Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Pptu.lefora.com
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Feb. 2011, p. 637–648
Copyright 2011, American Society for Microbiology. All Rights Reserved.
A Rapid, High-Throughput Viability Assay for
Blastocystis spp. Reveals
Metronidazole Resistance and Extensive Subtype-Dependent
Variations in Drug Susceptibilities䌤
Haris Mirza,1 Joshua D. W. Teo,1 Jacqui Upcroft,2 and Kevin S. W. Tan1*
Laboratory of Molecular and Cellular Parasitology, Department of Microbiology, Yong Loo Lin School of Medicine,
National University of Singapore, 5 Science Drive 2, Singapore 117596, Singapore,1
and Queensland Institute of
Medical Research, Brisbane, Queensland 4029, Australia2
Received 1 July 2010/Returned for modification 10 October 2010/Accepted 14 November 2010
Blastocystis is an emerging protistan parasite of controversial pathogenesis. Although metronidazole (Mz) is
standard therapy for Blastocystis infections, there have been accumulating reports of treatment failure, sug-
gesting the existence of drug-resistant isolates. Furthermore, very little is known about Blastocystis suscepti-
bility to standard antimicrobials. In the present study, we established resazurin and XTT viability microassays
for Blastocystis spp. belonging to subtypes 4 and 7, both of which have been suggested to represent pathogenic
zoonotic subtypes. The optimized resazurin assay was used to screen a total of 19 compounds against both
subtypes. Interestingly, subtype 7 parasites were resistant to Mz, a 1-position-substituted 5-nitroimidazole
(5-NI), while subtype 4 parasites were sensitive. Some cross-resistance was observed to tinidazole, another
1-position 5-NI. Conversely, subtype 4 parasites were resistant to emetine, while subtype 7 parasites were
sensitive. Position 2 5-NIs were effective against both subtypes, as were ornidazole, nitazoxanide, furazolidone,
mefloquine, quinicrine, quinine, cotrimoxazole (trimethoprim-sulfamethoxazole), and iodoacetamide. Both
subtypes were resistant to chloroquine, doxycycline, paromomycin, ampicillin, and pyrimethamine. This is the
first study to report extensive variations in drug sensitivities among two clinically important subtypes. Our
study highlights the need to reevaluate established treatment regimens for Blastocystis infections and offers
clear new treatment options for Mz treatment failures.
Blastocystis is an emerging enteric protistan parasite with
no
in vitro or
in vivo data to support this hypothesis. Despite
zoonotic potential (39, 57, 58). It is one of the most common
these controversies, interest in the parasite has increased in
parasites colonizing the human gut, with prevalences ranging
recent years, as signified by the establishment of organizations
between 10% of the population in developed countries and
like the
Blastocystis Research Foundation, which actively sup-
50% in developing countries (58). It frequently infects immu-
port studies on subtype-dependent variations in
Blastocystis
nocompromised individuals (27, 40, 59) and has a high preva-
pathobiology and treatment (6). The clinical significance of the
lence in impoverished children (35) and HIV/AIDS (27) and
intestinal parasite
Giardia intestinalis was recognized only after
cancer (59) patients. Individuals infected with
Blastocystis
it became possible to effectively eliminate it from the gut (33).
present with common intestinal symptoms, such as abdominal
To understand the role of
Blastocystis as a human pathogen,
pain, vomiting, and bloating, as well as mucous and watery
there is an urgent need to identify standardized and effective
diarrhea (58).
Blastocystis infections are commonly associated
treatment options for various
Blastocystis subtypes.
with dermatological disorders (25, 67) and irritable bowel syn-
At least 9 out of the 11 subtypes of
Blastocystis are known to
colonize the human gut (57). The identification of antibiotic-
Although metronidazole (Mz) treatment is considered first-
resistant subtypes of the parasite and development of new
line therapy for
Blastocystis infections, therapeutic intervention
therapeutic options to counter antimicrobial resistance require
is equivocal because of the large number of asymptomatic
a high-throughput screening tool. Conventional drug suscepti-
carriers and frequent reports of treatment failure (3, 23, 37, 53,
bility assays for
Blastocystis (16, 68, 72, 75) are not suitable for
55). The confusion concerning the status of
Blastocystis as a
high-throughput drug screening (HTS) because they are ex-
pathogen is primarily due to limitations of diagnostic tech-
pensive, laborious, time-consuming, potentially hazardous, and
niques, purported subtype-dependent variations in parasite
prone to bias. Since the incidence of
Blastocystis is higher in
virulence, and variable host responses (55). The variation in
developing countries (58), the cost and availability of sophis-
treatment response suggests the presence of metronidazole-
ticated equipment are also limitations for such screenings.
resistant (Mzr) subtypes of the parasite, but there are currently
In this study, we evaluated two high-throughput viability
assays and applied them to drug susceptibility microassays for
Blastocystis. Resazurin (7-hydroxy-3H-phenoxazin-3-one 10-
* Corresponding author. Mailing address: Laboratory of Molecular
oxide) is the active compound of a propriety solution, Alamar
and Cellular Parasitology, Department of Microbiology, Yong Loo Lin
blue (41). The resazurin assay measures intrinsic cellular met-
School of Medicine, National University of Singapore, 5 Science Drive
abolic activity, which reduces resazurin and changes its color as
2, Singapore 117596, Singapore. Phone: 65-6516 6780. Fax: 65-6776-
a measurable indicator of the number of viable cells that are
䌤 Published ahead of print on 22 November 2010.
present in a test sample (34, 47). Resazurin-based assays are
MIRZA ET AL.
ANTIMICROB. AGENTS CHEMOTHER.
TABLE 1. Sources of
Blastocystis isolates
metric measurements. For semiquantitative evaluation, the color change in eachwell was visually observed and recorded after 5 h.
Common hosts
b
Drug preparation. Compounds purchased from Sigma included Mz, ornida-
zole (Oz), ronidazole (Rz), furazolidone (FUR), mefloquine (MQ), quinacrine
Symptomatic human, SGH
a
(QC), quinine (QN), chloroquine (CQ), emetine (EM), doxycycline (DOX),
Symptomatic human, SGH
a
trimethoprim sulfate-sulfamethoxazole (TMP-SMZ), paromomycin (PAR), am-
Wistar rat, animal survey
c
picillin (AMP), pyrimethamine (PYR), and iodoacetamide (IA). Tinidazole (Tz)
Sprague-Dawley rat, animal survey
c Humans, rats
was purchased from AK Scientific, whereas nitazoxanide (NTZ) was purchased
a Isolated from symptomatic patients presenting at the Singapore General
from Romark Laboratory. C-17 is an experimental, chemically synthesized, 2-po-
Hospital (SGH) (36).
sition 5-nitroimidazole (NI) compound (66). Stock solutions of each compound
b Based on Tan (57).
to be tested were prepared fresh in dimethyl sulfoxide (DMSO). For drug
c Isolated during an animal survey (11).
sensitivity determination, stock solutions were diluted in prereduced
Blastocystismedium and transferred to 96-well plates. A total of 0.5 ⫻ 106 cells/well wereincubated for 24 h with different dilutions of the drugs ranging between 0 and 100
commonly used for drug susceptibility analysis of prokaryotic
g/ml. The final DMSO concentration was kept constant at 0.5%.
Confocal microscopy. Confocal micrographs of the parasites were taken in
(29) and eukaryotic (20, 34, 41, 46) cells. Much like resazurin,
order to determine whether the alteration in
Blastocystis redox activity under
the tetrazolium salt 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-
drug tension observed in previous assays was also associated with morphological
2H-tetrazolium-5-carboxanilide (XTT) is reduced by mito-
changes. Metronidazole-susceptible (Mzs) ST-4 (isolate WR-1) and Mzr ST-7
chondrial and cytoplasmic redox enzymes to a colored forma-
(isolate E) were treated for 24 h with a 12.5-g/ml concentration of FUR and
zan compound with a direct correlation with cell proliferation.
Mz. After drug exposure, the parasites were washed and resuspended in annexinV binding buffer (BioVision). Annexin V and propidium iodide (PI) (BioVision)
Tetrazolium compounds have also been widely utilized for
were then added to the cell suspension. Confocal imaging of cell suspensions was
cytotoxic evaluation of both prokaryotic (14) and eukaryotic
done using an Olympus Fluoview FV1000 (Japan) equipped with a dual filter set
(5) organisms.
for fluorescein isothiocyanate (FITC) and rhodamine. Images were captured
In the current study, we report that optimized resazurin and
using Olympus Fluoview version 1.6b.
Statistical analysis and validation of reproducibility. Before a particular assay
XTT redox-based assays are suitable for viability studies of the
was used for a full-scale HTS, smaller pilot screenings were used to predict its
parasite.
Blastocystis subtype 4 and subtype 7 isolates are most
usefulness for large-scale applications. The
Z⬘ factor predicts the robustness of
commonly found in rats and birds, respectively (57). Both
an assay for HTS by taking into account the mean and standard deviation of both
subtypes are known to colonize the human gut, and studies
positive and negative controls of the pilot screening (74). We calculated the
Z⬘
suggest that both subtypes have pathogenic potential (54). We
factors of both assays for
Blastocystis drug screening using the following equation:
Z⬘ factor ⫽ 1 ⫺ [(3
utilized the optimized assays to determine the susceptibility of
c⫹ ⫹ 3
c⫺)/ⱍ
c⫹ ⫺
c⫺ⱍ], where,
c⫹ is the positive control
(0.5% DMSO),
c⫺ is the negative control (6.25 g/ml FUR), is the standard
Blastocystis isolates to a range of antimicrobial agents. We
deviation, and is the mean.
observed extensive subtype-dependent variations in
Blastocys-
Assays having a
Z⬘ factor score between 0.5 and 1 are considered excellent for
tis susceptibility to a panel of conventional and experimental
antiprotozoal agents and identified Mz- and emetine (EM)-
Comparison of data sets with wide differences between their means should be
made using the coefficient of variation (
C ) instead of the standard deviation ().
resistant subtypes of the parasite. Importantly, we identified
It represents the in the context of the mean () and is another test used to
several new and potentially effective treatment options for Mzr
evaluate the robustness of an assay for HTS. We calculated the
C s of both assays
using the following formula (30):
C ⫽ /, where,
C is the coefficient of
variation, is the standard deviation of the positive control (5 ⫻ 105 parasites in200 l culture medium plus 0.5% DMSO), and is the mean of the positive
MATERIALS AND METHODS
control (5 ⫻ 105 parasites in 200 l culture medium plus 0.5% DMSO).
