Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Aspirin sensitivity and resistance: are different tests of platelet function comparable when taking aspirin ?
Update on Aspirin and Plavix Sensitivity and
Resistance Testing:
David L McGlasson, MS, MLS(ASCP)CM,
59th Clinical Research Division,, Wilford Hall Medical
Center, Lackland AFB, TX, 78236-5300
This information is for education only and is not a product endorsement.
• Aspirin irreversibly acetylates platelet cyclooxygenase,
preventing activation by blocking the prostaglandin pathway
• The platelet inhibiting effect of a single aspirin may be
detectable by platelet function assays within 24 hours
• Failure to detect aspirin-induced platelet suppression may
indicate physiological aspirin insensitivity, a phenomenon called "aspirin resistance"
• Aspirin resistance is a recognized cause of failed aspirin
therapy and may imply increased risk arterial thrombosis.
Why is it important ?
•
Aspirin is used for prevention of complications of vascular diseases
such as heart attack and strokes. Gender issues?
•
Studies have shown using Aspirin alone reduced recurrent non-fatal
stroke by 18%.
•
However, studies have shown about 5-40% (about 1-2 million) of patients
taking Aspirin may not be receiving full benefit because of resistance
•
Several studies have suggested a significant increase of major vascular
events associated with aspirin resistance. It may be reasonable to alter
therapy in the aspirin resistant population rather than continue to take a
drug that a test suggests is ineffective.
Mechanism of Action of ASA: Inhibits the
prostaglandin-producing enzyme cyclooxygenase which
converts arachidonic acid into prostaglandins.
ASPIRIN RESISTANCE
•
ASA resistance refers to less than expected
suppression of thromboxane A2 production by ASA.
Reported to be independently associated with an
increased risk of adverse cardiovascular events.
•
Clinical resistance: inability of ASA to protect
subjects from cardiovascular events such as an
acute MI.
•
Laboratory ASA resistance: refers to the lack of
anticipated effect of ASA on a laboratory assay of
its antiplatelet effect.
POSSIBLE CAUSES OF ASPIRIN RESISTANCE
•
Poor compliance by subjects.
•
Drug interaction: ibuprofen, naproxen.
•
Inadequate ASA dose.
•
Increased turnover of platelets.
•
Genetic polymorphisms of cyclo-oxygenase-1.
•
Up regulation of alternate (non-platelet) pathways of
thromboxane production.
•
No standardized approach to the diagnosis and
there are no proven effective treatments for aspirin
resistance that improve outcome. Yet!
Research Background
Eikelboom J et al: HOPE study: among patients with cardiovascular disease who
take aspirin with persistent high 11-dehydro-thromboxane B2, had a 3.5 fold
increase in the risk of death from heart attack
Grotemeyer K. H et al: two year follow up of aspirin responders and non
responders (180 Post-stroke patient):Major end point (CVA, MI, Vascular death)
seen in 4.4 % of aspirin responders but 40% in aspirin non-responders.
Gum P., Topol E, et al: A prospective, blinded determination of the natural
history of aspirin resistance among stable patients with cardiovascular disease
among patient with aspirin resistance, 24% experienced death, MI, or CVA
compared to 10% of patient who were not resistant
Faraday et al: Relation Between Atherosclerosis Risk Factor and Aspirin
Resistance in a Primary Prevention Population found that higher 11-DHT B2
levels is the only criteria associated with atherosclerosis risk factors suggesting
that this measurement may represent the most relevant approach for identifying
asymptomatic subjects who ASA treatment fail.
Research Background
Patrono et al: Low-Dose Aspirin for the Prevention of Atherthrombosis:
Benenfits fine for high risk subjects but may be marginal in low risk populations.
Rjdker PM et al: Women‟s Health study in healthy women gave surprising
results in that protection from stoke by 17% over men but no reduction in the
risk of MI. Reverse effect for men in protection from MI but low protection from
Becker DM et al: Women experienced the same or greater decrease in platelet
reactivity after ASA therapy, retaining modestly more platelet reactivity
compared with men.
Bhatt DK et al: Overall clopidogrel + ASA was not significantly more effective
than ASA alone in reducing MI, stroke and CVA.
Lordkipanidze M et al: Aspirin resistance: Truth or dare. ASA resistance is
poorly understood with testing not equivalent to each other. Like LA testing?
Research Background
Goodman T, Sharma P, Ferro A. The genetics of aspirin resistance: ASA may
not be effective in the prevention of thrombosis, depending on genetic makeup.
Genetic testing is not currently useful for predicting the effect of ASA clinically.
