Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Microsoft word - priclinbical_14.7.1

Vol 2 Issue 2 Jul –Dec 2011 76-80. Print ISSN – 2229 7502
Bioavailability studies of Pioglitazone with Antacid –
An Invivo Evaluation in Human Volunteers
S.Thirumurugu1*, V.Parthasarathy1, D.C.Arumainayagam2, and
1Department of Pharmacy, Annamalai University, Annamalai nagar, Tamilnadu,
2Department of Medicine, Rajah Muthiah Medical College & Hospital, Annamalai University,
Annamalai nagar, Tamilnadu, India- 608002.
ABSTRACT:
The intestinal absorption of oral-anti diabetic drugs in the treatment of type-II diabetes mellitus is
altered when they are concomitantly administered with antacids, and other antinuclear drugs, antibiotics
and others. A randomized cross over study in two phases and a washout period of 4 weeks was carried out
to evaluate the bioavailability of anti diabetic drug pioglitazone when used with Digene Gel (Magnesium
Hydroxide, Simethicone, Aluminium Hydroxide), a drug for management of problems in gastrointestinal
tract.The study has been approved by the institutional ethical committee of Raja muthiah medical college
and Hospital, Annamalai University. In the present study 10 diabetic patients received Digene Gel (10 ml)
for 5 days. After overnight fasting on 6th day a single dose of pioglitazone (30mg) was given. The blood
samples following the intake were taken at different time intervals of 1, 2, 3, 4, 5, 7, 9 and 12 hours. The
plasma samples (100µl) were injected into HPLC system after separation. The mobile phase comprised of
Methanol: acetonitrile: mixed phosphate buffer (pH 2.6) at a ratio of (40:12:48). Analyses were run using
C18 column (4.6 mm × 250 mm, 100 A) Luna. PHENOMINEX, USA was set at 30 C at a flow rate of
1.2 ml.min-1 with UV detector operating at a detection wave length of 269nm in HPLC and the
pharmacokinetic parameters were calculated by using the software Kinetica (Version 4.4.1Innaphase,
USA). The study reveals that the absorption of pioglitazone was delayed when it was concomitantly
administered with Digene Gel.
Keywords: Bioavailability, Anti diabetic drugs, Pioglitazone, Digene Gel, Pharmacokinetics, Concomitant
administration, Drug interaction.
The term diabetes mellitus describes a
suffering from diabetes and expected to rise 69.9
metabolic disorder of multiple etiologies
million by 2025 [21]. Chronic elevation of blood
characterized by chronic hyperglycemia with
glucose levels leads to many co-existing
disturbances of carbohydrate, fat and protein
complications like diabetic retinopathy, diabetic
metabolism resulting from defects in insulin
neuropathy, peptic ulcer, diabetic foot ulcer.
secretion, insulin action, or both [1]. Currently
Drug therapy in Type II diabetes becomes more
diabetic mellitus is a great threat to the world
complex as many individuals are on multiple
community with more than 100 million persons
drug therapy and administer many drugs during
suffering from diabetes. The prevalence and
the same period of time to treat secondary
incidence of diabetes is increasing in most
diabetic complications [3, 11, 13, and 16]. A
populations, being more prominent in developing
closer monitoring and supervision of drug
countries as follows, in USA more than 16
therapy is required so that drug related problems
million, in republic of China more than 14
can be prevented or detected at an early stage.
million, in Africa more than 20 million. India
An increasing number of drug related problems
leads the world largest number diabetic subjects
are caused by drug inter actions. [3, 12-16].
and is being termed the "diabetes capital of the
Currently clinicians come across the problem of
world ". With 40.9 million people currently
erratic absorption of oral anti diabetic drugs
Corresponding Author:- S.Thirumurugu Email: [email protected]
International Journal of Preclinical and Pharmaceutical Research
Vol 2 Issue 2 Jul –Dec 2011 76-80. Print ISSN – 2229 7502
when administered with other drugs prescribed for co-
for 5 days. After an overnight fast on day 6 a single dose of
existing diseases. Due to this, bioavailability of oral anti
Pioglitazone (PIOGLIT 30mg) was administered orally with
diabetic drugs is altered. Pioglitazone is a thiozolidinedione
150ml of water [2]. Volunteers received a standard meal 3h
compound used in the treatment type II diabetes. It is an
after dosing. Volunteers received light standard meals 7th h
insulin sensitizer that acts as agonist of the preoxsome
and 11th h after dosing.
