using sonication,the side effects are minimized and the therapeutic efficiency is in-
The main mechanisms of biological action for ultrasound in-
creased. Various nanosized carriers such as liposomes [16], poly-
clude the generation of thermal energy, sonoporation, the
meric micelles [17,18] and core–shell nanoparticles [15,19–21]
enhancement of local microjets due to inertial cavitation (which
have been reported as vehicles for passive targeting. Polymeric mi-
are further enhanced by multifrequency sonication [1–3] and the
celles [22] and core–shell nanoparticles [19,23] have received con-
enhanced permeability of blood capillaries [4–10]. All of these
siderable attention due to their self-assembly characteristics in an
mechanisms could potentially be used to enhance drug uptake lo-
aqueous solution. These properties offer the possibility for a un-
cally. Doxorubicin is of great importance in the treatment of leuke-
ique biodistribution of drugs to target solid tumors [18], and it
mia and solid tumors such as in breast and ovarian cancer and
has been reported that doxorubicin-conjugated block copolymer
sarcoma pulmonary metastasis, but its clinical use is hampered
micelles were effective in the treatment of solid tumors due to pro-
by its myelotoxicity and its cumulative cardiotoxicity when
longed circulation in the blood [17,24]. Generally, it has been re-
administered systemically [11–15]. By targeting this drug to the
ported that anticancer agents incorporated in polymeric micelleshave an enhanced blood circulation time (EPR: enhanced penetra-tion and retention effect) and a suppressive effect on the growth of
⇑ Corresponding author. Tel.: +98 21 82883893; fax: +98 21 88006544.
solid tumors [18,21–24]. Many polymeric micelles are convenient
E-mail address: [email protected] (M. Mokhtari-Dizaji).
to use because they can conveniently escape the reticuloendothe-
1 Present address: Assistant Professor in Medical Physics, Department of Medical
lial system (RES) and undergo renal extraction because of their
Physics, Semnan University of Medical Sciences, Semnan, Iran.
1350-4177/$ - see front matter Ó 2011 Elsevier B.V. All rights reserved.
doi:10.1016/j.ultsonch.2011.03.018
Author's personal copy
H. Hasanzadeh et al. / Ultrasonics Sonochemistry 18 (2011) 1165–1171
small size, which is approximately 20–100 nm [24–27]. Various
recorded with the hydrophone. All of the above conditions were
methods to quantify doxorubicin in biological fluids have been re-
applied in a continuous wave mode and for a sonication duration
viewed [28]. Reversed-phase liquid chromatography coupled with
of 3 min. Each recorded signal comprised of 32768 data points col-
fluorescence detection is the method of choice to assay of doxoru-
lected with a sampling rate of 10 MHz, which includes the primary
bicin [29–31], although alternative schemes such as electrochemi-
frequency and the pressure waves coming from the oscillating
cal detection [32], UV spectrophotometric [33] detection and mass
walls of the cavities. To extract the frequency contents, signals
spectrometry [34] have also been used. However, fluorescence
were analyzed in MATLAB software version 7.0.1 (Mathworks,
detection is often the most appropriate detection method for doxo-
USA) using an FFT function with a hamming window. Background
rubicin considering its simplicity of use, selectivity and sensitivity
noise was measured by placing the hydrophone in the usual mea-
surement conditions and setting the intensity of the unit to zero;
In this study, polymeric micelles were prepared using Pluron-
this background was subtracted from the signal amplitude in each
ic P-105, which is a triblock copolymer consisting of blocks of
measurement condition. The subharmonic peak in the FFT of the
PPO (Poly Propylene Oxide) and PEO (Poly Ethylene Oxide) in
measured pressure signal was recorded. Each experimental condi-
the form PEO37–PPO56–PEO37. After loading doxorubicin into
tion was repeated 5 times.
these micelles and checking the drug release upon sonicationin vitro, their anti-tumor effect was tested in vivo. Ultrasound
was used to enhance the intracellular drug uptake from micelles.
Using a spectrophotometric method, the drug content in several
Doxorubicin was obtained from Pharmacia (Italy). Pluronic P-
tissues was quantified.
105 was provided by the BASF Corp. (Mount Olive, NJ, USA). N-N-Diethylacrylamide (NNDEA) was obtained from Polysciences (War-rington, USA). N,N0-Bis(acryloyl)cystamine (BAC) was obtained
2. Materials and methods
from Fluka (Sigma–Aldrich, UK), and benzoyl peroxide (BP) wasobtained from Merck (Merck KGaA, Darmstadt, Germany).
2.1. Animal model
2.4. Preparation of micelles
Female inbred Balb/C (6–8 weeks) mice were purchased from
the breeding colony at the Pasteur Institute of Iran (Tehran, Iran).