Schwertner HA, McGlasson DL, Christopher M, Bush AC. Effects of different ASA
formulations on platelet aggregation times and plasma salicylate concentrations.
Feher G, Koltai K, Pappe E, et al: Aspirin resistance: possible roles of CV risk
factors, previous disease history, concomitant medication and
haemorrheological variables. Patients who demonstrated effective ASA
inhibition had a significantly lower fibrinogen level (330 mg/dl vs 380 mg/dl).
Cox D, Maree AO, Dooley M et al: Effect of enteric coating on antiplatelet activity
of low-dose ASA in healthy volunteers.
Research Background
•
Geske et al: Gender Variability of Urinary 11-DHT B2 levels in Diabetes
Mellitus. Healthy females had higher levels than males. DM patients had
higher levels than healthy controls. Female DM had higher levels than
healthy females and DM males. No difference between DM males and
healthy males. In response to ASA 325 healthy females levels were
higher than healthy males.
•
Gengo F et al: Prevalence of platelet non-responsiveness to ASa in
patients treated for secondary stroke prophylaxis and in patheits with
recurrent ischemic events. Prevalence of nonresponsiveness to ASA
was statisically higher in patients who suffered recurrent cerebral
ischemia while taking ASA compared with patients who remained withou
new ischemic symptoms.
• Assays that measure platelet response to aspirin may predict
clinical outcomes
• We compared four methods for monitoring 24-hour platelet
inhibition (single dose) and a 7 day dosing regimen in healthy
subjects by 81 mg and 325 mg (standard child and adult)
• We anticipated these assays will reveal a greater anti-platelet
effect of 325 mg compared to 81 mg of aspirin
• We further anticipated the assays were comparable in their
ability to detect aspirin effect
• We further anticipated that the 7-day dosing regimen would
reveal a greater anti-platelet effect compared to the 24-hour
Research Objectives
• To measure platelet response to aspirin using four
commercially available assays to determine:
1) Whether results of these assays compare and
validate each other
2) Whether the degree of platelet inhibition under different single doses of Aspirin (81 and 325mg)
Four commercially available assays were used in this study
•