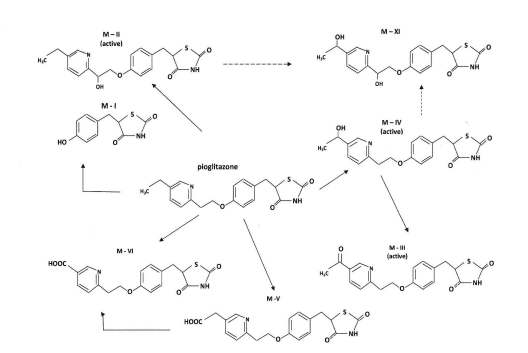
(PPAR - γ) [5, 8]. The main active metabolites are M – IV
(a hydroxyl derivatives) and M – III (a ketone derivatives);
2.4. PHARMACOKINETICS OF PIOGLITAZONE
the latter being formed from M –IV (Figure 1) [9]. Another
metabolite M – II also has pharmacological activity, but it
administered of Pioglitozone by orally at 1, 2, 3, 4, 5, 7, 9,
concentrations are low and it does not significantly
12 later through median capital vein, and collected in EDTA
contribute to the total amount of active species. The
treated vacationers tubes. Blood samples were immediately
circulating concentrations of the metabolites M - IV and M -
centrifuged at 5000rpm for 10 min to obtain plasma and
III are equal to or greater than those of the parent
stored at -20°C until analysis. Pioglitazone concentration
Pioglitazone and they have considerably longer half-life
was determined by addition of 100µl acetonitrile with 100µl
than Pioglitazone [2]. In vitro studies suggested that
of plasma to deprotinise the proteins. The mixture was
pioglitazone is metabolized by several cytochrome P450
vortex mixed for 5 min after which it was centrifuged at
(CYP) enzymes but mainly by CYP2C8 and CYP3A4 [5,
10000rpm for 10 min.100µl of supernatant liquid was
8]. The study will ensure that, if it shows no effect on
injected into the HPLC system for analysis [5.9] .The UV
pharmacokinetics of pioglitazone, it can be co-administered
detector was set at 269 nm for the present analysis. C18
for the better management of problems occurs in
column (4.6 mm × 250 mm, 100 A) Luna. PHENOMINEX,
gastrointestinal tract as co-existing diseases of type II
USA was set at 30 C. The mobile phase comprised of
Methanol: acetonitrile: mixed phosphate buffer (pH 2.6) at a
ratio of (40:12:48) at a flow rate of 1.2 ml.min-1[3].
Materials and Methods
2.1. Materials
2.5. Pharmacokinetic analysis
The standard drug pioglitazone was received as gift
Peak plasma concentration (Cmax), Time to Cmax (tmax), AUC
sample from Paris Dakner Microspheres (P) Ltd, Chennai,
from 0 to 12h (AUC0-12), t½. All the pharmacokinetic and
India. The test drugs were Pioglitazone 30 mg tablets
statistical data were calculated by using the software
(PIOGLIT), Cipla, and Digene Gel 10 ml, Abbott India
Kinetic, (Version 4.4.1, Innaphase, USA).
Limited. All other chemicals were used of analytical grade.
Freshly double distilled deionised water, filtered through
Results and discussion
0.2µm nylon filter (47 mm) in Millipore unit (USA), was
Currently the management of type II diabetes
used throughout the experiments. The drug analysis was
becoming more complex since the recommended global
carried out using HPLC system (Shimaddzu LC -10 AD)
approach of combination drug therapy has increased the risk
having gradient pump (LC 10 AD UP) Rheodyne injector
of pharmacokinetics interactions in patients with diabetes
port, and UV/Vis detector (SPD 10A VP). The data
[21]. The activity of one drug could alter the
interpretation was done with Shimadzu system controller
pharmacokinetics of another drug and it may be due to risk
(SCL – 10 AVP).
of the enzyme inverse reaction upon the plasma levels of
concomitantly administered drugs [22]. Pioglitazone is
2.2 Subjects
rapidly absorbed in GIT, its oral bioavailability exceeds
Ten diabetic patients (men age range from (21-30)
80%, and it is extensively metabolized by hydroxylation and
weight range (57-79kg) participated in the study after
oxidation to form active and inactive metabolites in the liver
obtaining a written informed consent and were ascertained
[23]. In vivo studies suggest that the drug is metabolized by
to be healthy by medical history Clinical examination and
several cytochrome P450 (CYP) enzymes, but mainly by
routine laboratory tests. No one even on medication. Study
CYP2C8 and CYP3A4 [22]. The main active metabolites of
protocol was approved by ethics committee for studies in
Pioglitazone are M-IV (a hydroxyl derivative) and M-III (a
healthy subjects and primary care of the Rajah Muthiah
ketone derivative). The M-III being formed from M-IV
Medical College and hospital, Chidambaram.