Polymeric micelles were prepared using Pluronic P-105, which
Mice were housed under standard conditions (constant tempera-
is a triblock copolymer consisting of blocks of poly (propylene
ture, humidity and 12 h dark–light cycles) and had access to food
oxide) (PPO) and poly (ethylene oxide) (PEO) in the form PEO37–
and water ad libitum. The tumor model was a syngenic of murine
PPO56–PEO37. A solution of NaCl and 10 wt.% Pluronic P-105 in dis-
spontaneous breast adenocarcinoma, which was chopped into
tilled water was added to a round-bottom balloon, which was stir-
fresh pieces of about 2–3 mm diameter and then transplanted into
red for 20 min while immersed in a water bath under a nitrogen
the flank region of the mice. Tumors reached a diameter of about
purge at a temperature of 65 °C. A mixture of BAC, BP and NNDEA
7–9 mm in 7–10 days, at which point they were ready to be used
(26:1:55 weight ratio) was added to the above solution, and it was
in experiments. All animal experiments and protocols were evalu-
allowed to stir at this temperature under a nitrogen purge for 3.5 h.
ated and approved by the Animal and Ethics Review Committee of
After turning the nitrogen off, the mixture was allowed to poly-
the Tarbiat Modares University (Tehran, Iran).
merize for 19.5 h. The size distribution of the micelles was mea-sured by dynamic light scattering (DLS) (Malvern Instruments
2.2. Ultrasonic setup and sonication conditions
Ltd., Malvern, UK).
Two ultrasonic systems were used in a cubic Perspex water tank
2.5. Loading doxorubicin into micelles
(25 � 20 � 20 cm3) in an orthogonal geometry. The first systemwas designed in our lab (Tarbiat Modares University, Ultrasound
To load doxorubicin into injectable micelles, it was necessary to
Lab, Tehran, Iran) in collaboration with the Pars Nahand Engineer-
find the optimal drug loading into micelles through a standard
ing company (Pardis Technology Park, Tehran, Iran) to operate in
curve, which was measured using a UV spectrophotometer (Shima-
the low kHz range with a center frequency of 27.7 kHz (nominal
dzu, Model RF-1500, Japan) at 350 nm.
frequency of 28 kHz), a bandwidth of 421 Hz and a probe diameter
To obtain the curve, several known concentrations (0–0.12 mg
of 60 mm. The second system was a 3-MHz therapeutic unit
of doxorubicin in a 1 ml solution of micelles) were prepared, and
(SM3670, Shrewsbury Medical Ltd., Shropshire, UK) with a 30-
their fluorescence emissions were read at 350 nm. It is notable that
mm-diameter probe and 5-cm2 effective radiation area (ERA).
at higher concentrations, the fluorescent emission decreases due to
Probes were held fixed in the tank wall through a pair of circular
self-quenching of the doxorubicin. Fluorescence intensity (counts)
holes in such a way that the central beam axis for each probe
versus doxorubicin concentration (mg/ml) yielded a straight line of
was perpendicular to the other. Acoustic calibration for the power
positive slope for increasing concentrations. The results show that
and intensity of the devices was carried out in degassed water in
there is a significant correlation between the fluorescence intensity
the tank using a radiation force balance (Shrewsbury Medical Co.,
and the doxorubicin concentration (R2 = 0.96, correlation is signif-
Shropshire, UK, ±10%) for the therapeutic unit and the hydrophone
icant at 0.01). A linear regression analysis was applied between the
method in the cubic chamber for our unit (PA124, Precision Acous-
fluorescence intensity and the doxorubicin concentration and the
tics Ltd., Dorchester, Dorset, UK; calibration range: 10 kHz–3 MHz
results are shown in Fig. 1.
with a sensor diameter of 25 mm). Different single frequency son-
To examine drug release in vitro, a custom exposure chamber
ication conditions including 28 kHz (0.02 and 0.04 W/cm2) and
was constructed according to the spectrophotometric characteris-
3 MHz (1 and 2 W/cm2) were demonstrated (Hasanzadeh et al.,
tics of doxorubicin obtained above (kex = 350 nm, kem = 560 nm);
2010). In addition, a dual frequency combination (28 kHz + 3 MHz)
this chamber recorded the change in light emission due to doxoru-
at the above-mentioned intensities was studied. All reported
bicin release under sonication (Fig. 2). A digital camera (Sony, DSC
experimental intensity values consist of the spatial average/tem-
P-93, Japan) with a multi-band filter (Alexa FluorÒ 350/488/594,
poral average (ISATA). In each sonication condition, subharmonic
USA) was used to record images (BMP images with dimensions
signal amplitudes at 14 kHz and 1.5 MHz in the water tank were
of 640 � 480 pixels), and a background was recorded without son-
Author's personal copy
H. Hasanzadeh et al. / Ultrasonics Sonochemistry 18 (2011) 1165–1171
the complex. The complex was stored at 4 °C, and its stability wasmeasured for one month.