Whole blood aggregometry: examines platelet aggregation by using
platelet agonists Collagen, ADP, Arachidonic Acid.
•
PFA-100: tests platelet aggregation by measuring time to occlude an
aperture. (Closure time)
•
Verify/Now Accumetrics: studies platelet function by using arachidonic
acid reagent. ASA inhibits platelet function and does not react to AA.
Platelet aggregation is quantified as ARU (aspirin resistance units).
•
Aspirin-works: Measure level of urine 11-Dehydrothromboxane
(metabolite of Thromboxane A2) in pg/mg of creatinine.
• If these platelet function assays are found to be comparable, we may
be able to choose the most time efficient, cost-effective approach to
obtain this information.
• Data obtained can be used to distinguish aspirin resistant and aspirin
sensitive individuals.
• If aspirin resistance is associated with increased risk of recurrent
stroke, CVA, MI etc., then using platelet function assays could detect
such individuals (who could then be offered other anti-thrombotic
Plan for Data Analysis
• Whole blood platelet aggregation (WBPA) using
Chronolog- 570Vs aggregometer was the gold standard test
• The other 3 assays results were compared to
WBPA to validate equivalency
Chronolog WBA®
• Records whole blood platelet activation by platelet
aggregation impedance
• Whole blood platelet aggregation is the reference
method for aspirin detection
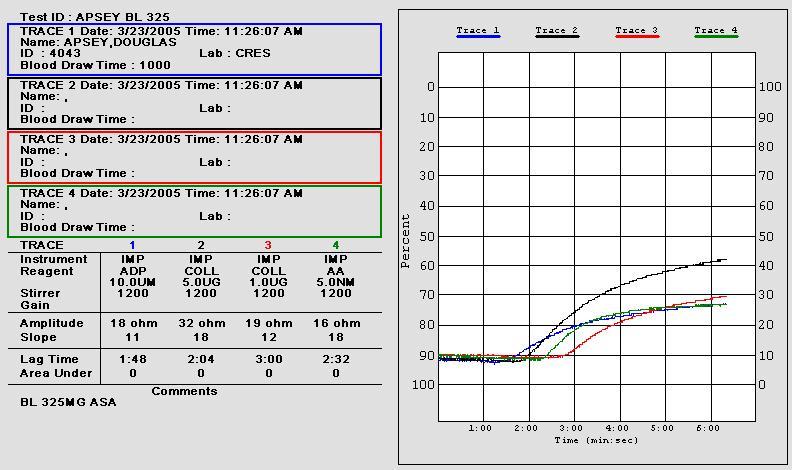
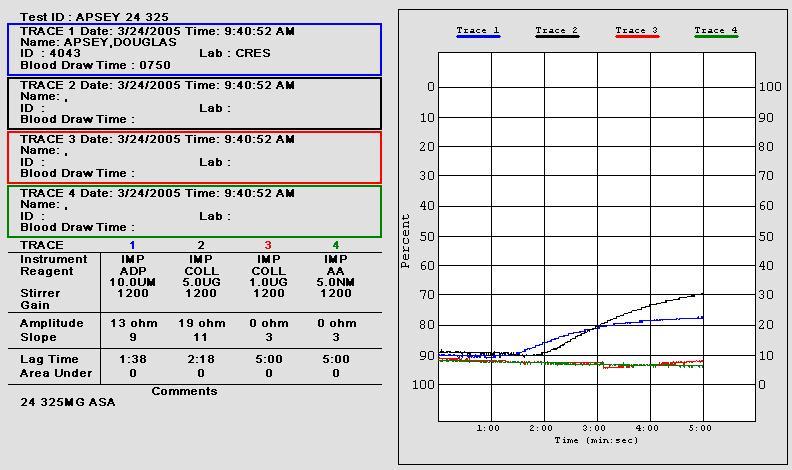
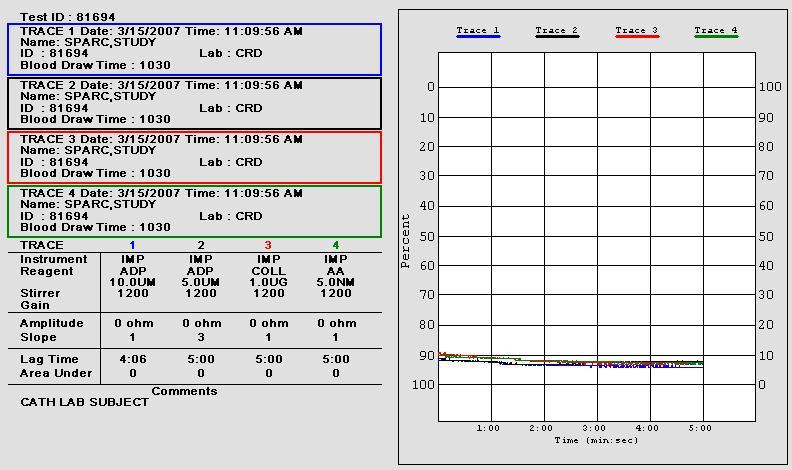
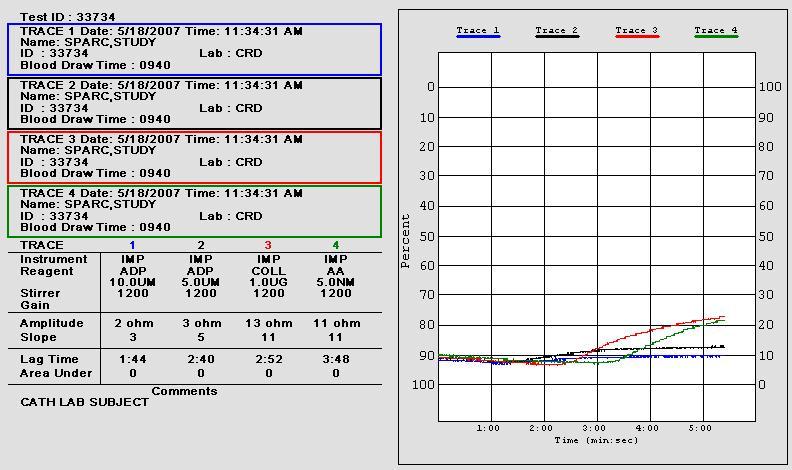
• 10 uL of aggregation agonists 1.0 µg/mL collagen (Coll)
and 0.5 mM arachidonic acid (AA) were added to 1:1 saline/whole blood suspensions
• Aggregation impedance 8 ohms indicates aspirin
PLATELET AGGREGATION
Platelet rich plasma
(light transmission
aggregometry) LTA
–Measures change in light
transmission upon
addition of agonist
–Considered by some the
gold standard
–Labor intensive, not
specific
–Sensitivity variable
–Correlates with clinical
events
WHOLE BLOOD AGGREGATION
Measures impedance: Superior to PRP?
Evaluates platelets in a physiologic milieu in the
presence of RBC and WBC which are know to modulate platelet function.
Faster and uses less specimen making it better
for children and hard to draw subjects.
Higher sensitivity to medication responses.