(Figure 1). The circulating concentrations of the metabolites
M-IV and M-III are equal to or greater than those of the
2.3. Study design
parent Pioglitazone and they have considerably longer half-
A randomized cross over study with two phases
lives than Pioglitazone [2] .The Digene Gel (Each 10 ml
and a washout period of 4weeks was carried out. Volunteers
contains Magnesium hydroxide IP 185 mg, Simethicone IP
took 10ml of Digene Gel orally once daily at 20.00 h (8 am)
50 mg, Dried Aluminium hydroxide Gel IP 830 mg) .
International Journal of Preclinical and Pharmaceutical Research
Vol 2 Issue 2 Jul –Dec 2011 76-80. Print ISSN – 2229 7502
The effect of Digene Gel on the pharmacokinetics of
3). The study has been carried out with five volunteers.
Pioglitazone was assessed using a randomized, two cross
Antacid delays the absorption of Pioglitazone and alters tmax.
over study with wash out period of 4 weeks. Volunteers
There is no interaction in metabolism of Pioglitazone.
took 1o ml of Digene Gel orally once daily at 20.00 hrs
Physiologic factors like pH of GIT, Gastric emptying time,
(8pm) for 5 days. After an overnight fasting on 6 day at 9.00
intestinal transit time, and body posture, emotional status
am single dose of 30mg Pioglitazone (PIOGLIT) was
etc., to be discussed and conformed. Hence the absorption
administered orally with 150ml of water. The blood samples
of Pioglitazone delayed by the concomitant administration
were drawn before and after administration of Pioglitazone.
of Digene Gel and may produce hyperglycemia in the
The separated plasma was analyzed in HPLC system. The
systemic circulation. It may lead to the increasement of
data obtained from the analysis shows that of Digene Gel
other complications like adverse reaction and toxicity of
delays the absorption of Pioglitazone due to that increases
the Cmax, AUC and t½ of Pioglitazone after 2hrs (Figure 2 &
The metabolism of pioglitazone in humans [9]. The metabolite M-XI is a previously unrecognized metabolite. The image
Courtesy: Tiina Jaakkola, et al., Department of Clinical Pharmacology, University of Helsinki and Helsinki University Central
Hospital, Helsinki, Finland
International Journal of Preclinical and Pharmaceutical Research
Vol 2 Issue 2 Jul –Dec 2011 76-80. Print ISSN – 2229 7502
Plasma concentration time curve of pioglitazone alone
Plasma concentration time curve of pioglitazone
concomitantly administred with Antacid
Plasma Concentration – time curve of Pioglitazone after its
Plasma Concentration time curve of Pioglitazone after its
oral administration (30mg) with Digene Gel (10ml) in pre-
oral administration (30mg) in human volunteers. The
treated human volunteers. The experiments were carried out
experiments were carried out by using by the plasma
by using by the plasma samples of diabetic patients. Each
samples of diabetic patients. Each point represents the mean
point represents the mean ± standard deviation (n=10)
± standard deviation (n=10)
Table. 1 Pharmacokinetic parameters of Pioglitazone inAntacid pretreated human volunteers
Pharmacokinetics parameter
Pioglitazone alone
Pioglitazone with Antacid
AUC0-t (ng *h/mL)
382.0.13±18.483
Volunteers took 30mg Pioglitazone (PIOGLIT) orally once daily for 5 days. After an overnight fast on the day 6 a single dose of 10ml of Digene Gel was administered orally there after pharmacokinetics of Pioglitazone was carried out. Tmax - Time to Reach; Cmax - Peak Plasma Concentration; AUC - Area Under the Plasma Concentration Curve; t1/2 - Half Life.