Intensity (Count) = 4590.5Concentration+ 52.82
2.7. Biodistribution and in vivo study
Because the goal of the current study was to work below the le-
vel of hyperthermia, it was necessary to measure the temperature
rise due to sonication in vivo. Three female Balb/C mice were se-lected and placed into the sonication condition after being anes-
escence intensity (Count) 100
thetized. The temperatures of the tumors and their surroundingenvironment were monitored every second during sonication with
a portable digital thermometer containing a dual thermocouple in-
put. The tissue temperature was monitored invasively using K-type
Doxorubicin concentration (mg/ml)
wire thermocouples (TP-01, Lutron Electronic Enterprise Co., Tai-wan, �200–1372 °C, 0.1 mm thickness, ±0.1 °C). Each of these mic-
Fig. 1. Micellar drug loading standard curve.
rothermometers had two wire thermocouples placed in theanimal's body. The temperature changes (°C) at different depths
ication. The frequency and intensity were selected previously using
in mice versus duration of sonication under dual frequency sonica-
a subharmonic analysis method to enhance the acoustic cavitation
tion conditions was evaluated for different depths relative to the
at 28 kHz and 3 MHz [35].
skin in the center of the tumor (close to the ultrasonic probes) to
Each sonication protocol was run 5 times, and the RMSE (root
about 4 cm below the skin until animal posterior layer in 1 cm
mean square error) values of the images were used to compare be-
steps. All of the thermocouples provided inputs to the control unit,
which contained a microprocessor connected to a computer via anRS-232 port. The temperatures were recorded every second (as
.doc files) using thermometer software (Multilogger Thermometer
CHY502A, Taiwan), which was connected to a PC, and the temper-
ature rise under sonication conditions was recorded every 30 s for20 min. The water temperature was controlled using a digital ther-
In the above equation, x i and x0 are signal amplitudes of the
mometer and an electrical heater and was fixed at 32 °C. According
images in each group and the background images, respectively. Fi-
to our temperature measurements, a 2.5 min sonication time was
nally, the average of the RMSEs of the image components was used
deemed suitable for the next phase of our in vivo study. We have
to compare the ability of different sonication conditions to release
evaluated a control group without sonication but we did not have
the drug from the micelles.
any temperature changes. Therefore, that obtained results are due
Doxorubicin was stirred into the micellar solution to load it into
to sonication rather than a vasculature lesion provoked by probe
the micelles; to separate the free drug from the encapsulated drug,
insert in tumor mass.
the solution was filled into a dialysis bag (30 mm diameter, 5 kDa
To study the drug distribution in animals, 9 female Balb/C mice
cut off, BioGene, Mashhad, Iran) and dialyzed against water for 2 h.
with tumor diameters of about 7–9 mm were randomly divided
The correct time was determined by sampling from the dialysis bag
into 3 groups as follows: IV injection of free doxorubicin, IV injec-
at different times until the drug fluorescence no longer changed.
tion of micellar doxorubicin and IV injection of micellar doxorubi-
According to the standard curve of the drug in micelles, the opti-
cin with sonication under the optimum conditions mentioned
mum drug loading into micelles was determined. All experiments
above. After drug injection via the tail vein at a dose of 1.3 mg/kg
were performed in triplicate.
(the amount of drug injected was 26 lg for each animal in differentgroups), animals were anesthetized and placed in a special cage inthe vicinity of the probes in the ultrasonic field; in only the sonica-
2.6. Stability studies of the micellar drug
tion group, the tumor region was exposed for 2.5 min to the se-lected sonication protocol, which was determined by the
To investigate the stability of the micellar drug, the fluorescence
subharmonic amplitude analysis (Fig. 3).
amplitude of the micellar drug at 350 nm was measured over time,
The concentration of total doxorubicin in plasma is maximum
and the amplitude of this emission from each day was normalized
at 2 min following injection and decreased bi-exponentially to
to day zero (the time of drug encapsulation) to find the stability of
underdetectable levels after 24 h [15,28,33]. Therefore animalswere exposed 2 min following injection. Based on previous study,the distribution of doxorubicin into tumors was maximum 24 hfollowing injection. The levels of doxorubicin in the tumors in-creased until 24 h [28]. Therefore, after 24 h, the animals were sac-rificed, blood was collected using a heparinated syringe from theheart, and tissues (heart, liver, spleen, kidney and tumor) were dis-sected and lyophilized. The blood was centrifuged (1500 rpm for10 min), and the plasma was separated and kept for later analysis.
Tissue extracts were obtained according to available protocols [36].
The doxorubicin concentration in the tissues and plasma wasdetermined by a fluorescence assay using a UV spectrophotometer(Shimadzu, Model RF-1500, Japan) at 350 nm. Standard curves foreach tissue were established by adding known amounts of doxoru-
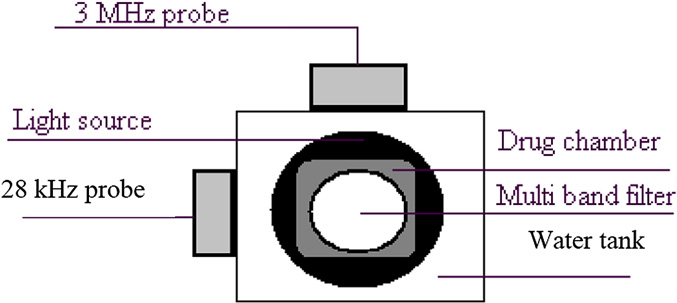
Fig. 2. Setup for the measurement of doxorubicin release in vitro. Elements include
bicin to tissue extracts of control mice to obtain various concentra-
the 3-MHz probe, the 28-kHz probe, the filter on which the camera was mounted,
tions; these curves were used later to obtain the drug
the light source that is placed at the bottom and the drug chamber that was placedin the water box on the light source.
concentration in different tissues from the experimental groups.