Cell culture. Four axenized isolates of
Blastocystis were used (Table 1). All
Assays with a
C of ⬍1 are considered low variance and fit for HTS (30).
four isolates were subtyped previously by small-subunit rRNA gene analyses
The final validation step was the screening of the dose-dependent antiproto-
(39). Isolates WR-1 and S-1 belong to subtype 4, while isolates B and E belong
zoal activity of Mz against 4 different isolates of
Blastocystis repeated twice in
to subtype 7, according to a recent
Blastocystis sp. classification system (52).
triplicate. The results were statistically compared for reproducibility.
Cultures of all four isolates were maintained as described previously (36). In
The statistical significance of variations between the drug susceptibility values
brief, the parasites were maintained in 10 ml of prereduced Iscove's modified
of 4 isolates was determined using one-way analysis of variance (ANOVA). A
Dulbecco's medium (IMDM) containing 10% horse serum in an anaerobic jar
one-way ANOVA test is ideal to test the statistical significance of the variations
(Oxoid) with an AnaeroGen gas pack (Oxoid) at 37°C. The parasites were
observed between means of three or more groups of data.
subcultured alternately at 72 and 96 h. Under these culture conditions, all fourparasites exhibited noncystic vacuolar morphology. This morphological state isadvantageous for assessment of MZ resistance because
Blastocystis cysts are
known to be resistant to the drug (73), complicating our study. Cultures wereharvested from log-phase
in vitro cultures for viability studies in 96-well plates.
Resazurin and XTT result in fluorimetric and colorimetric
Microculture technique. In order to establish and validate the analytical meth-
reactions with Blastocystis in a cell density-dependent manner.
ods for
Blastocystis viability determination, the microculture conditions wereoptimized for standard 96-well plates. Subtype 7 parasites (isolate B) were
For semiquantitative analysis, visible color changes were ob-
employed for the optimization experiments. Several parasite numbers between
served after 5 h of incubation of resazurin and XTT with
103 and 106 cells were incubated in
Blastocystis culture medium in a final volume
Blastocystis sp. subtype 7 in 200 l parasite culture medium.
of 200 l/well in standard 96-well plates, unless otherwise stated. The 96-well
Several shades of resazurin dye, ranging from blue to pink,
plates were then incubated at 37°C under anaerobic conditions for 24 h unless
developed with increasing cell density. Similarly, XTT devel-
otherwise stated. After 24 h, the cultures were incubated with redox dyes for anadditional 3 h and 5 h for quantitative and semiquantitative evaluation, respec-
oped shades ranging from yellow to deep orange with increas-
tively. Unless otherwise stated, a 5% final dilution of the resazurin dye solution
ing cell density. Minimums of 105 parasites/well were needed
(Sigma) was used for resazurin assays, whereas XTT (Sigma) was used at a final
to obtain visual evidence of color change for both dyes, al-
concentration of 50 g/ml. At the end of incubation, readings of resazurin
though the color change was more obvious in the resazurin dye
fluorescence were taken at 550-nm excitation and 570-nm emission wavelengths,while XTT assay measurements were made at an absorbance wavelength of 450
than with XTT.
nm. A Tecan Infinite M200 reader was used for both fluorimetric and colori-
For quantitative analysis, fluorescence and absorbance mea-
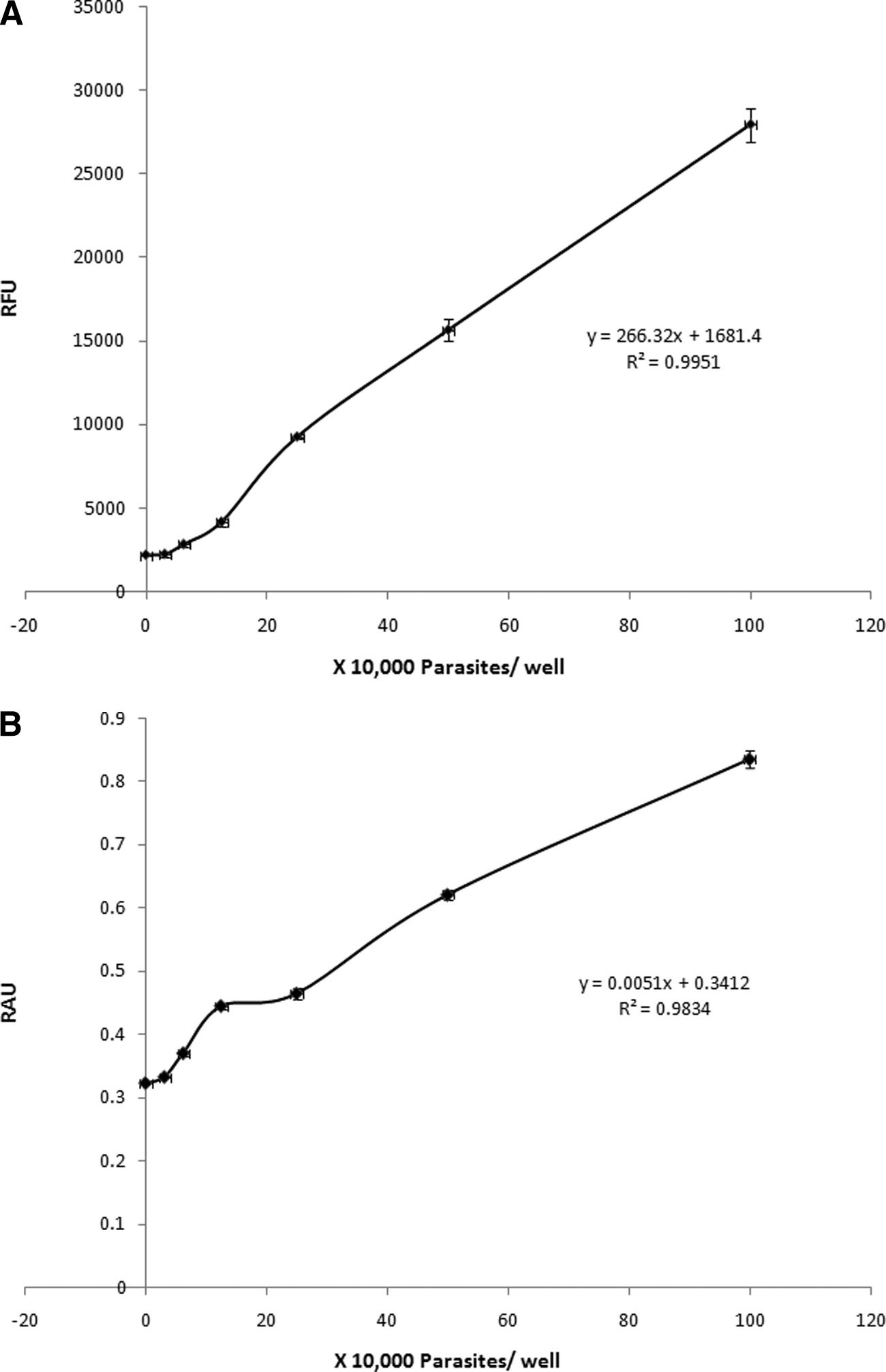
BLASTOCYSTIS METRONIDAZOLE RESISTANCE AND SUSCEPTIBILITY
FIG. 1. Correlation between the number of subtype 7 parasites and relative fluorescence units (RFU) (A) and relative absorbance units (RAU)
(B) after 24 h of incubation and 3 h of development with resazurin and XTT, respectively. Each point represents an average of 6 values derivedfrom two independent sets of experiments. The error bars represent standard errors.
surements were taken for resazurin and XTT dyes, respec-
linear range of cell density versus dye reduction for both assays
tively, after 3 h of incubation. Negligible changes in absorbance
(Fig. 1) and provides visible color changes in a short time.
and fluorescence measurements were observed between the
Blastocystis requires 200-l/well volumes for optimal meta-
blank medium control and up to 104 parasites/well (Fig. 1), but
bolic activity. For viability assays, cells should be at their op-
a linear increase in fluorimetric, as well as colorimetric, mea-
timal metabolic activity. A recent study reported an increase in
surements was noted from 104 parasites to 106 parasites/well
metabolic activity of Acanthamoeba with a reduction of the
(Fig. 1). The R2 values for resazurin and XTT dyes were cal-
culture volume from 200 to 100 l/well (34). In this study, a
culated to be 0.995 and 0.983, respectively (Fig. 1; see Table 3).