CONCLUSION
activity by CYP3A4 mediated inhibition. This finding
The present study was carried out with an attempt
indicates that Digene Gel delays the absorption of
to investigate any possible interaction occurs between
pioglitazone during absorption phase. There is no inter
Digene Gel and Pioglitazone in the treatment of Type II
individual variation in AUC of Pioglitazone from 0–12 hrs
diabetes with the problems of gastrointestinal tract. The
as compared to standard phase (pioglitazone alone). The
increase in tmax and t1/2 of Pioglitazone was found to be
chromatograms. Digene Gel was found to delay the
higher when it is co-administered administered with Digene
absorption of pioglitazone upto 4 hrs. From 2 hr onwards as
Gel and it may increase the blood glucose lowering efficacy
compared to standard phase (pioglitazone alone), decreases
of Pioglitazone. This may lead to accumulation of drug in
elimination rate (t½) of pioglitazone in plasma (Table 1) and
the body, which may lead to toxicity. Therefore, it is
also revealed that Cmax of pioglitazone was not affected
advisable to monitor blood glucose level when starting the
much. It clarifies that there is no change in the intestinal
therapy with Digene Gel to adjust the required dosage of
microsomal activity but changes the hepatic microsomal
International Journal of Preclinical and Pharmaceutical Research
Vol 2 Issue 2 Jul –Dec 2011 76-80. Print ISSN – 2229 7502
In conclusion, the present study suggests that
substantial increase in plasma concentration of pioglitazone
Digene Gel delays the concentration of pioglitazone causes
and it may lead the risk of toxicity in diabetic patients.
REFERENCES
1. Report of a World Health Organisation Consultation. Definition, Diagnosis, Classification of Diabetes mellitus and its
Complications. WHO/NCD/NCS/99.2
2. Tiina Jaakkola, Janne T. Backman, Mikko Neuvonen, Jouko Laitila & Pertti J. Neuvonen. Effect of Rifampicin on the
pharmacokinetics of pioglitazone, British Journal of Clinical Pharmacology of Helsinki University Central Hospital, Helsinki, Finland.
3. Pattana Sripalakit AB, Penporn Neamhom B, Aurasorn Saraphnhotiwitthaya C. High-performance liquid
chromatographic method for the determination of pioglitazone in human plasma using ultraviolet detection and its application to pharmacokinetic study. Journal of Chromatography B, 843, 2006, 164-169.
4. Sudhir N. Umathe, Pankaj V. Dixit, Vijendra Kumar, Kuldeep U. Bansod, Manish M. Wanjari Quercetin pretreatment
increases the bioavailability of pioglitazone in rats: Involvement of CYP3A inhibition. Journal homepage: www.Elsevier. Com/locate/biochempharm.
5. Guillaume Hoizey, Denis Lamiable, Thierry Trenque and Arnaud Robinet. Identification and Quantification of 8
Sulfonylureas with Clinical Toxicology Interest by Liquid Chromatography-Ion-Trap Tandem Mass Spectrometry and Library Searching. Clinical Chemistry, 51 (9), 2005, 1666-1672.
6. Scheen AJ, De Magalhaes AC, Salvatore T, Lefebvre PJ. Reduction of the acute bioavailability of metformin by the
alpha-glucosidase inhibitor acarbose in normal man. Eur J Clin Invest, 24 Suppl 3, 1994, 50-4.
7. Naggar VF, Khalil SA. In vitro study of antidiabetics with antacids and adforbents. Pharmazie, 35(7), 1980, 46. 8. Yukiyoshi Fujita, Yasuhiko Yamada, Makiko Kusama and Toshimasa Yamauchi. Sex differences in the
pharmacokinetics of pioglitazone in rats. Comparative Biochemistry and physiology, 136, 2003, 85-94.
9. Mikko Niemi, Janne T. Backman, Mikko Neuvonen and Pertti J. Neuvonen. Effect of rifampicin on the
pharmacokinetics and pharmacodynamics of nateglinide in healthy subjects. J. Chromatography. B 817, 2005, 277-286.
10. Kirchheiner J. Roots I. Goldammer M. and Rosenkranz B. Effect of genetic polymorphisms in cytochrome p450 (CYP)
2CP and CYP2C8 on the pharmacokinetics of oral antidiabetic drugs: Clinical relevance. Clin Pharmacokinet., 44 (12), 2005, 1209-25.
11. Marathe PH. Arnold ME, Meeker J, Greene DS, Barbhaiya RH. Pharmacokinetics and bioavailability of a
metformin/glyburide tablet administered alone and with food. J Clin Pharmacol., 40(12), 2000, 1494-502.
12. Tashtoush BM,Al-Qashi ZS, Najib NM. In vitro and in vivo evaluation of glibenclamide in solid dispersion systems.
Drug Dev Ind Pharm., 30(6), 2004, 601-607.