Author's personal copy
H. Hasanzadeh et al. / Ultrasonics Sonochemistry 18 (2011) 1165–1171
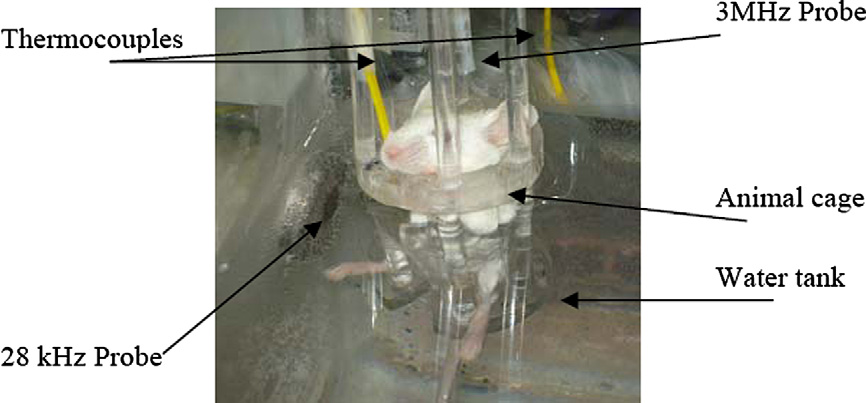
Fig. 3. Animal sonication setup. A side view of the animal cage in the sonication condition. The 28-kHz probe, 3-MHz probe and thermocouples are shown.
2.8. Statistical analysis
Statistical analysis was performed using Microsoft Excel 2003
and SPSS 16 (SPSS/PC Inc., Chicago, IL). The drug content in tissuesis presented as the mean ± SD. To analyze differences between
groups, a one way ANOVA was used with a level of significanceof 0.05 (P-value < 0.05).
Fig. 4 shows the results from dynamic light scattering (DSL),
which gives a particle size distribution of the synthesized micelles.
As shown in Fig. 4, polymeric micelles had small particle sizes with
Time after drug loading (Day)
an average size of 14 nm.
The stability of the encapsulated drug was evaluated over one
Fig. 5. Stability of the micellar drug versus time (days).
month. Fig. 5 shows the fluorescence amplitude of the micellardrug at 350 nm. The amplitude of this emission measurement ateach day was normalized to the value from day zero. As shown
in Fig. 5, the encapsulated drug is stable for the first 4 days, andabout 90% of the maximum possible stability was maintained over
the first ten days.
The results of a subharmonic amplitude measurement at 14 kHz
during both single frequency sonication and simultaneous dual-
frequency sonication at different intensities are shown in Fig. 6.
For each measurement condition, the amplitude of the processed
signal was extracted and recorded after the subtraction of back-
ground noise for each frequency component. One of the interesting
results of this frequency combination is the synergistic effects in
dual frequency sonication using 28 kHz (0.04 W/cm2) and 3 MHz
(2 W/cm2) frequencies. The amplitude increase of dual frequency
28 (0.04) kHz+3 (2)MH
28 (0.02) kHz+3 (1)MHz
Fig. 6. Signal amplitudes (mV) at 14 kHz (a subharmonic of 28 kHz) of the 28 kHzand 3 MHz sources applied alone and in combination and presented as mean ± SD.
The background has been subtracted from all values. The values in the parenthesesare intensities in W/cm2.
irradiation with respect to single frequency irradiation is due tothe synergistic effect and creates an enhanced cavitation activity
Mean intensity (%)
from the combined fields.
It has been previously shown by the authors that the 14 kHz
subharmonic amplitude when applying a dual frequency combina-
tion of 28 kHz (0.04 W/cm2) and 3 MHz (2 W/cm2) in the continu-ous mode was about 5 times higher than that obtained from the
Size distribution of micelles (nm)
algebraic sum of single 28 kHz and 3 MHz irradiation. RMSE values
Fig. 4. Result of DLS (dynamic light scattering) on the synthesized micelles.
of the images from dual frequency sonication (28 kHz (0.04 W/
Author's personal copy
H. Hasanzadeh et al. / Ultrasonics Sonochemistry 18 (2011) 1165–1171
Table 1The standard linear regression functions for each tissue given as fluorescence
intensity (X: count) versus concentration of doxorubicin (Y: lg/ml).
Linear regression function
Correlation of coefficient
28 kHz+3 MHz
regression functions. Fluorescence intensity (counts) versus con-centration of doxorubicin (lg/ml) for each tissue yielded a straight
Fig. 7. RMSE values of the images (Mean ± SD) from dual frequency sonication
line of positive slope for increasing concentrations. The results
(28 kHz (0.04 W/cm2) and 3 MHz (2 W/cm2)).
show that there is a significant correlation between the fluores-cence intensity and the concentration of doxorubicin (R > 0.96, cor-
cm2) and 3 MHz (2 W/cm2)) in vitro are shown in Fig. 7. Drug re-
relation is significant at 0.01) for each tissue.
lease from the micelles is due to the cavitation activity, which is
These curves were later used to obtain the drug concentration
enhanced in a dual frequency field; this property is observable in
in different tissues in the studied groups. Table 2 shows the results
the RMSE values.