decrease in volume per well resulted in a drop in Blastocystis
A density of 5 ⫻ 105 parasites/well was chosen as the optimal
metabolic activity (Fig. 2). Blastocystis, an anaerobic organism
cell density for further experiments because it lies within the
(57), should have higher metabolic activity in high well vol-
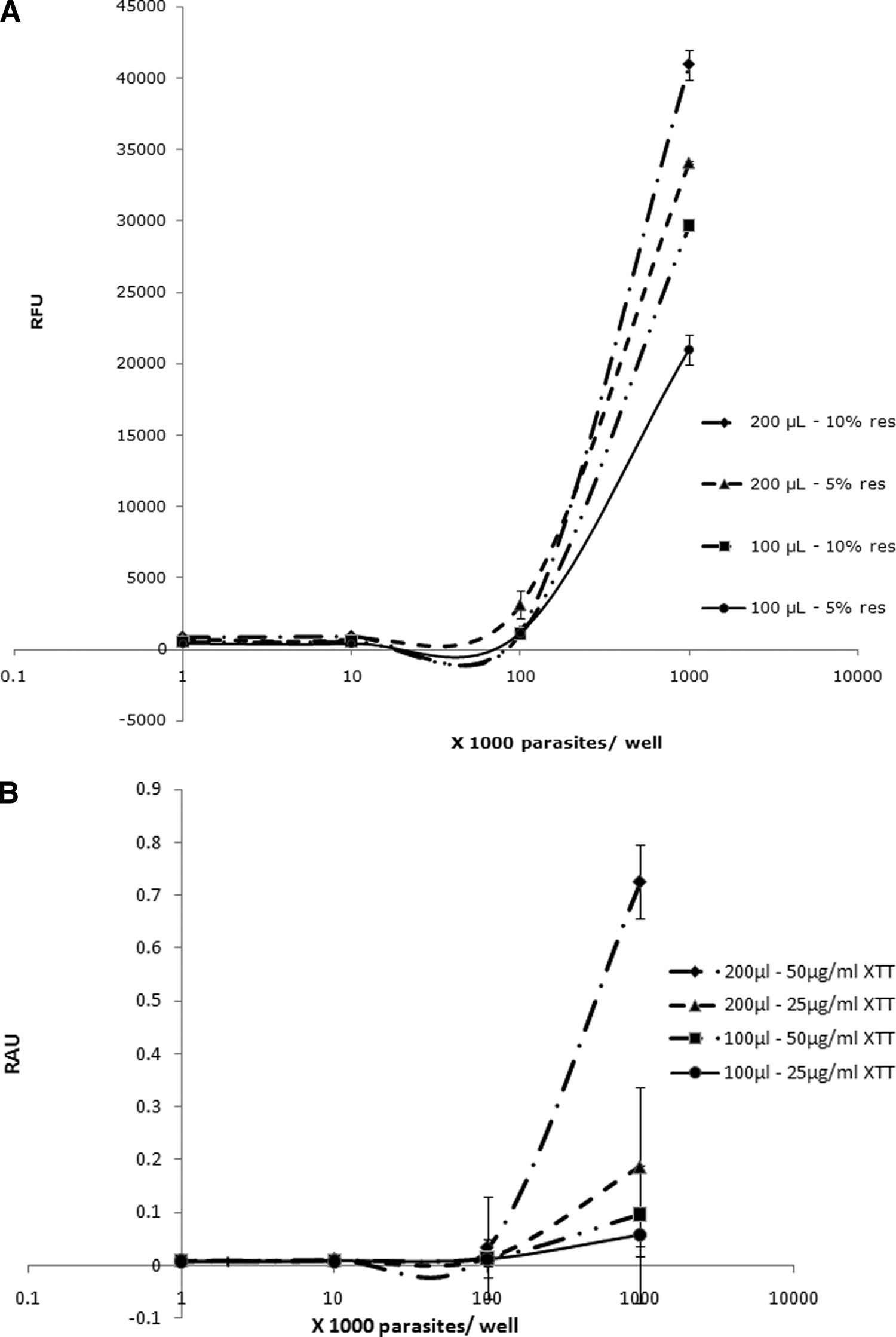
MIRZA ET AL.
ANTIMICROB. AGENTS CHEMOTHER.
FIG. 2. Correlation of total volume per well and dye concentration with relative fluorescence units (RFU) (A) and relative absorbance units
(RAU) (B) for resazurin (res) and XTT dyes, respectively. Higher volumes per well and dye concentrations resulted in higher sensitivity ofresazurin and XTT, denoted by higher RFU and RAU readings, respectively. Each point represents a mean of 6 values derived from twoindependent sets of experiments. The error bars represent standard errors.
umes as opposed to Acanthamoeba, which is an aerobic pro-
croplate growth conditions, exhibited an increasing degrada-
tozoan (34). Therefore, a 200-l/well volume was used in all
tion of resazurin over time, suggesting a rise in the redox
subsequent experiments (Table 2 and Fig. 2).
activity of the culture (Fig. 3). This increase in redox activity
Blastocystis exhibits exponential growth in microcultures.
could be due to an increase in either parasite numbers or
Blastocystis sp. subtype 7, when incubated under optimal mi-
metabolic activity. The redox activity of the parasite cultures
BLASTOCYSTIS METRONIDAZOLE RESISTANCE AND SUSCEPTIBILITY
TABLE 2. Optimized parameters for resazurin and XTT assays
TABLE 3. Statistical evaluation of the quality of resazurin and
XTT assaysa
Z⬘ factor
C (%)b
Growth medium .IMDM ⫹ 10% HSa ⫹
a Ideal HTS parameters are a Z⬘ factor of ⬎0.5 (74) and a C of ⬍10% (30).
b C %, coefficient of variance of cell controls.
Volume/well .200 l
Linearity of the dye reduction-versus-parasites/well curve.
Temperature .37°CCulture conditions.Anaerobic
Contact time with dye (h)
resazurin and XTT assays (Table 3). Both assays exhibited
statistical reproducibility for dose-dependent activity assays ofantimicrobial agents against Blastocystis (Fig. 4).
Excitation/emission (nm)
Blastocystis exhibits subtype-dependent variation in suscep-
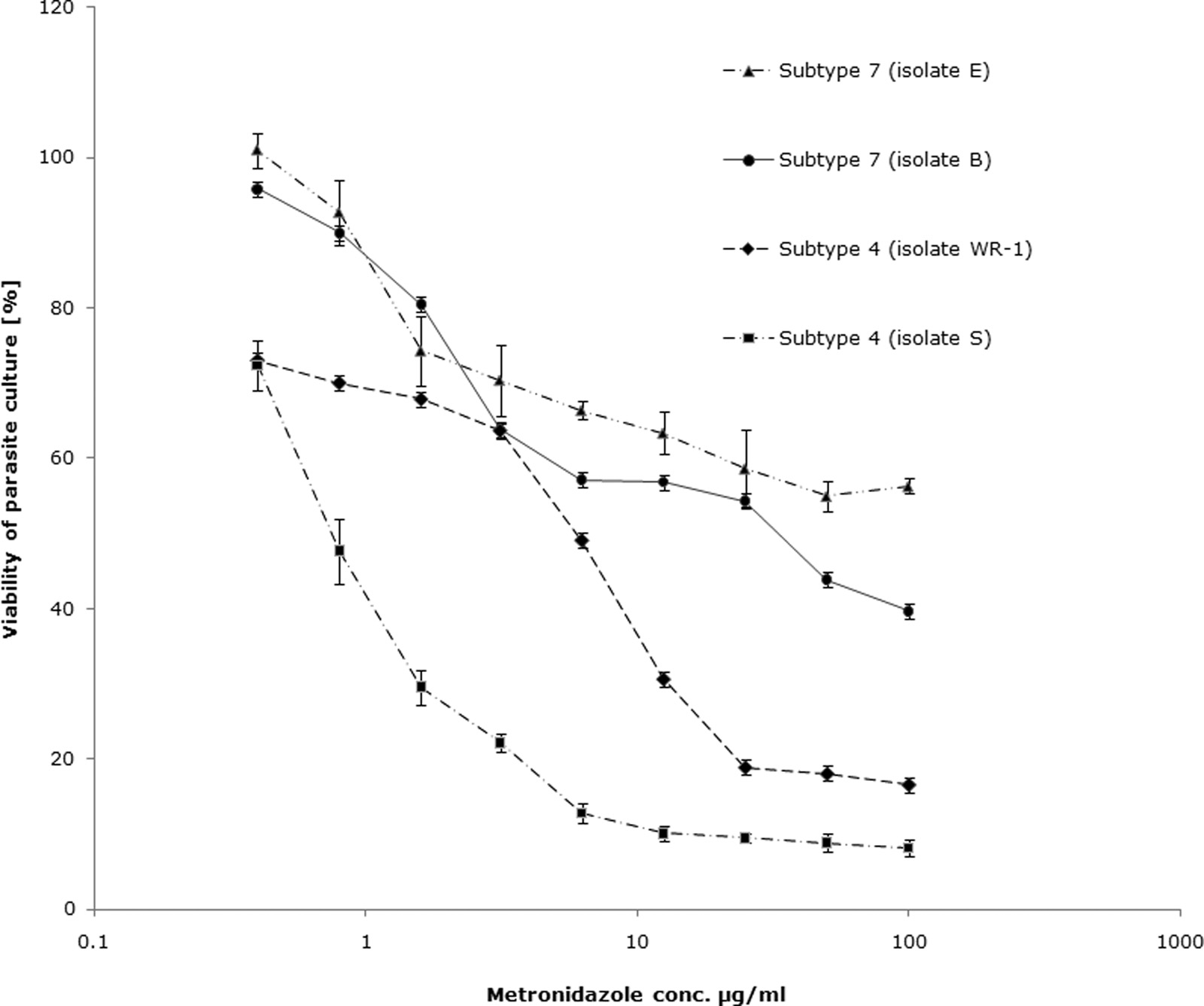
tibility and resistance to Mz. Using the optimized resazurin
assay, the 50% inhibitory concentrations (IC s) of Mz against
Optimal cell density (parasites/well).0.5 ⫻ 106
subtype 4 and subtype 7 isolates of Blastocystis were calculated.
Mz inhibited 50% of growth of subtype 4 isolates WR-1 and
HS, heat-inactivated horse serum.