Does not require centrifugation thus avoiding
injury to platelets and loss of giant platelets.
1. Whole blood
sample is
diluted with
0.9 % saline,
1:1 in cuvette
2. Electrode is
placed in
sample
Methods 3. Platelets form a
monolayer on the
electrode
4. Voltage is run
through the
electrode and
resistance baseline
is assigned a value
of zero ohms
5. Agonist is added
to stimulate
aggregation
Amount of
aggregation is
directly
proportional to
the change in
resistance in ohms
WHOLE BLOOD LUMI-AGGREGOMETRY
vs OPTICAL-LUMI
After 3 Days of Aspirin Treatment @ 325 mg
Whole Blood Aggregation
Optical Aggregation
Arachidonic
Arachidonic
1:00 2:00 3:00 4:00 5:00 6:00 7:00 8:00
Courtesy of: Anna M. Dyszkiewicz-Korpanty, MD, University of Texas Southwestern Medical Center at Dallas, Department of Medicine
WHOLE BLOOD [Impedance] AGGREGOMETRY
and the
Effect of ASA on
Platelets,
and WBC's (L)
* p<0.02
* * p<0.001
PRP + RBC PRP + WBC
Reference: Platelet Aggregation in Human Whole Blood After Chronic Administration of Aspirin, De La Cruz et al, Thrombosis Res 46;133-140,1987
Dade-Behring PFA-100®
• Records platelet-induced whole blood interval to
occlusion of an aperture in a biochemically active
membrane cartridge producing "closure time" (CT).
Alternative to Ivy Bleeding Time.
• Specimens first assayed with ADP/collagen
impregnated cartridges
• If ADP/collagen CT 145 s, aspirin effect was
assessed by epinephrine/collagen (EPI/COLL)
impregnated cartridges
• CT 175 seconds is anticipated aspirin response
PFA-100 CARTRIDGES
Collagen/Epinephrine (CEPI) is the
primary screening cartridge
Collagen/ADP (CADP) indicates if a
platelet dysfunction observed with
CEPI is due to ASA or may reflect
Plavix effect.
Requires PFA -100 instrument
Uses cartridges coated with collagen and
epinephrine or collagen and ADP
Rapid, easy to perform
Whole blood – platelet count dependent.
Hematocrit dependent. May be affected by high
fibrinogen and vWF.
Sensitivity variable
Clinical outcomes studies limited
Qualitative – results measured in closure time
(sec)


PFA-100 Test Procedure
Pipette 800 µL of
Using the
citrated whole
Place the cassette
integrated
blood into the
onto the carousel
keypad, initiate
sample reservoir of
of the analyzer.
the test run.
The Solution: A diagnostic test that can help physicians
determine if aspirin therapy is working for their patients.
Verify Now by Accumetrics
• RAPID
Result available in less than 10
minutes
Whole blood - no sample
preparation
Automatic sampling from closed
tube
Factory calibrated reagents
CLIA - moderately complex; filed for
waived status
FDA Cleared
Reimbursement/ CPT code
• ACCURATE
A quantitative reference point
measured in Aspirin Reactive Units
Correlates to optical platelet aggregometry
Insert assay device
Add blood sample
Result in minutes
Clinical Lab Cardiac Cath Lab Point of Care Doctors' Office
Ultegra® Aspirin Test Results
If a patient result is <550 ARU, then
If a patient result is >550 ARU, then
platelet dysfunction has been
no platelet dysfunction has been
detected, indicating that Aspirin IS
detected, indicating that the anti-
working.
platelet effect may not have been
achieved or Aspirin IS NOT Working
Verify Now
• Verify/Now Accumetrics Ultegra instrument • Cartridge containing fibrinogen-coated microparticles in
a proprietary tube using Arachidonic Acid as agonist.