of tissue spectrofluorimetric measurements from three different
Fig. 8 shows the temperature rise due to sonication with com-
groups: free doxorubicin, micellar doxorubicin and micellar
bined ultrasound irradiation (28 kHz (0.04 W/cm2) + 3 MHz (2 W/
doxorubicin with 28 kHz (0.04 W/cm2) and 3 MHz (2 W/cm2) dual
cm2)) in 30 s intervals for 20 min at several depths in the animal
frequency sonication in continuous mode. Based on spectrofluori-
body. True to expectations, the temperature rise is more serious
metric analysis, doxorubicin content (lg) was presented as
at a 1 cm depth, which is closer to the ultrasonic probes than in
mean ± SD in different tissues.
the other regions. The increase is also seen to some extent at the
The drug content in the group that received micellar drugs in
2 cm depth, but the other two depths do not change temperature
their tumor tissue was significantly higher (3.34 times) than in
significantly. The 1 cm thermocouple was placed into tumor tissue,
the group receiving doxorubicin in its free form (p < 0.05). In other
and the other three were placed outside the tumor. From this data,
non-tumor tissues, the drug content in the micellar group was low-
the duration of sonication in future experiments was selected to be
er (1.35 times) than in the group that received free doxorubicin
2.5 min; as Fig. 8 illustrates, this length of time causes a tempera-
(p < 0.05).
ture rise of no more than 4 °C outside the tumor. The results indi-cate that a 2.5 min sonication causes a temperature rise below the
level of hyperthermia (T 6 42 °C). We have evaluated a controlgroup without sonication but we did not have any temperature
Ultrasound is a unique tool in the field of drug delivery because it
enables improved penetration ability, which allows substances to
To study the drug distribution in animal tissues, three forms of
reach deep regions in the body, and it is non-ionizing by nature. In
doxorubicin were injected into female Balb/C mice that had tumors
addition, it may be used to enhance intracellular drug uptake from
of about 7–9 mm diameter: free doxorubicin, micellar doxorubicin
micelles. The biological action of ultrasound encompasses several
and micellar doxorubicin with sonication under optimum condi-
mechanisms of action including the generation of thermal energy,
tions. The doxorubicin concentration in tissues and in the plasma
sonoporation, the enhancement of local microjets due to inertial
were determined by a fluorescence assay; the results are shown
cavitation (which is enhanced in a multifrequency sonication field
in Table 1. In this table, standard curves for each tissue were estab-
[1,2]) and the enhancement of the permeability of blood capillaries
lished by adding known amounts of doxorubicin to tissue extracts
[4–10]. To inhibit an unwanted temperature increase in critical tis-
of control mice to obtain standard measurements at different
sues above their tolerances, the sonication time was selected as
concentrations. The results are shown and are fit with linear
2.5 min. It is also expected that under multifrequency sonicationconditions, enhanced cavitation activity will be observed.
The amount of drug collected from the different groups was
12.64 ± 1.94 lg for the micellar doxorubicin injection group and16.09 ± 1.07 lg for the micellar doxorubicin injection with sonica-
tion group. The amount of drug injected was 26 lg for each animal
in the different groups. The difference between the amounts of col-
lected drug and injected drug might be due to the fact that we did
not collect animal urine and we used plasma instead of serum. Fur-
thermore, to collect the drug from several tissue extracts, only the
filtered supernatant of centrifuged tissue extracts was used to
track the drug, but the sediments may also contain some drugs. Fi-nally, the drug content of tissues such as the muscle, lung, gut and
brain was not measured. All of these issues may cause a decrease in
drug collection.
Time (min)
It is also apparent in Table 2 that sonication of the micellar form
of the drug caused a significant increase (2.60 times) in drug up-
Fig. 8. The mean ± SD of temperature changes (°C) at different depths in mice (1, 2,
take by the tumor and a decrease in drug uptake by other tissues
3 and 4 cm) versus duration of sonication (min) under dual frequency sonicationconditions (3 MHz and 28 kHz).
(from 1.5 times for the spleen to 9.6 times for the heart). It is ex-
Author's personal copy
H. Hasanzadeh et al. / Ultrasonics Sonochemistry 18 (2011) 1165–1171
Table 2Doxorubicin content (lg) presented as mean ± SD in different tissues and in different groups studied 24 h after injection.
Micellar doxorubicin
Micellar doxorubicin + sonication
pected that the accumulation and uptake of the drug from the
[2] R. Feng, Y. Zhao, C. Zhu, T.J. Mason, Enhancement of ultrasonic cavitation yield
micellar form in the tumor tissue will be higher (8.69 times) than
by multi-frequency sonication, Ultrason. Sonochem. 9 (2002) 231–236.
[3] A.H. Barati, M. Mokhtari-Dizaji, H. Mozdarani, S.Z. Bathaie, Z.M. Hassan, Effect
the free form of the drug because of the EPR (Enhanced Penetration
of exposure parameters on cavitation induced by low-level dual-frequency
and Retention) effect. Moreover, sonication causes an increase in
ultrasound, Ultrason. Sonochem. 14 (2007) 783–789.
drug uptake because of sonoporation, which is observed in the
[4] G. Husseini, G. Myrup, W. Pitt, D. Christensen, N. Rapoport, Factors affecting
acoustically triggered release of drugs from polymeric micelles, J. Contr.
group that was sonicated under optimum conditions.