S-1 at concentrations of 5.5 ⫾ 2.89 g/ml and 1.9 ⫾ 1.32 g/ml,respectively (Table 4; Fig. 4 and 5). These values were within
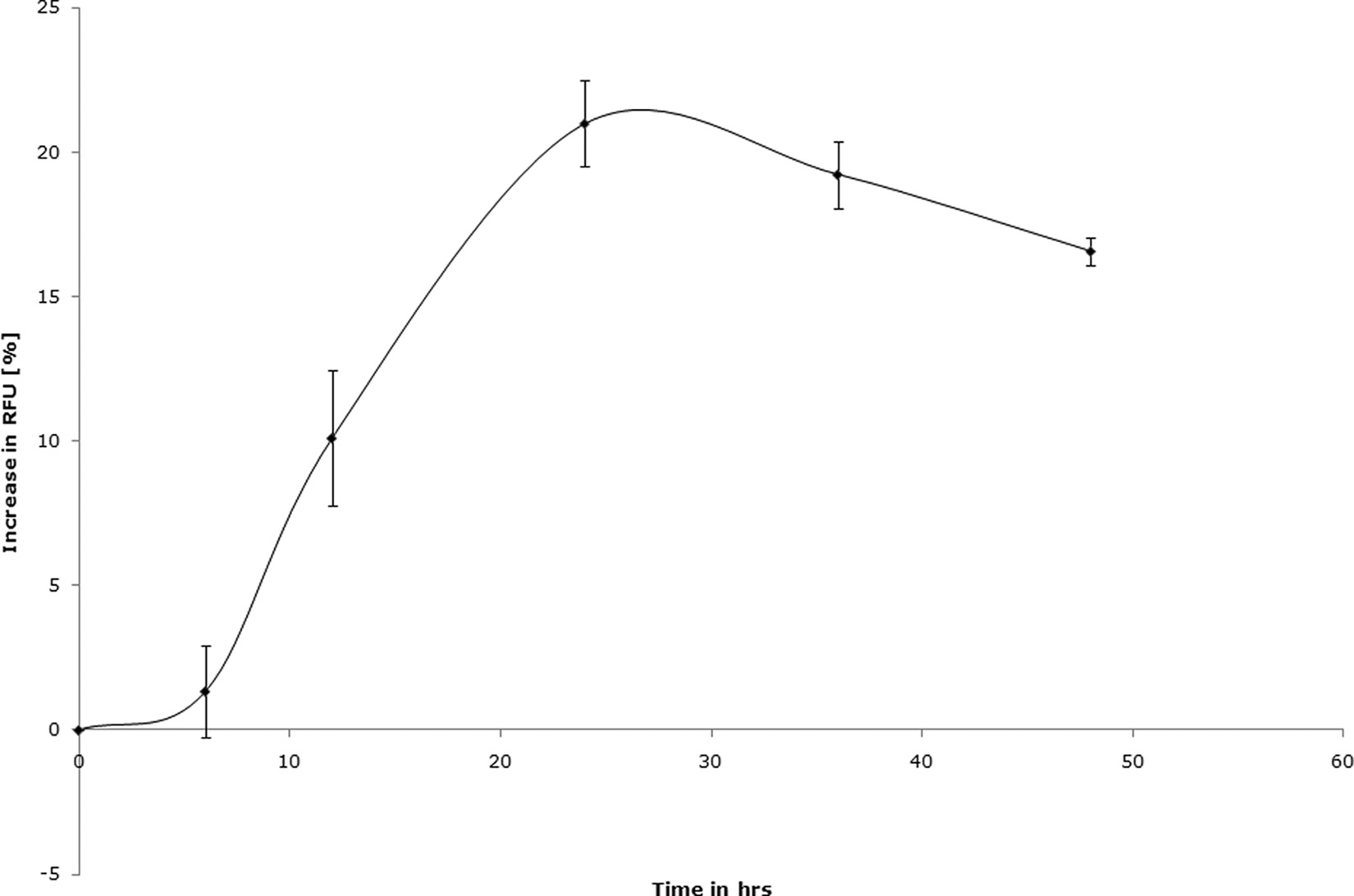
peaked at 24 h, followed by a drop, suggesting a slowing down
the range of previously reported values of Mz susceptibility for
of the culture growth or metabolism due to overcrowding. The
Blastocystis (16, 75). The IC
of Mz against isolate B (subtype
24-h time point was chosen for drug susceptibility assays (Table
7) was 32.5 ⫾ 3.4 g/ml. This value is significantly higher than
2). The complete optimized parameters for both resazurin and
of subtype 4 isolates (P ⬍ 0.01) and exceeds the
XTT assays are summarized in Table 2.
average fecal Mz concentration of 9.5 g/ml (26). Isolate E of
Resazurin and XTT are suitable for HTS of antimicrobials
subtype 7 exhibited minimal susceptibility to Mz (Table 4 and
against Blastocystis. HTS quality control parameters, i.e., a Z⬘
Fig. 4), even at concentrations as high as 100 g/ml. These
factor of ⬎0.5 (74) and a C of ⬍10% (30), were met by both
results suggest that isolates B and E of subtype 7 are Mzr
FIG. 3. Blastocystis subtype 7 exhibits a time-dependent increase in redox activity when cultured in a 96-well plate under the resazurin assay
conditions described in this study. The starting parasite density was 0.5 ⫻ 106 cells in 200 l of IMDM supplemented with 10% horse serum and0.5% DMSO. The redox activity of the culture peaked at 24 h, followed by a steady decline. A drug contact duration of 24 h was chosen basedon these results. Each point represents a mean of 6 values derived from two independent experiments, with each experiment conducted intriplicate. The error bars represent standard errors.
MIRZA ET AL.
ANTIMICROB. AGENTS CHEMOTHER.
FIG. 4. Graph representing percent inhibition of Blastocystis subtype 4 and 7 cultures by Mz using the resazurin assay. The IC s of Mz against
subtype 4 isolates were found to be significantly lower than those of subtype 7 isolates (P ⬍ 0.01). Mz induced 50% inhibition of subtype 7 isolateB cultures at a concentration (conc.) of 32.5 ⫾ 3.4 g/ml, whereas isolate E cultures exhibited only minimal inhibition even at concentrations ashigh as 100 g/ml. Each point represents a mean of six readings derived from two independent experiments. The error bars represent standarderrors.
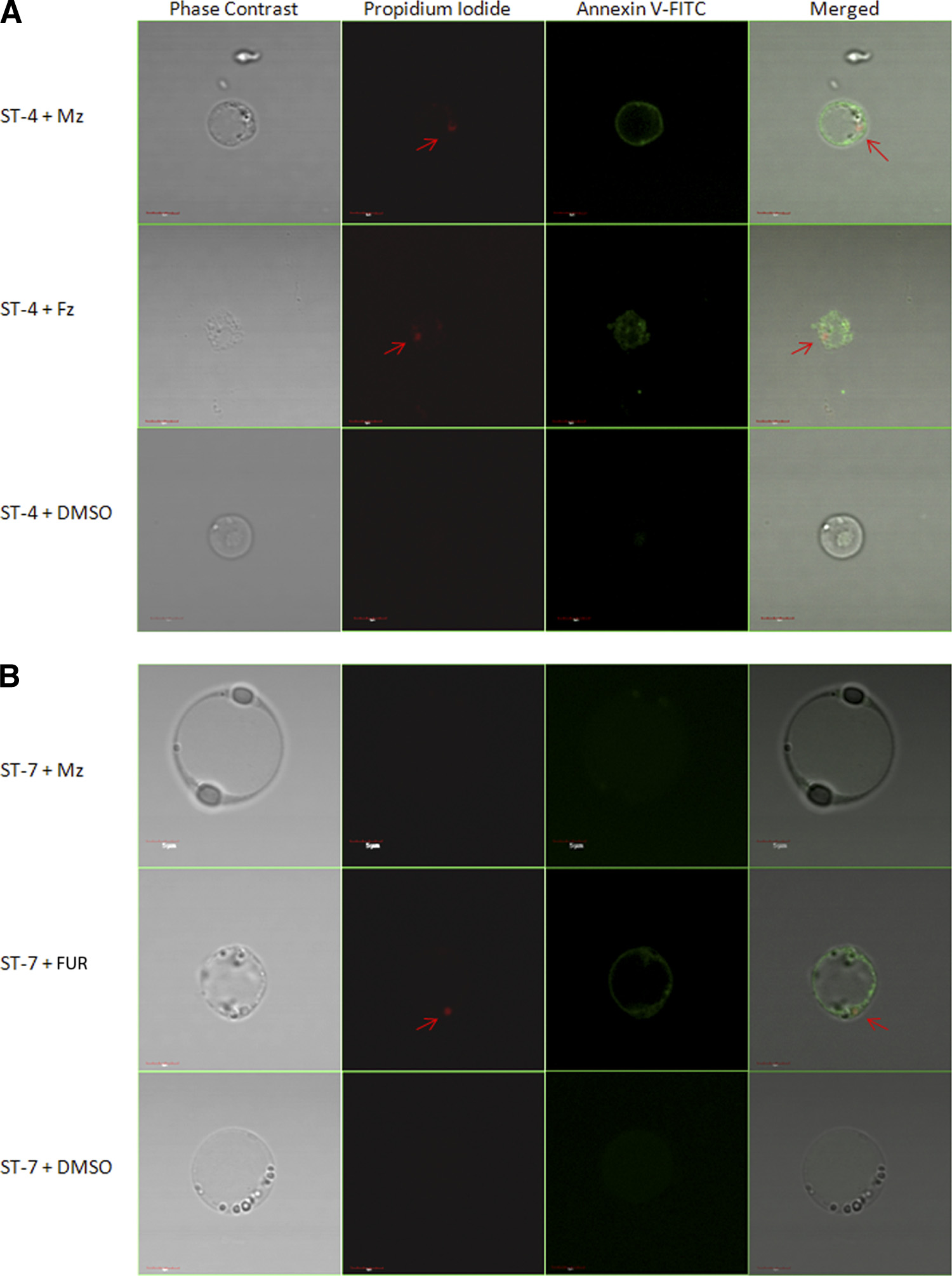
strains of Blastocystis. The XTT assay further confirmed these
are impermeable to both PI and annexin V (71). Mzs ST-4
strains to be Mzr (Table 4).