• Whole blood • Rapid, easy to perform • Sensitivity and specificity variable • Clinical outcomes studies limited • Qualitative – results measured as aspirin response units
Mapping of ARU to % Inhibition
VerifyNow® P2Y12
– Result available in <3 minutes
– Whole blood - no sample preparation
– Automatic sampling from closed tube
– Factory calibrated reagents
– More specific than optical aggregometry
– Can measure % platelet inhibition without weaning patient off drug
• COST-EFFECTIVE
– Reimbursement
– CPT code 85576 (2 times)
– FDA cleared
Results are based on the rate and extent of
platelet aggregation and are reported in P2Y12
Reaction Units (PRU) and % platelet inhibition
PRU result is „P2Y12-
-Base result is „Maximal
mediated platelet
platelet aggregation‟ via
aggregation‟ via
Thrombin Receptor
adenosine
Activating Peptide (TRAP)
diphosphate (ADP)
pathway which is
independent of aspirin and
clopidogrel
VerifyNow® P2Y12 Advantages
• Greater specificity for clopidogrel than test methods
using ADP alone, e.g., optical aggregometry
• Ability to measure % platelet inhibition in patients
on clopidogrel without first withdrawing clopidogrel
• Rapid - Time to result <3 minutes
ADP activates platelets via two ADP
receptors: P2Y12 and P2Y1…
Tests using ADP alone measure ADP-induced
platelet aggregation via both P2Y12 & P2Y1
which may
over-estimate
the degree of
aggregation, by
as much as
25%
PGE1 minimizes
contribution of
P2Y1
aggregation
VerifyNow® P2Y12 Result Calculations
ADP-mediated platelet
activation determines the
PRU value
platelet activation
% platelet inhibition
Baseline PRU – Post-PRU
Baseline PRU
Baseline PRU
Urinary 11-dehydrothromboxane B2
Urinary 11-dehydrothromboxane B2
• Requires ELISA equipment and urinary creatinine
• Random urine specimen that can be frozen until
ready for testing.
• Sensitivity good
• Specificity uncertain
• Labor intense, not rapid. Two hour specimen
incubation. Recently FDA approved. Established
test in optimized format
• Quantitative - Results reported as pg 11-
dehydrothromboxane B2/mg creatinine
• May be used to guide incremental aspirin therapy
VASP.P2Y12
Vasodilator Stimulated Phosphoprotein
• Dedicated to the monitoring of specific platelet ADP receptor
(P2Y12) antagonists: Thienopyrdines
• Regulated by cAMP cascade
• cAMP activated by PGE1 (1)
• Inhibited by ADP through P2Y12 receptors (2)
• VASP phosphorylation correlates with P2Y12 receptor
inhibition. Non-phosphorylation state correlates with the
active form of P2Y12 receptor.
• Thienopyrdines can be demonstrated with PLT VASP/P2Y12
(3). Performed by flow cytometry on citrated blood.
VASP.P2Y12
Vasodilator Stimulated Phosphoprotein
• The aim of the assay is to evaluate the efficacy of Plavix
therapy.
• Uses the PRI or platelet reactivity index expressed as a
percentage to measure the difference in VASP fluorescence
intensity between resting +PGE1 and activated +ADP
platelets.
• Aleil B et al: J Thromb Haemost, 2005; 1:85-92 measuring
VASP for clopidogrel resistance in patients with ischemic
cardiovascular diseases found the following:
– 85.8 to 6.6% PRI with the 85.8 being poor responders and 6.6% good
responders. 30% of treated subjects were in range with bad
responders. (PLAVIX RESISTANCE?)
Tests Requiring Blood Specimen
• Advantages
– Point of Care
– Rapid results
• Disadvantages
– Preanalytical variables
– Lack of standardization
– Test must be run within 3-4 hours
– Limited clinical outcomes data
(except platelet aggregation)
Tests Requiring Blood Specimen
Additional considerations
• Platelet function tests requiring whole blood may be
impacted by:
– Platelet count
– Hematocrit
– Fibrinogen - elevated levels (Lower fibrinogen levels
have shown greater ASA response). Values above 380
mg/dl have been shown to affect assay.