Release 69 (2000) 43–52.
Urva et al. studied the biological distribution of doxorubicin and
[5] G. Husseini, D. Christensen, N. Rapoport, W. Pitt, Ultrasonic release of
showed that there is a significant decrease in drug content in the
doxorubicin from Pluronic P105 micelles stabilized with an interpenetratingnetwork of N,N-diethylacrylamide, J. Contr. Release 83 (2002) 303–305.
plasma in the first 30 min following injection; the spleen had the
[6] G. Husseini, M.D. Rosa, T. Gabuji, Y. Zeng, D. Christensen, W. Pitt, Release of
highest drug concentration relative to plasma 72 h after injection
doxorubicin from unstablized and stabilized micelles under the action of
followed by the liver, kidney, lung, gut, heart, muscle, testes and
ultrasound, J. Nanosci. Nanotechnol. 7 (2007) 1028–1033.
[7] A. Marine, M. Muniruzzaman, N. Rapoport, Acoustic activation of drug delivery
brain [31]. The high uptake ability of liver tissue was observed in
from polymeric micelles: effect of pulsed ultrasound, J. Contr. Release 71
the study of Bibby et al., which used Balb/C mice and a metastatic
(2001) 239–249.
mammary cell line [28]. Al-Abd et al. used doxorubicin and its
[8] Z. Gao, H. Fain, N. Rapoporrt, Controlled and targeted tumor chemotherapy by
polyphosphazene hydrogel mixture in their study on drug distribu-
micellar encapsulated drug and ultrasound, J. Contr. Release 102 (2005) 203–222.
tion. They dissected tissues 1 h after intratumoral drug injection
[9] I. Larina, B. Evers, T. Ashitkov, C. Bartels, K. Larin, R. Esenaliev, Enhancement of
and analyzed them for drug content; they showed that in the
drug delivery in tumors by using interaction of nanoparticles with ultrasound
hydrogel mixture group, the tumor had the highest drug content,
radiation, Technol. Cancer Res. Treat. 4 (2005) 217–226.
[10] J. Nelson, B. Roeder, J. Carmen, F. Roloff, W. Pitt, Ultrasonically activated
while the kidney, liver, spleen, gut, lung, heart and brain were at
chemotherapeutic drug delivery in a rat model, Cancer Res. 62 (2002) 7280–
lower concentrations [29]. In another study on the doxorubicin
profile of rat plasma, it was shown that in the first 40 min after
[11] A. Kümmerle, T. Krueger, M. Dusmet, C. Vallet, Y. Pan, H.B. Ris, L.A. Decosterd, A
validated assay for measuring doxorubicin in biological fluids and tissues in an
injection, the drug content in the plasma reduces to about 1/50
isolated lung perfusion model: matrix effect and heparin interference strongly
its primary value [37]. In a study on nude Balb/C mice that used
influence doxorubicin measurements, J. Pharm. Biomed. Anal. 33 (2003) 475–
two different micellar forms of doxorubicin as well as free doxoru-
[12] P.E. Colombo, M. Boustta, S. Poujol, F. Pinguet, P. Rouanet, F. Bressolle, M. Vert,
bicin, it was shown that one of the micellar forms of the drug had
Biodistribution of doxorubicin-alkylated poly (l-lysine citramide imide)
the highest drug uptake, while the remaining groups showed max-
conjugates in an experimental model of peritoneal carcinomatosis after
imal uptake by the liver [33]. In the present study, the group that
intraperitoneal administration, Eur. J. Pharm. Sci. 31 (2007) 43–52.
[13] V. Alakhov, E. Klinski, S. Li, G. Pietrzynski, A. Venne, E. Batrakova, T. Bronitch, A.
received doxorubicin in its free form showed the highest drug con-
Kabanov, Block copolymer-based formulation of doxorubicin. From cell screen
tent in the spleen and lower drug content in the tumor, while the
to clinical trials, Colloids. Surf. B. Biointerfaces 16 (1999) 113–134.
group that received the micellar drug showed the highest drug
[14] A.M.M. Osman, M.M. Nemnem, A.A. Abou-Bakr, O.A. Nassier, M.T. Khayyal,
concentration in the tumor. This problem can be explained by
Effect of methimazole treatment on doxorubicin-induced cardiotoxicity inmice, Food Chem. Toxicol. 47 (2009) 2425–2430.
the characteristics of tumor tissues. The physiological and intersti-
[15] A. Fundar, R. Cavalli, A. Bargoni, D. Vighetto, G.P. Zara, M.R. Gasco, Non-stealth
tial properties of tumors create slower lymphatic drainage than
normal tissue, which causes extravasation of macromolecules to
pharmacokinetics and tissue distribution after i.v. administration to rats,Pharmacol. Res. 42 (2000) 337–343.