(isolate WR-1) exhibited nuclear incorporation of PI and an-
An Mzs isolate of Blastocystis exhibits typical morphological
nexin V binding after 24 h of exposure to a 12.5-g/ml con-
features of cell death after exposure to Mz, as opposed to an
centration of Mz, suggesting a breach in the parasite cell mem-
Mzr isolate. Our findings, based on resazurin and XTT assays,
brane (Fig. 6A). No changes were observed in Mzr ST-7
indicate suppression of parasite redox activity under drug ten-
(isolate E) after Mz treatment (Fig. 6B). MZs and Mzr isolates
sion. Concomitantly, to determine whether Blastocystis under-
exhibited cell death morphology after treatment with a 12.5-
goes morphological changes after drug exposure, parasites
g/ml concentration of FUR (Fig. 6A and B), whereas neither
were stained with propidium iodide and annexin V-FITC. Both
of the isolates incorporated PI or annexin V after treatment
PI and annexin V stain only dying parasites (71). PI binds to
with the DMSO control (Fig. 6A and B). These findings sug-
the parasite nuclear material (71). Annexin V binds with high
gest that after treatment with Mz, morphological alterations
affinity to phosphatidylserine (PS). PS is located at the cytoso-
typical of dying cells were observed in the Mzs isolate, while the
lic face of the cell membrane and has access to annexin V only
Mzr isolate remained unaffected.
when it becomes exposed at cell death (71). Healthy parasites
Mzr isolates of Blastocystis exhibit cross-resistance with a
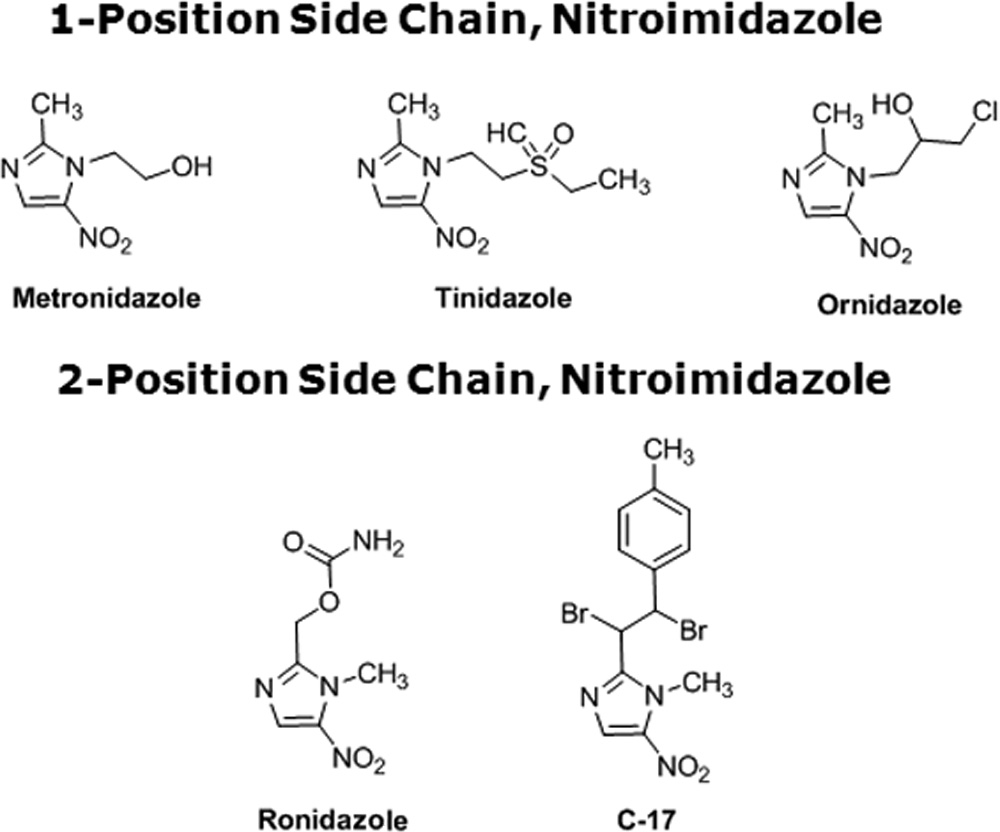
1-position-substituted 5-NI. Tz, a compound closely related to
Mz due to the presence of its side chain at position 1 of the
values of Blastocystis susceptibility to Mz
imidazole ring (Fig. 5), was effective in killing both Mzr and
Mzs isolates. Interestingly, Mzs subtype 4 isolates WR-1 and S
关g/ml (M)兴
exhibited IC s (0.51 ⫾ 0.02 and 0.3 ⫾ 0.1 g/ml, respectively)
Subtype 7 isolates
Subtype 4 isolates
of Tz lower than those of Mzr subtype 7 isolates B and E
(5.13 ⫾ 0.16 and 9.33 ⫾ 0.45 g/ml, respectively) (P ⬍ 0.01)
32.5 ⫾ 3.4 (189.8)
5.5 ⫾ 2.89 (32.16)
0.75 ⫾ 0.04 (4.38)
(Table 5). Even within subtype 7, the IC
of Tz for Mzr isolate
29 ⫾ 3.4 (169.36)
1.76 ⫾ 0.39 (10.27)
1.1 ⫾ 0.08 (6.4)
E was significantly higher than that for isolate B (Table 5).
a NS, not susceptible to drug concentrations of ⱕ100 g/ml.
These findings in Blastocystis suggest a cross-resistance pattern
BLASTOCYSTIS METRONIDAZOLE RESISTANCE AND SUSCEPTIBILITY
ineffective against all four isolates of the parasite (data notshown).
Cysteine protease inhibition causes parasite death. The sig-
nificance of cysteine proteases in Blastocystis pathobiology iswell reported (36, 48, 58, 71). In this study, Inhibition of cys-teine protease activity of the parasite by IA resulted in com-plete inhibition of all four isolates with similar IC s, suggest-
ing the importance of cysteine proteases in parasite survival.
We found both resazurin and XTT assays to be suitable for
high-throughput analysis of drug susceptibility in Blastocystisisolates. The HTS parameters (a Z⬘ factor of ⬎0.5 and a C of
⬍10%) provide a highly conservative estimate of the sensitivityof an assay (30, 74). The high Z⬘ factor value, low C , and
reproducibility of both resazurin and XTT assays suggest thatthey are robust and suitable for HTS. The option of semiquan-titative visual evaluation of color gives these assays the flexi-
bility to be applied in the field without the need for sophisti-
cated equipment. The suppression of metabolic activityobserved in these redox assays was also found to be associatedwith morphological signs of cell death (71), i.e., nuclear incor-poration of PI and annexin V binding to the cell membrane,
similar to those exhibited by other parasites (7, 12). Oz, an-
further validating these assays in determining drug susceptibil-
other closely related 5-NI (Fig. 5), despite having a position 1
ities. Considering the large number of variant Blastocystis iso-
side chain, was found to be equally effective against both Mzr
lates and the predominance of the parasite in developing coun-
and Mzs isolates. Interestingly, Mzr subtype 7 isolates exhibited
tries (58) with limited research funding, these assays will be
significantly higher susceptibility to the position 2 side chain
particularly useful due to their low cost and high yield.
5-NIs Rz and C-17 (Fig. 5) than to position 1 5-NI. No signif-
Subtype 7 isolates were shown to be resistant to Mz and
icant subtype-dependent variation in Blastocystis susceptibility
cross-resistant to Tz, the 1-position-substituted 5-NI of choice
to position 2 5-NIs was observed.
to treat a wide variety of anaerobic organisms (4, 22). This is
Blastocystis subtype 4 exhibits EM resistance. EM is an an-
consistent with previous reports of cross-resistance between
tiamoebic agent with limited clinical use, reported to be effec-
the two drugs in Trichomonas (12, 31) and Giardia (7, 61). In
tive against Blastocystis in vitro (16, 75). Our study found EM to
these organisms, resistance is proposed to be due to downregu-
be effective against Mzr subtype 7 isolates (Table 6). Subtype 4
lation of the enzymes PFOR (65) and thioredoxin oxidoreduc-
isolates S and WR-1, on the other hand, exhibited no inhibition
tase (28), which in conjunction with the electron acceptor
even at the highest test concentrations of 100 g/ml, suggesting
ferredoxin are believed to activate the 5-NI prodrugs to the
EM resistance in subtype 4 isolates.
toxic radical states inside the parasite (28, 65). However, this
Blastocystis exhibits subtype-dependent variations in suscep-
mechanism of activation has not been shown for Blastocystis,
tibility to NTZ, MQ, and QC. NTZ, a well-documented pyru-
although PFOR and other oxidoreductase enzymes are present
vate-ferredoxin oxidoreductase (PFOR) inhibitor (43), was
in the organism (70). The subtype 4 isolates showed no con-
found to be more effective against Mzr strains of the parasite in
vincing uniformity in susceptibility to Mz and Tz, indicating
this study (Table 6). Subtype 7 (avian) isolates were signifi-
that new, unknown mechanisms of activation and/or resistance
cantly more sensitive to NTZ than subtype 4 (rodent) isolates
may be involved.
(P ⬍ 0.01). Similarly, the anti-malarial MQ and a closely re-
All isolates were similarly susceptible to another 1-position
lated drug, QC, were also found to be significantly more ef-
5-NI, Oz. Compared to Mz, the drug has significantly higher
fective against subtype 7 isolates than subtype 4 (Table 6).
efficacy against Mzr isolates of Blastocystis (P ⬍ 0.01), as ob-
No subtype-dependent variations in FUR and QN suscepti-
served in other parasites (10, 64) and also reported for Blas-
bility. Both Mzr and Mzs isolates exhibited sensitivity to FUR
tocystis previously (16). However, its superior efficacy against
and QN (Table 6), two well-known antiprotozoal agents.
Mzs isolates is not as obvious, again suggesting new, unknown
Higher susceptibility of Blastocystis spp. to a TMP/SMZ
mechanisms of activation and/or resistance to 1-position 5-NIs
ratio of 1:2 than to one of 1:5. SMZ and TMP are administered
in the parasite. Oz is frequently used to treat amoebiasis in
in two different ratios for protozoan infections. TMP/SMZ
India (21). Although the IC s of Oz against all four isolates
ratios of 1:5 and 1:2 were tested for Blastocystis inhibition. All
tested here (4.9 to 6.44 M) were higher than the MIC of the
isolates exhibited susceptibility to both combinations, but all
drug against Entamoeba (0.25 M) (10), its effectiveness
four isolates were significantly more sensitive (P ⬍ 0.01) to a
against both Mzr and Mzs isolates suggests the drug would be
TMP/SMZ ratio of 1:2 than to one of 1:5 (Table 6).
a useful alternative to Mz to treat Blastocystis infections.