– Factor VIII – elevated levels
– vWF – elevated levels
Urinary 11-dehydrothromboxane B2
• Metabolite not formed by platelet
• High concentration
• Longer circulating half-life
• Minimal pre-analytical variables
• Specimen stable 72 hours at room temperature
• Major clinical outcomes study to support the test
• Standardization of test
– to outcome studies
– between laboratories
– Disadvantages: Liver disease, renal disease may
affect results
Comparison of The 24-hour Sensitivity of Four
Platelet Function Assays to A Single Aspirin
DL McGlasson, G Fritsma, M Chen, Z Knight, M Dobbs, 59th Clinical
Research Squadron and Department of Neurology Wilford Hall
Medical Center, Lackland AFB, TX and University of Alabama
Birmingham, Division of Laboratory Medicine, Birmingham, AL
Aspirin Response Assays
• Assays that measure platelet response to aspirin may
predict aspirin‟s cardioprotective effect
• We compared four methods for monitoring 24-hour
platelet inhibition in healthy subjects by a single 81
mg and 325 mg (standard child and adult) aspirin
• We anticipated these assays would reveal a greater
24-hour anti-platelet aspirin effect of 325 mg
compared to 81 mg
• We further anticipated that the assays were
comparable in their ability to detect the aspirin effect
Subjects and Procedure
Fifty normal healthy volunteers were enrolled. None
had taken aspirin or other NSAIDs for 14 days
20 females, mean age 33.1 (18-51)
30 males, mean age 36.6 (20-58)
1. Baseline citrated whole blood and urine
2. Subjects observed to ingest a single 81 mg aspirin
3. Citrated blood and urine obtained 24 hours after dosing
Process repeated 14 days with single 325 mg aspirin
Materials and Methods
Chronolog WBA®
• Records whole blood platelet aggregation by
impedance
• Whole blood platelet aggregation is chosen as the
reference method for aspirin response detection
• 10 uL of aggregation agonists 1.0 µg/mL collagen
(Coll) and 0.5 mM arachidonic acid (AA) were added
to 1:1 saline/whole blood suspensions
• Post-aspirin aggregation impedance 8 ohms
indicates anticipated aspirin response
Materials and Methods
11-dehydro Thromboxane B2
• Urine 11-dehydrothromboxane B2 (11-DHT) is an
end product of the platelet arachidonic acid
prostaglandin pathway whose urine concentration
reflects in vivo platelet activity
• Aspirin inhibits the prostaglandin pathway and
decreases urine 11-DHT production
• 50% 11-DHT reduction from baseline indicates
aspirin effect
• Urine 11-DHT is measured using random urine
when normalized to urine creatinine
Materials and Methods
• Arachidonic acid (AA)-impregnated cartridge
aggregates platelets
• Aggregation time interval expressed as aspirin
reaction units (ARUs)
• Post-aspirin aggregation impedance 550
ARUs indicates response
Materials and Methods
Dade-Behring PFA-100®
• Records platelet-induced whole blood interval to
occlusion of an agonist-impregnated cartridge aperture
producing closure time (CT)
• Specimens first assayed with ADP/collagen
impregnated cartridges
• If ADP/collagen CT 145 s, aspirin effect was assessed
by epinephrine/collagen (EPI/Coll) impregnated
cartridges
• EPI/Coll CT 175 seconds is anticipated aspirin
response
Materials and Methods
24-Hour Response to 81 mg
and 325 mg Aspirin: Means
Chronolog WBA®
VerifyNow®
PFA-100®
Reference Method
1.0 ug Coll 0.5 mM AA
Baseline
978.4 pg/mg 643.7 ARU
24-h Response
16.1 *
510.7 pg/mg* 600.7 ARU*
170.0 CT*
to 81 mg
Baseline
884.5 pg/mg 646.2 ARU
24-h Response
13.6 *
349.1 pg/mg* 465.3 ARU*
258.2 CT*
In all assays, 81 mg to 325 mg baselines are not significantly different at p < 0.05
*All aspirin responses significant at p < 0.05
24-Hour Response to 81 mg
and 325 mg Aspirin: Action Limits
Chronolog WBA®
VerifyNow®
PFA-100®
Reference Method
Action Limit
550 ARU EPI/COLL
Reduction
CT 175 s
Response to 11 (22.4%) 12 (24.5%) 23 (46.9%) 10 (20.4%) 18 (36.7%)
81 mg Aspirin
Response to 44 (89.8%) 44 (89.8%) 39 (80.0%) 43 (87.8%) 39 (80.0%)
325 mg Aspirin
24-Hour Response to 81 mg
and 325 mg Aspirin: Action Limits
Subjects Responsive to Aspirin By Assay Method (N = 49)
Agg: 1 ug Coll
Agg: .5 mM AA
24-hour Response to 325 mg Aspirin
• There was no significant gender effect at baseline
or 24 hours for 11-DHT and VerifyNow in either the
81 or 325 mg arm (data not displayed)
• The systems equivalently recorded an average
85.5% 24-hour individual subject responses to 325
mg aspirin relative to action limits
24-hour Response to 81 mg Aspirin
• The systems recorded a significant mean reduction
of platelet function 24 hours after a single dose of
81 or 325 mg aspirin
• The ratio of individual subject responses to 81 mg
aspirin relative to action limits averaged 30.2%
• The 11-DHT individual subject responses to 81 mg
aspirin, 46.9%, is the most sensitive
• The Dade-Behring PFA-100 individual subject
responses to 81 mg aspirin, 36.7%, is the second
most sensitive
Discussion
Predictive Values of Methods
• The predictive value of 11-DHT, VerifyNow, and PFA-100
compared to aggregation, averages 39% at 81 mg aspirin
• The predictive values of 11-DHT and VerifyNow compared to
aggregation at 325 mg aspirin are 86.8% and 93.0%,
respectively
• 11-DHT and VerifyNow duplicate the reference method‟s
ability to identify the 24-hour platelet response to 325 but
not 81 mg aspirin
• These data may be confirmed using a 7-day dosage
schedule
Analysis
• Platelet inhibition across 3 assays seems to be dose
dependent (81mg vs 325 mg) at 24 hours.