the interstitial spaces. Additionally, the lack of vascular permeabil-
[16] G.J.R. Charrois, T.M. Allen, Drug release rate influences the pharmacokinetics,
ity factors in tumor cells makes the tumor vasculature abnormally
biodistribution, therapeutic activity, and toxicity of pegylated liposomal
leaky to macromolecules, and this inability of tumor tissue to elim-
doxorubicin formulations in murine breast cancer, Biochim. Biophys. Acta1663 (2004) 167–177.
inate macromolecules may explain the accumulation of the poly-
[17] M. Yokoyama, M. Miyauchi, N. Yamada, T. Okano, Y. Sakurai, K. Kataoka, S.
mer conjugates seen in tumors. The most notable result was the
Inoue, Polymer micelles as novel drug carrier: adriamycin-conjugated
group that received the micellar drug in combination with sonica-
poly(ethylene glycol)-poly(aspartic acid) block copolymer, J. Contr. Release11 (1990) 269–278.
tion. A dual frequency sonication condition was used to reduce the
[18] M. Yokoyama, M. Miyauchi, N. Yamada, T. Okano, Y. Sakurai, K. Kataoka, S.
cavitation area [35], and as a result, it was possible to minimize the
Inoue, Characterization and anticancer activity of the micelle-forming
cavitation region and control the sonoporation region in vivo,
polymeric anticancer drug adriamycin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer, Cancer Res. 50 (1990) 1693–1700.
which causes enhanced drug uptake locally. It was observed that
[19] Y.I. Jeong, H.S. Na, K.O. Cho, H.C. Lee, J.W. Nah, C.S. Cho, Antitumor activity of
tumor drug uptake in this group was much more than in the group
adriamycin-incorporated polymeric micelles of poly([gamma]-benzyl l-
that only received the micellar drug.
glutamate)/poly(ethylene oxide), Int. J. Pharm. 365 (2009) 150–156.
It is concluded that local sonication with a dual frequency sys-
[20] H.L. Wong, A.M. Rauth, R. Bendayan, X.Y. Wu, In vivo evaluation of a
new polymer-lipid hybrid nanoparticle (PLN) formulation of doxorubicin
tem causes the drug to release from the micellar carriers and in-
in a murine solid tumor model, Eur. J. Pharm. Biopharm. 65 (2007)
creases drug uptake ability. We hypothesize that cavitation
events caused by dual frequency sonication can disrupt micelles
[21] R.K. Subedi, K.W. Kang, H.K. Choi, Preparation and characterization of solid
lipid nanoparticles loaded with doxorubicin, Eur. J. Pharm. Sci. 37 (2009) 508–
and release the drug into the aqueous environment.
[22] K. Kazunori, S.K. Glenn, Y. Masayuki, O. Teruo, S. Yasuhisa, Block copolymer
micelles as vehicles for drug delivery, J. Contr. Release 24 (1993) 119–132.
[23] R. Gref, Y. Minamitake, M.T. Peracchia, V. Trubetskoy, V. Torchilin, R. Langer,
Biodegradable long-circulating polymeric nanospheres, Science 263 (1994)1600–1603.
[1] W. Lauterborn, T. Kurz, R. Geisler, D. Schanz, O. Lindau, Acoustic cavitation,
[24] M. Yokoyama, T. Okano, Y. Sakurai, H. Ekimoto, C. Shibazaki, K. Kataoka,
bubble dynamics and sonoluminescence, Ultrason. Sonochem. 14 (2007) 484–
Toxicity and antitumor activity against solid tumors of micelle-forming
Author's personal copy
H. Hasanzadeh et al. / Ultrasonics Sonochemistry 18 (2011) 1165–1171
polymeric anticancer drug and its extremely long circulation in blood, Cancer
mouse plasma and tissues, J. Chromatogr. B. Anal. Technol. Biomed. Life Sci.
Res. 51 (1991) 3229–3236.
877 (2009) 837–841.
[25] A. Miwa, A. Ishibe, M. Nakano, T. Yamahira, S. Itai, S. Jinno, H. Kawahara,
[32] R. Ricciarello, S. Pichini, R. Pacifici, I. Altieri, M. Pellegrini, A. Fattorossi, P.
Development of novel chitosan derivatives as micellar carriers of taxol, Pharm.
Zuccaro, Simultaneous determination of epirubicin, doxorubicin and their
Res. 15 (1998) 1844–1850.
principal metabolites
by high-performance
[26] Y.I. Son, J.S. Jang, Y.W. Cho, H. Chung, R.W. Park, I.C. Kwon, I.S. Kim, J.Y. Park,
chromatography and electrochemical detection, J. Chromatogr. B. Biomed.
S.B. Seo, C.R. Park, S.Y. Jeong, Biodistribution and anti-tumor efficacy of
Sci. Appl. 707 (1998) 219–225.
doxorubicin loaded glycol-chitosan nanoaggregates by EPR effect, J. Contr.