Nonsusceptibility of Blastocystis to broad-spectrum antibi-
Similarly to Oz, 2-position 5-NIs, the commercially available
otics. PAR, PYR, CQ, DOX, and AMP were found to be
poultry drug Rz and the experimental drug C-17, were uni-
MIRZA ET AL.
ANTIMICROB. AGENTS CHEMOTHER.
FIG. 6. Confocal micrographs of Blastocystis stained with propidium iodide (arrow) and annexin V-FITC. (A) Mzs ST-4 (WR-1) exhibited
nuclear incorporation of PI and annexin V-FITC binding after 24 h of exposure to 12.5 g/ml Mz. (B) Mzr ST-7 (isolate E) did not exhibit theseclassical signs of cell death after Mz treatment. Both Mzs and Mzr isolates exhibited PI incorporation and annexin V-FITC binding after 24-htreatment with 12.5 g/ml FUR, while no changes were observed in healthy parasites incubated with DMSO. Bars, 5 m.
formly effective against the isolates of both subtypes tested.
1.54 M, suggesting the potential of the drug as a broad-
These 2-position 5-NIs exhibited significantly higher efficacy
spectrum antiprotozoal agent against Mzr parasites. Two-po-
against Mzr isolates than 1-position 5-NIs (P ⬍ 0.01), as ob-
sition 5-NIs may prove to be effective alternatives to treat
served in Giardia and Trichomonas (66). Again, the improved
Blastocystis infections in cases of Mz treatment failure.
efficacy of 2-position 5-NIs against Mzs subtype 4 isolates is not
The susceptibility of the Mzr subtype 7 isolates to NTZ and
as obvious, suggesting a different mechanism of action in Blas-
the reduced susceptibility to the Mzs subtype 4 isolates are also
tocystis than in other organisms (66). The IC
evidence for different mechanisms of action of NTZ in Blas-
Giardia was recently reported to be 0.5 M (17), whereas
tocystis than in Giardia and Trichomonas, where cross-resis-
against Trichomonas it exhibited a MIC of 6.3 M (66). In this
tance between Mz and NTZ is apparent (2). These data sug-
of C-17 against Blastocystis ranged from 0.89 to
gest that Mz treatment failures in blastocystosis may well
BLASTOCYSTIS METRONIDAZOLE RESISTANCE AND SUSCEPTIBILITY
values of Blastocystis for 5-NIs by resazurin assay
关g/ml (M)兴
Subtype 7 isolates
Subtype 4 isolates
1-Position 5-NIsa
32.5 ⫾ 3.4 (189.8)
5.5 ⫾ 2.89 (32.16)
0.75 ⫾ 0.04 (4.38)
5.13 ⫾ 0.16 (20.52)
9.33 ⫾ 0.45 (37.32)
0.51 ⫾ 0.02 (2.04)
0.3 ⫾ 0.1 (1.2)
1.42 ⫾ 0.02 (6.44)
1.23 ⫾ 0.15 (5.58)
1.1 ⫾ 0.3 (4.9)
1.15 ⫾ 0.05 (5.22)
2-Position 5-NIsb
0.52 ⫾ 0.02 (2.6)
0.31 ⫾ 0.08 (1.55)
0.32 ⫾ 0.1 (1.6)
0.37 ⫾ 0.08 (1.85)
0.63 ⫾ 0.1 (1.56)
0.36 ⫾ 0.13 (0.89)
0.42 ⫾ 0.08 (1.04)
0.5 ⫾ 0.05 (1.24)
a Side chain at position 1 of the imidazole ring of 5-NI.
b Side chain at position 2 of the imidazole ring of 5-NI.
c NS, not susceptible to drug concentrations of ⱕ100 g/ml.
d FDA-approved antimicrobial agent.
e Veterinary antiparasitic agent.
f Experimental antiparasitic agent effective against Trichomonas and Giardia (66).
respond to NTZ, as in the case of Cryptosporidium parvum
parasites are not known, although they have been suggested to
infections. C. parvum infections do not respond well to Mz
act on protozoan cell membranes (62). The activity of QC
(19), and NTZ is the treatment of choice, with in vitro IC s of
against Blastocystis has been reported previously (16, 68), but
⬍10 g/ml (60), similar to the IC s of the drug against both
the current study is the first to report the potential usefulness
Mzr and Mzs isolates of Blastocystis in this study. Recent in
of MQ as an anti-Blastocystis drug.
vitro (68) and clinical data (55) also suggest the usefulness of
EM is an effective antiamoebic agent with unpleasant side
the drug in Blastocystis infections.
effects. It targets ribosomes and limits protein synthesis (43).
Another alternative to treat Mzr Blastocystis isolates is FUR,
The in vitro activity of EM against Blastocystis has been eval-
which was equally effective against all isolates in this study.
uated in two previous studies. While both studies suggested its
FUR is a nitrofuran commonly used to treat giardiasis (49). It
effectiveness against Blastocystis, Zierdt et al. reported strain-
is activated inside the cell by NADH oxidase and generates
to-strain variation in the susceptibility of the parasite to the
toxic products that interfere with DNA processes in the para-
drug (75). The multidrug resistance (MDR) phenotype of En-
site (9). The IC s of FUR against both Mzr and Mzs isolates
tamoeba histolytica exhibits resistance to a wide range of drugs,
of Blastocystis were found to be similar to that against Giardia
including EM, while responding to Mz (43), but no such MDR
(2 M) (5).
phenotypes have been reported in Blastocystis spp. Our study
The prophylactic antimalarial MQ and a closely related
describes the existence of EM resistance in Mzs isolates of
drug, QC, were also found to be more effective against Mzr
Blastocystis, suggesting that MDR phenotypes might be present
subtype 7 isolates than Mzs subtype 4 isolates. These findings
in the parasite. Clinically, however, EM has limited use be-
are surprising because in Giardia, cross-resistance against QC
cause of its severe side effects (32, 56).
has been observed between Mzr (8) and Tzr (63) strains, sug-
TMP and SMZ are often prescribed in combination at a
gesting a different mode of action of the drug in Blastocystis.
1:5 ratio as an alternative to Mz in Blastocystis infections.
The exact mechanisms of action of these drugs against luminal
Clinical studies suggest that this drug combination success-
TABLE 6. IC-50 values of anti-protozoal agents effective against Blastocystis isolates using the resazurin assay
关g/ml (M)兴
Subtype 7 (Mzr) isolates
Subtype 4 (Mzs) isolates
0.62 ⫾ 0.07 (2.01)
1.14 ⫾ 0.49 (3.7)
4.15 ⫾ 0.41 (13.48)
0.65 ⫾ 0.05 (2.88)
1.06 ⫾ 0.4 (4.7)
0.49 ⫾ 0.01 (2.17)
0.475 ⫾ 0.05 (2.1)
1.49 ⫾ 0.83 (3.93)
1.85 ⫾ 0.88 (4.88)
4.7 ⫾ 0.35 (12.4)
5.1 ⫾ 0.58 (13.46)
1.9 ⫾ 0.2 (4.75)
5.1 ⫾ 0.47 (12.75)
4.9 ⫾ 0.53 (12.25)
5.1 ⫾ 1.1 (15.7)
4.3 ⫾ 2.4 (13.24)
3.2 ⫾ 0.52 (9.8)
5.4 ⫾ 1.4 (16.63)
1.03 ⫾ 0.4 (2.13)
1.32 ⫾ 0.9 (2.73)
TMP:SMZ 1:2b
TMP:SMZ 1:5b
0.34 ⫾ 0.05 (1.83)
0.2 ⫾ 0.03 (1.08)
0.33 ⫾ 0.06 (1.78)
0.26 ⫾ 0.02 (1.4)
a N/S, not susceptible to ⱕ100-g/ml concentration of the drug.
b FDA-approved antimicrobial agent.
c Antiparasitic agent with adverse side effects; not currently used in clinical practice.
d Carcinogenic cysteine protease inhibitor; not clinically useful.
MIRZA ET AL.
ANTIMICROB. AGENTS CHEMOTHER.
fully eradicates Blastocystis infections in 95% to 100% of
of our findings across different life cycle stages of the par-
cases (53, 54). There are no reports of the effectiveness of a
asite. Despite this limitation, this is the first study suggesting
1:2 combination against Blastocystis. Our findings suggest
subtype-dependent variation in the parasite response to che-
the superiority of a 1:2 combination over a 1:5 combination
with no subtype-dependent variation in susceptibility. We
In conclusion, this study describes two cost-effective as-
suggest that the 1:2 combination is likely to be more effec-
says for high-throughput antimicrobial susceptibility analy-
tive than the 1:5 combination in treatment of clinical infec-
sis of Blastocystis. Using one of these assays, we demon-
tions of Blastocystis.
strated for the first time subtype-dependent variations in the
Cysteine proteases play an important role in the cell cycle
susceptibility of Blastocystis to six different antiprotozoal
and pathophysiology of protozoan parasites. Blastocystis cys-
agents. We identified 4 new potential therapeutic options
teine proteases have been reported to cleave human secretory
against Blastocystis, namely, MQ, TMP-SMZ (1:2), Oz, and
IgAs (58) and to induce upregulation of proinflammatory cy-
FUR. Furthermore, we confirmed the antiprotozoal activi-
tokines (48). A prosurvival role of legumain, a cysteine pro-
ties of 10 compounds already reported to be effective against
tease, has also been reported recently for Blastocystis (71).