• Out of 38 individuals whose WBPA showed no significant
changes at 81 mg, 31 of those individual become
responders at 325 mg.
• % of aspirin resistance may be high in this study secondary
to one time dose effect. If subjects were to take aspirin on
daily basis, % of aspirin resistance may drop.
• Initial responders may develop aspirin tolerance according
to some studies when taking aspirin chronically.
Comparison of Four Commercial Platelet
Function Assays‟ Ability to Detect Response
to 7 Days of Aspirin at 81 and 325 mg Doses
DL McGlasson, G Fritsma, M Chen, Z Knight, M Dobbs
59th Clinical Research Squadron and Department of Neurology
Wilford Hall Medical Center, Lackland AFB, TX and
University of Alabama Birmingham
Division of Laboratory Medicine, Birmingham, AL
Aspirin Response
• We compared the ability of four commercial platelet
function assays to detect the 7-day aspirin (ASA)
response in normal subjects taking 81 or 325 mg
• Laboratory detection of inadequate ASA-induced
platelet suppression may indicate physiological
insensitivity, called "aspirin resistance"
• ASA resistance is a recognized cause of failed ASA
therapy and may predict arterial thrombosis risk
• We anticipated the assays would reveal a dosage effect
for 325 mg compared to 81 mg ASA
• We anticipated the assays are comparable in their
ability to detect ASA response
Materials and Methods
We consented forty-five normal healthy volunteers.
None had taken ASA or other NSAIDs for 14 days
22 females, mean age 33.1 (18-51)
23 males, mean age 36.6 (20-58)
1. Baseline 3.2% Na citrate whole blood and urine
2. Subjects provided a single 81 mg aspirin for 7 days
3. Na citrate whole blood and urine obtained 24 hours
after final dose
Repeated 14 days with single 325 mg ASA for 7 days
Mean Responses to
7-Day ASA at 81 mg and 325 mg
Chronolog WBA® WBPA
Accumetrics Dade-Behring
Reference Method
PFA-100®
1 ug/mL Coll 500 M AA
Baseline
538.0 pg/mg 634.5 ARU
pre-81 mg
7-d response
161.7 pg/mg* 436.3 ARU*
226.7 CT*
to 81 mg
Baseline
642.4 pg/mg 647.6 ARU
pre-325 mg
7-d response
206.7 pg/mg* 425.8 ARU*
250.0 CT*
No pre-81 mg to pre-325 mg baselines are significantly different at p < 0.05
*All 7-day responses significant at p < 0.05
Percent 7-Day Response to 81 mg
and 325 mg ASA by Action Limits
N and (%) ASA Responders
Chronolog WBA® WBPA
Accumetrics Dade-Behring
Reference Method
PFA-100®
1 ug/mL Coll
500 M AA
EPI/COLL
Action Limit
8 aggregation
≤ 550 ARU
reduction
CT ≥175 s
Response to 32 (71.1) 38 (84.4) 35 (77.8) 44 (97.8) 32 (71.1)
Response to 38 (84.4) 43 (95.6) 39 (86.7) 43 (95.6) 35 (77.8)
325 mg ASA
Numerical 7-Day Response to 81 mg
and 325 mg ASA by Action Limits
Subjects Responsive to ASA by Assay Method (N = 45)
Agg: 1 ug Col
Agg: .5 mM
Discussion
• Mean platelet response to ASA at 81or 325 mg ASA for 7 days
for all platforms were significant
• Verify/Now is the most sensitive to 81 mg and 325 mg ASA
• WBPA using 1.0 µg/mL collagen, 11-DHT and PFA-100 detected
the most instances of ASA resistance
• Positive predictive values were comparable for 11-DHT, PFA-
100, and Verify/Now at 81 and 325 mg
• These data provide support for these methods to use in clinical
settings to distinguish aspirin responders vs. non responders
• We recommend continued testing on clinical populations to
confirm the dosage effect and compare platforms to clinical
Potential study limitations
Other possible mechanisms of clinical aspirin resistance
Patient non-compliance and underdosing
COX 2 expression inducing production of THX-A2
Glycoprotein IIb/IIIa polymorphisim
Erythrocyte/Leukocyte/platelet interaction
Elevated fibrinogen and vonWillebrand‟s factor
Type II Diabetics do not respond as well to ASA
Cigarette smoking and hypercholesterolemia
Platelet inhibition may not be constant over an extended time with a fixed dose
of Aspirin
Aspirin resistance in the single dose study may be higher because of one time
dose effect. Percent of aspirin resistance may be reduced if given aspirin on
daily basis.