[33] D. Kim, Z.G. Gao, E.S. Lee, Y.H. Bae, In vivo evaluation of doxorubicin-loaded
Release 91 (2003) 135–145.
polymeric micelles targeting folate receptors and early endosomal pH in drug-
[27] H.S. Yoo, T.G. Park, Biodegradable polymeric micelles composed of
resistant ovarian cancer, Mol. Pharm. 6 (2009) 1353–1362.
doxorubicin conjugated PLGA–PEG block copolymer, J. Contr. Release 70
[34] R.D. Arnold, J.E. Slack, R.M. Straubinger, Quantification of doxorubicin and
(2001) 63–70.
metabolites in rat plasma and small volume tissue samples by liquid
[28] D.C. Bibby, J.E. Talmadge, M.K. Dalal, S.G. Kurz, K.M. Chytil, S.E. Barry, D.G.
chromatography/electrospray tandem mass spectroscopy, J. Chromatogr. B.
Shand, M. Steiert, Pharmacokinetics and biodistribution of RGD-targeted
Anal. Technol. Biomed. Life Sci. 808 (2004) 141–152.
doxorubicin-loaded nanoparticles in tumor-bearing mice, Int. J. Pharm. 293
[35] H. Hasanzadeh, M. Mokhtari-Dizaji, S.Z. Bathaie, Z.M. Hassan, V. Nilchiani, H.
(2005) 281–290.
Goudarzi, Enhancement and control of acoustic cavitation yield by low level
[29] A.M. Al-Abd, N.H. Kim, S.C. Song, S.J. Lee, H.J. Kuh, A simple HPLC method for
dual frequency sonication: a subharmonic analysis, Ultrason. Sonochem. 18
doxorubicin in plasma and tissues of nude mice, Arch. Pharm. Res. 32 (2009)
(2011) 394–400.
[36] J.G. Shiah, Y. Sun, C.M. Peterson, J. Kopecek, Biodistribution of free and N-(2-
[30] T.E. Mürdter, B. Sperker, K. Bosslet, P. Fritz, H.K. Kroemer, Simultaneous high-
hydroxypropyl) methacrylamide copolymer-bound mesochlorin e6 and
performance liquid chromatographic determination of a glucuronyl prodrug of
adriamycin in nude mice bearing human ovarian carcinoma OVCAR-3
doxorubicin, doxorubicin and its metabolites in human lung tissue, J.
xenografts, J. Contr. Release 61 (1999) 145–157.
Chromatogr. B. Biomed. Sci. Appl. 709 (1998) 289–295.
[37] Q. Zhou, B. Chowbay, Determination of doxorubicin and its metabolites in rat
[31] S.R. Urva, B.S. Shin, V.C. Yang, J.P. Balthasar, Sensitive high performance liquid
serum and bile by LC: application to preclinical pharmacokinetic studies, J.
chromatographic assay for assessment of doxorubicin pharmacokinetics in
Pharm. Biomed. Anal. 30 (2002) 1063–1074.
Source: http://www.pnec.co.ir/userfiles/bm_attaches/Ultrasonics%20Sonochemistry.pdf
HARMONIZING STANDARDS AS INSTITUTIONS Monika Tothova and James F. Oehmke (Paper Presented on the 7th International Conference on "Institutions in Transitions", Kranjska Gora, Slovenia, June 19-20, 2003) Integration efforts of the Central and Eastern European (CEE) countries are gradually materializing. Czech Republic, Estonia, Hungary, Latvia, Lithuania, Poland, Slovakia and Slovenia, and in addition to Cyprus and Malta, are to join the EU on May 1 2004 after the Accession Treaty is ratifiedthusiasm is apparent in every accession country, less optimistic opinions are also voiced. One of the first short- and medium-term priorities in each country was to establish and consolidate standardization and conformity assessment structures. While the concept of "deeper integration beyond abolition of import tariffs and quotas, to further measures to remove market segmentation and promote integration" (Venables 2000) is by and large not questioned, critical views on potential losses of national standards harming national identity – i.e., what constitutes "rum", and how to address cheeses made from non-pasteurized sheep milk – are seen as well. The issue of "lost national standards" is repeated: in 1987 the EU (then European Community) published its visionary plan on new, standardized Europe of 1992; a gain of 7 per cent of European income was estimated from harmonization (Emerson 1988, p.6). Even then British were opposed to some parts of it, claiming "brilliant green mushy peas" and "pink sausages" are part of their national identity, and eventually succeeded in getting the necessary exemptions (Krugman and Obstfeld 2002). Although the harmonization issue has been on the tables in Brussels at least since the late 1980s, it yet has to be concluded. Over the years "new" approach replaced the "old" one: instead of imposing technical solutions, the EU legislation is limited to establishing the essential requirements which products must meet (EC 2003). Differences in quality standards are protected by World Trade Organization's (WTO) recognition of countries' right to adopt the standards they consider appropriate – i.e., for human, animal or plant life or health, for the protection of the environment or to meet other consumer interests assuming their use is justified and they are not used as barriers to trade (WTO 2003). Although countries are urged to apply international food standards (when existing), suggestions of Codex Alimentarius result is a variety of standards and technical regulations across the world and consequent welfare losses. Reaching out for an example from outside Europe, tolerance levels for
INTERNET GOVERNANCE PAPERS PAPER NO. 1 — JULY 2013 Reimaging the Internet: The Need for a High-level Strategic Vision for Internet Governance Mark Raymond and Gordon Smith INTERNET GOVERNANCE PAPERS PAPER NO. 1 — JULY 2013Reimaging the Internet: The Need for a High-level Strategic