Blastocystis. We also demonstrated in vitro Mz and EM
Accumulating data in recent years suggest the therapeutic po-
resistance in Blastocystis. By assessing the susceptibility of
tential of protease inhibitors in parasitic infections (1, 42).
the parasite to different 5-NIs, we also demonstrated that
Several cysteine protease inhibitors are being investigated as
5-NI resistance could be overcome in Blastocystis with more
potential chemotherapeutic agents against parasites as diverse
effective 5-NI compounds. Based on our findings, there is
as Plasmodium (42, 44, 50), trypanosomes (18), and schisto-
clearly a need to reevaluate currently established treatment
somes (69). In this study, we found all four isolates to be highly
regimens for Blastocystis infections.
susceptible to IA, a cysteine protease inhibitor, irrespective oftheir susceptibility to Mz. These findings suggest a potential
role of cysteine protease inhibitors as a therapeutic option for
This work was supported by a generous grant from the National
Blastocystis isolates resistant to conventional antiprotozoal
Medical Research Council (NMRC/1071/2006). H.M. and J.D.W.T.
are graduate students supported by National University of Singapore
PAR is a broad-spectrum aminoglycoside (13). Although
(NUS) research scholarships. This work was also supported in part byU01 Cooperative Research Agreement AI75527 from the National
clinical studies suggest its effectiveness in the treatment of
Institutes of Health. The study was facilitated by the commissioning of
Blastocystis infections (3, 45, 67), in vitro data are equivocal (68,
synthesis of C-17 by the NIH from the Southern Research Institute.
72). In this study, PAR was found to be ineffective against the
We are grateful to Martin Lear and Oliver Simon for providing the
isolates of both subtypes tested. The high clinical efficacy of the
chemical structures of 5-nitroimidazoles.
drug against Blastocystis could be due to its broad-spectrum
antibiotic activity (13). Although predominantly used for par-
1. Abdulla, M. H., K. C. Lim, M. Sajid, J. H. McKerrow, and C. R. Caffrey.
asitic infections, PAR is also bactericidal (15). It might act by
2007. Schistosomiasis mansoni: novel chemotherapy using a cysteine protease
destruction of the gut bacterial flora essential for Blastocystis
inhibitor. PLoS Med. 4:e14.
2. Adagu, I. S., D. Nolder, D. C. Warhurst, and J. F. Rossignol. 2002. In vitro
survival (57).
activity of nitazoxanide and related compounds against isolates of Giardia
All four isolates tested were found to be nonsensitive to
intestinalis, Entamoeba histolytica and Trichomonas vaginalis. J. Antimicrob.
several other broad-spectrum antibiotics, PAR, PYR, CQ,
3. Armentia, A., et al. 1993. Urticaria by Blastocystis hominis. Successful treat-
DOX, and AMP. This feature could be exploited for the
ment with paromomycin. Allergol. Immunopathol. 21:149–151.
isolation and axenization of Blastocystis from clinical sam-
4. Bassily, S., Z. Farid, N. A. el-Masry, and E. M. Mikhail. 1987. Treatment of
intestinal E. histolytica and G. lamblia with metronidazole, tinidazole and
ornidazole: a comparative study. J. Trop. Med. Hyg. 90:9–12.
Clinical (54) and animal infection (24) studies, as well as in
´, E., R. A. da Luz, M. Vermeersch, P. Cos, and L. Maes. 2007. A new
vitro data (36), suggest a subtype-dependent variation in the
quantitative in vitro microculture method for Giardia duodenalis trophozo-
ites. J. Microbiol. Methods 71:101–106.
pathobiology of Blastocystis. Although strain-to-strain variation
6. Boorom, K. F., et al. 2008. Oh my aching gut: irritable bowel syndrome,
in parasite susceptibilities to drugs has been reported previ-
Blastocystis, and asymptomatic infection. Parasit. Vectors 1:40.
ously, subtype-dependent variation in parasite responses to
7. Boreham, P. F. L., N. C. Smith, and R. W. Shepherd. 1988. Drug resistance
and treatment of giardiasis, p. 3–7. In P. M. Wallis and B. R. Hammond
chemotherapeutic agents has not been described before. To
(ed.), Advances in Giardia research. University of Calgary Press, Calgary,
the best of our knowledge, this is the first study of its kind
Alberta, Canada.
suggesting a variation in parasite susceptibilities to six common
8. Brasseur, P., and L. Favennec. 1995. Two cases of giardiasis unsuccessfully
treated by albendazole. Parasite 2:422.
antiparasitic agents between isolates of two subtypes known to
9. Brown, D. M., J. A. Upcroft, and P. Upcroft. 1996. A H O-producing NADH
infect humans (54). It will be interesting to conduct a more
oxidase from the protozoan parasite Giardia duodenalis. Eur. J. Biochem.
241:155–161.
extensive evaluation analyzing variability in the drug responses
10. Chintana, T., P. Sucharit, V. Mahakittikun, C. Siripanth, and W. Suphad-
of different isolates across all 11 subtypes of the parasite.
tanaphongs. 1986. In vitro studies on the sensitivity of local Entamoeba
Although the vacuolar form is the most commonly re-
histolytica to anti-amoebic drugs. Southeast Asian J. Trop. Med. Public
Health 17:591–594.
ported form of the parasite, Blastocystis is also known to
11. Chen, X. Q., et al. 1997. A survey of Blastocystis sp. in rodents. Lab. Anim.
exist in amoeboid, granular, and cyst forms. Blastocystis cysts
have been reported to be Mzr, suggesting that different
12. Crowell, A. L., K. A. Sanders-Lewis, and W. E. Secor. 2003. In vitro metro-
nidazole and tinidazole activities against metronidazole-resistant strains of
forms might respond differently to drug pressure (73). Since
Trichomonas vaginalis. Antimicrob. Agents Chemother. 47:1407–1409.
there are no standardized methods available for maintaining
13. Davidson, R. N., M. den Boer, and K. Ritmeijer. 2009. Paromomycin. Trans.
R. Soc. Trop. Med. Hyg. 103:653–660.
axenic cultures of other Blastocystis forms, only vacuolar
14. De Logu, A., et al. 2003. Comparison of the susceptibility testing of
forms were evaluated in this study, limiting the application
clinical isolates of Mycobacterium tuberculosis by the XTT colorimetric
BLASTOCYSTIS METRONIDAZOLE RESISTANCE AND SUSCEPTIBILITY
method and the NCCLS standards method. Int. J. Antimicrob. Agents
42. Olson, J. E., G. K. Lee, A. Semenov, and P. J. Rosenthal. 1999. Antimalarial
effects in mice of orally administered peptidyl cysteine protease inhibitors.
15. Donald, P. R., et al. 2000. Early bactericidal activity of paromomycin (amino-
Bioorg. Med. Chem. 7:633–668.
sidine) in patients with smear-positive pulmonary tuberculosis. Antimicrob.
43. Orozco, E., L. A. Marchat, C. Go
´mez, C. Lo
´pez-Camarillo, and D. G. Pe
Agents Chemother. 44:3285–3287.
2009. Drug resistance mechanisms in Entamoeba histolytica, Giardia lamblia,
16. Dunn, L. A., and P. F. Boreham. 1991. The in-vitro activity of drugs against
Trichomonas vaginalis, and opportunistic anaerobic protozoa, p. 549–559. In
Blastocystis hominis. J. Antimicrob. Chemother. 27:507–516.
G. A. Jacoby, R. Elston, P. R. Bonneau, and I. M. Douglas (ed.), Antimi-
17. Dunn, L. A., et al. 2010. A new-generation 5-nitroimidazole can induce
crobial drug resistance; mechanisms of drug resistance, vol. 1. Humana
highly metronidazole-resistant Giardia lamblia in vitro. Int. J. Antimicrob.
Press, Totowa, NJ.
44. Parikh, S., et al. 2005. Antimalarial activity of human immunodeficiency
18. Engel, J. C., P. S. Doyle, and J. H. McKerrow. 1999. Trypanocidal effect of
virus type 1 protease inhibitors. Antimicrob. Agents Chemother. 49:2983–
cysteine protease inhibitors in vitro and in vivo in experimental Chagas
disease. Medicina (Buenos Aires) 59:171–175.
45. Pasqui, A. L., et al. 2004. Chronic urticaria and Blastocystis hominis infection:
19. Gargala, G. 2008. Drug treatment and novel drug target against Cryptospo-
a case report. Eur. Rev. Med. Pharmacol. Sci. 8:117–120.
ridium. Parasite 15:275–281.
46. Perrot, S., H. Dutertre-Catella, C. Martin, J. M. Warnet, and P. Rat. 2003.
20. Glass, R. H., et al. 1991. The resazurin reduction test provides an assessment
A new nondestructive cytometric assay based on resazurin metabolism and
of sperm activity. Fertil. Steril. 56:743–746.
an organ culture model for the assessment of corneal viability. Cytometry A
¨ven, A. 2003. Amebiasis in the newborn. Indian J. Pediatr. 70:437–438.
47. Petrenko, Y. A., N. A. Gorokhova, E. N. Tkachova, and A. Y. Petrenko. 2005.
Harder, A., G. Greif, and A. Haberkorn. 2001. Chemotherapeutic ap-
The reduction of Alamar Blue by peripheral blood lymphocytes and isolated
proaches to protozoa: Giardia, Trichomonas and Entamoeba—current level
mitochondria. Ukr. Biokhim. Zh. 77:100–105.
of knowledge and outlook. Parasitol. Res. 87:785–786.
48. Puthia, M. K., J. Lu, and K. S. Tan. 2008. Blastocystis ratti contains cysteine
23. Haresh, K., K. Suresh, A. Khairul Anus, and S. Saminathan. 1999. Isolate
proteases that mediate interleukin-8 response from human epithelial cells in
resistance of Blastocystis hominis to metronidazole. Trop. Med. Int. Health
an NF-kappaB-dependent manner. Eukaryot. Cell 7:435–443.
49. Quiros-Buelna, E. 1989. Furazolidone and metronidazole for treatment of
24. Hussein, E. M., A. M. Hussein, M. M. Eida, and M. M. Atwa. 2008. Patho-
giardiasis in children. Scand. J. Gastroenterol. Suppl. 169:65–69.
physiological variability of different genotypes of human Blastocystis hominis
50. Rosenthal, P. J., G. K. Lee, and R. E. Smith. 1993. Inhibition of a Plasmo-
Egyptian isolates in experimentally infected rats. Parasitol. Res. 102:853–
dium vinckei cysteine proteinase cures murine malaria. J. Clin. Invest. 91:
25. Katsarou-Katsari, A., et al. 2008. Acute urticaria associated with amoeboid
51. Rossignol, J. F., S. M. Kabil, M. Said, H. Samir, and A. M. Younis. 2005.
forms of Blastocystis sp. subtype 3. Acta Derm. Venereol. 88:80–81.