13. Thokcho IS and BD. Rajkumari Modification of Hypoglycemic Action of Glibenclamide by doxycycline in albino rats.
Indian Journal of Pharmacology, 25, 1993, 251.
14. Hanefeld M. Pharmacokinetics and clinical efficacy of pioglitazone. Int J Clin Pract., 121, 2001, 19-25. 15. Scheen Ajde Magalhaes AC, Salvator T, Lefebvre PJ. Reduction of the acute bioavailability of merformin by the alpha-
glucosidase inhibitor acarbose in normal man. Eur J Clin Invest., 24 (3), 1994, 50-54.
16. Jonkman JH, van Lier JJ, van Heiningen PN, Lins R, Sennewald R, Hogemann A. Pharmacokinetic drug interaction
studies with candesartan cilexetil. J Hum Hypertens., 11(2), 1997, S31-35.
17. Hanefeld M. Pharmacokinetics and Clinical Efficacy of Pioglitzone. Int J Clin Pract, 121, 2001, 19-25. 18. Eckland DA Danhof M. Clinical Pharmacokinities of Pioglitzone. Exp Clin Endocrinal Diabetes, 108(2), 2000, 234-42. 19. Niemi M, Kivisto KT, Backma JT, Neovoven PJ. Effect of Rifampicyin on the Pharmacokinetics and Pharmacodynamic
of Glimeperide. Br J Clin, Pharmacol, 50, 2000, 591-5.
20. Bottorf M, Hansten P. Long- term safety of hepatic hydroxymethyl glutaryl coenzyme AReductase inhibitors: the role of
metabolism- monograph for physicians. Arch intern Med, 2000, 2273-2280.
21. Sudhir N. Umathe, Pankaj V Dixit et all: Quercetin pretreatment increases the bioavailability of pioglitazone in rats:
Involvement of CYP3A4 inhibition. Biochemical Pharmacology, 75, 2008, 1670 – 1676.
22. Michael A. Wynalda J. Matthew Hutzler, et al. Invitro metabolism of clidamycin in Human liver and intestinal
microcosms. Pharmacia, 2003.
23. Kivistq KT, Kroemer HK, and Eichelbaum M. The role of human cytochrome P450 enzymes in the metabolism of
anticancer agents : Implications for drug interactions. Br J Clin Pharmacol., 40(6), 1995, 523 -530.
24. Kroon LA. Drug interactions with smokining. Am J Health Syst Pharm., 64 (18), 007, 1917 – 1921.
International Journal of Preclinical and Pharmaceutical Research
Source: http://www.preclinicaljournal.com/download.php?id=98&f=98_76-80.pdf
Cult Med Psychiatry (2010) 34:132–168DOI 10.1007/s11013-009-9163-1 Post-Soviet Placebos: Epistemology and Authorityin Russian Treatments for Alcoholism Published online: 5 December 2009 ! Springer Science+Business Media, LLC 2009 The dominant modalities of treatment for alcoholism in Russia are suggestion-based methods developed by narcology—the subspecialty of Russianpsychiatry which deals with addiction. A particularly popular method is the use ofdisulfiram—an alcohol antagonist—for which narcologists commonly substituteneutral substances. Drawing on 14 months of fieldwork at narcological clinics inSt. Petersburg, this article examines the epistemological and institutional conditionswhich facilitate this practice of ‘‘placebo therapy.'' I argue that narcologists'embrace of such treatments has been shaped by a clinical style of reasoning specificto a Soviet and post-Soviet psychiatry, itself the product of contested Soviet politicsover the knowledge of the mind and brain. This style of reasoning has facilitatednarcologists' understanding of disulfiram as a behavioral, rather than a pharmaco-logical, treatment and has disposed them to amplify patients' responses throughattention to the performative aspects of the clinical encounter and through man-agement of the treatment's broader reputation as an effective therapy. Moreover,such therapies have generally depended upon, and helped to reinforce, clinicalencounters premised on a steeply hierarchical physician–patient relationship.
EDUCATIONAL STRATEGIES FOR CHILDREN WITH EMOTIONAL AND CENTER FOR EFFECTIVE COLLABORATION AND PRACTICE AMERICAN INSTITUTES FOR RESEARCH March 2000 Mary Magee Quinn David Osher Beth DeHaven Bader Robert Tate Published byCenter for Effective Collaboration and PracticeAmerican Institutes for ResearchWashington, DC