Some people might show biochemical platelet inhibition at baseline without
administration of antiplatelet drugs.
Clinical Implications
• For individuals who do not respond to 81mg ASA when tested by these
methods, titrating up aspirin dose may be needed to achieve sufficient
platelet inhibition over several days and retest
• For those who are aspirin non-responders when testing by these
methods with 325 mg aspirin (including urine 11-dehdyrothromboxane),
alternate anti-platelet therapy may be indicated
• Initial responders may develop aspirin tolerance according to some
studies when taking aspirin chronically.
• There are needs for randomized double blind studies to show that by
giving alternate anti-platelet therapy in patients with a history of vascular
events on ASA and shown to be biochemically ASA resistant, the risk of
further events is decreased when compared to those individuals
continued with aspirin
Clinical Implications
• Caveat emptor
– There are no clinical studies to date showing that patients who are
aspirin resistant in vitro do not derive some clinical benefit and
protection from taking ASA at any accepted dose
– Because of the complexity of the platelet activation process, one
single test is unlikely to adequately reflect all aspects of platelet
function that are relevant to clinical events
– We need prospective controlled studies to test the hypothesis that
biochemical aspirin resistance translates to clinical aspirin
resistance
McConnell J, et al. Urinary 11-dehydrothromboxane B2 and coagulation activation markers measured within 24 h of
human ischemic stroke. Neuroscience Letters 2001;313:88-92.
Halushka MK Halushka PV. Why are some individuals resistant to the cardioprotective effects of aspirin? Could it be
thromboxane A2? Circulation 2002;105:1620-2.
Eikelboom JW, et al. Aspirin-resistant thromboxane biosynthesis an the risk of myocardial infarction, stroke, or
cardiovascular death in patients at high risk for cardiovascular events. Circulation 2002;9:1650-5.
Bruno A, et al. Aspirin and urinary 11-dehydrothromboxane B2 in African American stroke patients. Stroke 2002;:57-60.
Grau, AJ et al. Platelet function under aspirin, clopidogrel, and both after ischemic stroke: a case-crossover study.
Stroke 2003;Apr:849-855.
Yusuf, S et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment
elevation. NEJM 2001;345(7):494-502.
Hart, RG and RD Bailey. An assessment of guidelines for prevention of ischemic stroke. Neurology 2002;59:977-982.
Are patients actually receiving aspirin's stroke prevention benefits? Neurology Reviews 2003;Apr:6-7.
Helgason, C et al. Development of aspirin resistance in persons with previous ischemic stroke. Stroke 1994;25(12):2331-
Koudstaal, P et al. Increased thromboxane biosynthesis in patients with acute cerebral ischemia. Stroke 1993;24(2):219-
Undas, A et al. Simvastatin depresses blood clotting by inhibiting activation of prothrombin, factor V, and factor XIII and
by enhancing factor Va inactivation. Circulation 2001;103:2248-2253.
Halushka MK and PV Halushka. Why are some individuals resistant to the cardioprotective effects of aspirin? Could it be
thromboxane A2? Circulation 2002;105:1620-1622.
McGlasson DL, Chen MA, Fritsma GA, Knight ZA, Dobbs M. Urinary 11-dehydrothromboxane B2 levels in healthy
individuals following a single dose response to two concentrations of aspirin. J of Clinical Ligand Assay. Fall 2005. 28(3),
147-150.
Source: http://www.safmls.org/2011/2011%20Presentations/W%204/Update%20on%20Aspirin%20and%20Plavix%20Sensitivity.pdf
Nutritional Supplementation in Pregnancy It goes without saying that Nutrition in Pregnancy is vitally important for the developing fetus. Dietary requirements are increased substantially in a pregnant woman, and if she does not have enough in the way of proteins, vitamins and minerals in her own body stores, then her baby could be compromised. It has been clearly established that Pregnant women in Australia are not meeting their daily
Interuniversity Progress Test for Medicine All rights reserved. All rights to intellectual ownership of the contents of the interuniver-sity progress test and the item bank are with the Medical Faculties of the universities of Amsterdam, Maastricht, Nijmegen, Groningen and Leiden. The use of progress test questions by a party for other purposes than the joint composition of the progress test is only allowed with the prior written permission of the Interuniversity Progress Test Com-