Effects of nitazoxanide in persistent diarrhea and enteritis associated with
26. Krook, A., B. Lindstro
¨m, J. Kjellander, G. Ja
¨rnerot, and L. Bodin. 1981.
Blastocystis hominis. Clin. Gastroenterol. Hepatol. 3:987–991.
Relation between concentrations of metronidazole and Bacteroides spp in
52. Stensvold, C. R., et al. 2007. Terminology for Blastocystis subtypes—a con-
faeces of patients with Crohn's disease and healthy individuals. J. Clin.
sensus. Trends Parasitol. 23:93–96.
53. Stensvold, C. R., M. C. Arendrup, H. V. Nielsen, A. Bada, and S. Thorsen.
27. Kurniawan, A., et al. 2009. Intestinal parasitic infections in HIV/AIDS pa-
2008. Symptomatic infection with Blastocystis sp. subtype 8 successfully
tients presenting with diarrhoea in Jakarta, Indonesia. Trans. R. Soc. Trop.
treated with trimethoprim-sulfamethoxazole. Ann. Trop. Med. Parasitol.
Med. Hyg. 103:892–898.
28. Leitsch, D., D. Kolarich, and M. Duche
ˆne. 2010. The flavin inhibitor
54. Stensvold, C. R., et al. 2009. Blastocystis: unravelling potential risk factors
diphenyleneiodonium renders Trichomonas vaginalis resistant to metro-
and clinical significance of a common but neglected parasite. Epidemiol.
nidazole, inhibits thioredoxin reductase and flavin reductase, and shuts
off hydrogenosomal enzymatic pathways. Mol. Biochem. Parasitol. 171:
55. Stensvold, C. R., H. V. Smith, R. Nagel, K. E. Olsen, and R. J. Traub. 2010.
Eradication of Blastocystis carriage with antimicrobials: reality or delusion?
29. Leonard, B., et al. 2008. Inter- and intra-assay reproducibility of microplate
J. Clin. Gastroenterol. 44:85–90.
Alamar Blue assay results for isoniazid, rifampicin, ethambutol, streptomy-
56. Sugie, H., R. Russin, and M. A. Verity. 1984. Emetine myopathy: two case
cin, ciprofloxacin, and capreomycin drug susceptibility testing of Mycobac-
reports with pathobiochemical analysis. 7:54–59.
terium tuberculosis. J. Clin. Microbiol. 46:3526–3529.
57. Tan, K. S. 2008. New insights on classification, identification, and clinical
30. Li, Q., C. Maddox, L. Rasmussen, J. V. Hobarth, and L. E. White. 2009.
relevance of Blastocystis spp. Clin. Microbiol. Rev. 21:639–665.
Assay development and high-throughput antiviral drug screening against
58. Tan, K. S., H. Mirza, D. W. T. Joshua, B. Wu, and P. A. MacAry. 2010.
Bluetongue virus. Antiviral Res. 83:267–273.
Current views on the clinical relevance of Blastocystis spp. Curr. Infect. Dis.
¨fmark, S., C. Edlund, and C. E. Nord. 2010. Metronidazole is still the drug
of choice for treatment of anaerobic infections. Clin. Infect. Dis. 50(Suppl.
59. Taçsova, Y., B. Sahin, S. Koltaçs, and S. Paydaçs. 2000. Clinical significance
and frequency of Blastocystis hominis in Turkish patients with hematological
32. Marino, A., R. Costa, and G. De Natale. 1990. Cardiotoxicity of emetine.
malignancy. Acta Med. Okayama 54:133–136.
Clin. Ter. 133:131–143.
60. Theodos, C. M., J. K. Griffiths, J. D'Onfro, A. Fairfield, and S. Tzipori. 1998.
33. Markell, E. K. 1995. Is there any reason to continue treating Blastocystis
Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and
infections? Clin. Infect. Dis. 21:104–105.
in animal models. Antimicrob. Agents Chemother. 42:1959–1965.
34. McBride, J., P. R. Ingram, F. L. Henriquez, and C. W. Roberts. 2005.
61. Upcroft, J. A., and P. Upcroft. 1993. Drug resistance and Giardia. Parasitol.
Development of colorimetric microtiter plate assay for assessment of anti-
microbials against Acanthamoeba. J. Clin. Microbiol. 43:629–634.
62. Upcroft, J. A., R. Mitchell, N. Chen, and P. Upcroft. 1996. Albendazole
35. Mehraj, V., J. Hatcher, S. Akhtar, G. Rafique, and M. A. Beg. 2008. Preva-
resistance in Giardia is correlated with cytoskeletal changes but not with
lence and factors associated with intestinal parasitic infection among chil-
a mutation at amino acid 200 in beta-tubulin. Microb. Drug Resist.
dren in an urban slum of Karachi. PLoS One 3:e3680.
36. Mirza, H., and K. S. Tan. 2009. Blastocystis exhibits inter- and intra-subtype
63. Upcroft, J. A., R. W. Campbell, and P. Upcroft. 1996. Quinacrine resistant
variation in cysteine protease activity. Parasitol. Res. 104:355–361.
Giardia duodenalis. Parasitology 112:309–313.
37. Moghaddam, D. D., E. Ghadirian, and M. Azmi. 2005. Blastocystis hominis
64. Upcroft, J. A., R. W. Campbell, K. Benakli, P. Upcroft, and P. Vanelle. 1999.
and the evaluation of efficacy of metronidazole and trimethoprim/sulfame-
Efficacy of 5-nitroimidazoles against metronidazole-susceptible and resistant
thoxazole. Parasitol. Res. 96:273–275.
Giardia, Trichomonas and Entamoeba spp. Antimicrob. Agents Chemother.
38. Muzaffar, J., K. Madan, M. P. Sharma, and P. Kar. 2006. Randomized,
single-blind, placebo-controlled multicenter trial to compare the efficacy and
65. Upcroft, P., and J. A. Upcroft. 2001. Drug targets and mechanisms of resis-
safety of metronidazole and satranidazole in patients with amebic liver ab-
tance in anaerobic protozoa. Clin. Microbiol. Rev. 14:150–164.
scess. Dig. Dis. Sci. 51:2270–2273.
66. Upcroft, J. A., et al. 2006. 5-Nitroimidazole drugs effective against metron-
¨l, C., et al. 2005. Molecular phylogenies of Blastocystis isolates from
idazole-resistant Trichomonas vaginalis and Giardia duodenalis. Antimicrob.
different hosts: implications for genetic diversity, identification of species,
Agents Chemother. 50:344–347.
and zoonosis. J. Clin. Microbiol. 43:348–355.
67. Valsecchi, R., P. Leghissa, and V. Greco. 2004. Cutaneous lesions in Blasto-
40. Noureldin M. S., A. A. Shaltout, E. M. El Hamshary, and M. E. Ali. 1999.
cystis hominis infection. Acta Derm. Venereol. 84:322–323.
Opportunistic intestinal protozoal infections in immunocompromised chil-
68. Vdovenko, A. A., and J. E. Williams. 2000. Blastocystis hominis: neutral red
dren. J. Egypt. Soc. Parasitol. 29:951–961.
supravital staining and its application to in vitro drug sensitivity testing.
41. O'Brien, J., I. Wilson, T. Orton, and F. Pognan. 2000. Investigation of the
Parasitol. Res. 86:573–581.
Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian
69. Wasilewski, M. M., K. C. Lim, J. Phillips, and J. H. McKerrow. 1996.
cell cytotoxicity. Eur. J. Biochem. 267:5421–5426.
Cysteine protease inhibitors block schistosome hemoglobin degradation in
MIRZA ET AL.
ANTIMICROB. AGENTS CHEMOTHER.
vitro and decrease worm burden and egg production in vivo. Mol. Biochem.
susceptibility of Blastocystis hominis isolated from patients with irritable
bowel syndrome. Br. J. Biomed. Sci. 61:75–77.
70. Wawrzyniak, I., et al. 2008. Complete circular DNA in the mitochondria-like
73. Zaman, V., and M. Zaki. 1996. Resistance of Blastocystis hominis cysts to
organelles of Blastocystis hominis. Int. J. Parasitol. 38:1377–1382.
metronidazole. Trop. Med. Int. Health 1:677–678.
71. Wu, B., J. Yin, C. Texier, M. Roussel, and K. S. Tan. 2010. Blastocystis
74. Zhang, J. H., T. D. Chung, and K. R. Oldenburg. 1999. A simple statistical
legumain is localized on the cell surface, and specific inhibition of its
parameter for use in evaluation and validation of high throughput screening
activity implicates a pro-survival role for the enzyme. J. Biol. Chem.
assays. J. Biomol. Screen. 4:67–73.
75. Zierdt, C. H., J. C. Swan, and J. Hosseini. 1983. In vitro response of Blas-
72. Yakoob, J., W. Jafri, N. Jafri, M. Islam, and M. A. Beg. 2004. In vitro
tocystis hominis to antiprotozoal drugs. J. Protozool. 30:332–334.
Source: http://pptu.lefora.com/attach/view/ma/538c5553af7c7e50946605d8e9aab306e7fb600c.pdf
Universität Ferrara Das Forschungszentrum für Hygienekontrolle in hochsterilen führte eine einjährige Studie (2010 - 2011) durch zum Thema "TESTUNG VON BIOSTABILISATIONSTECHNIKEN BEI BENUTZUNG VON PROBIOTISCHEN PRODUKTEN VON CHRISAL FÜR DIE REINIGUNG UND DESINFEKTION VON KRANKENSTATIONEN" Medical Sciences Group JE Übersetzungskopie TEST VON BIOSTABILISATIONSTECHNIKEN FÜR DIE REINIGUNG UND DESINFEKTION VON KRANKENSTATIONEN
The World Anti-Doping Code PROHIBITED LIST The official text of the Prohibited List shall be maintained by WADA and shall be published in English and French. In the event of any conflict between the English and French versions, the English version shall prevail. This List shall come into effect on 1 January 2009 The Prohibited List 2009 20 September 2008






