Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Pone.0010914 1.9
Design of Group IIA Secreted/Synovial Phospholipase A2Inhibitors: An Oxadiazolone Derivative SuppressesChondrocyte Prostaglandin E2 Secretion
Jean-Edouard Ombetta1., Natacha Thelier2., Chang Zhi Dong3, Ste´phanie Plocki3, Lydia Tsagris2,
Franc¸ois Rannou2,4, France Massicot5, Atime´ Djimde´3, Elissar El-Hayek2, Yiming Shi3, Franc¸oise
Heymans3, Nohad Gresh6, Caroline Chauvet2*
1 Laboratoire de Chimie Organique, Faculte´ de Pharmacie, Universite´ Franc¸ois Rabelais, Tours, France, 2 Laboratoire de Pharmacologie, Toxicologie et Signalisation
Cellulaire, INSERM UMR-S-747, UFR Biome´dicale des Saints Pe res, Universite´ Paris Descartes, Paris, France, 3 Equipe de Pharmacochimie, ITODYS, CNRS UMR7086,
Universite´ Paris Diderot, Paris, France, 4 Service de re´e´ducation, AP-HP, Hoˆpital Cochin, Paris, France, 5 Laboratoire de Chimie-Toxicologie analytique et cellulaire, EA4463,
Faculte´ de Pharmacie, Universite´ Paris Descartes, Paris, France, 6 Laboratoire de Chimie et Biochimie Pharmacologique et Toxicologique, CNRS UMR8601, UFR Biome´dicale
des Saints Pe res, Universite´ Paris Descartes, Paris, France
Group IIA secreted/synovial phospholipase A2 (GIIAPLA2) is an enzyme involved in the synthesis of eicosanoids such asprostaglandin E2 (PGE2), the main eicosanoid contributing to pain and inflammation in rheumatic diseases. We designed, bymolecular modeling, 7 novel analogs of 3-{4-[5(indol-1-yl)pentoxy]benzyl}-4H-1,2,4-oxadiazol-5-one, denoted C1, aninhibitor of the GIIAPLA2 enzyme. We report the results of molecular dynamics studies of the complexes between thesederivatives and GIIAPLA2, along with their chemical synthesis and results from PLA2 inhibition tests. Modeling predictedsome derivatives to display greater GIIAPLA2 affinities than did C1, and such predictions were confirmed by in vitro PLA2enzymatic tests. Compound C8, endowed with the most favorable energy balance, was shown experimentally to be thestrongest GIIAPLA2 inhibitor. Moreover, it displayed an anti-inflammatory activity on rabbit articular chondrocytes, as shownby its capacity to inhibit IL-1b-stimulated PGE2 secretion in these cells. Interestingly, it did not modify the COX-1 to COX-2ratio. C8 is therefore a potential candidate for anti-inflammatory therapy in joints.
Citation: Ombetta J-E, Thelier N, Dong CZ, Plocki S, Tsagris L, et al. (2010) Design of Group IIA Secreted/Synovial Phospholipase A2 Inhibitors: An OxadiazoloneDerivative Suppresses Chondrocyte Prostaglandin E2 Secretion. PLoS ONE 5(6): e10914. doi:10.1371/journal.pone.0010914
Editor: Sudha Agarwal, Ohio State University, United States of America
Received January 19, 2010; Accepted April 1, 2010; Published June 1, 2010
Copyright: ß 2010 Ombetta et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permitsunrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This study was supported in part by INSERM, University Paris Descartes, Arthritis Foundation Courtin and a program of Paris Centre University (ParisDescartes and Paris Diderot). The authors thank the computer center CINES-Montpellier for computer time and technical support. The authors also thank theLigue Nationale Contre le Cancer for support. NT was supported by Assistance Publique-Hopitaux de Paris. The funders had no role in study design, datacollection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests: The authors have declared that no competing interests exist.
* E-mail:
[email protected]
. These authors contributed equally to this work.
ever, COX-2 is absent in most tissues under normal restingconditions but is induced in inflamed tissues and is responsible for
Inflammation is a multi-faceted process involving numerous
increased PGE2 production. This activation has motivated the
enzymes, such as phospholipases A2 (PLA2s) and cyclo-oxygenases
development of selective COX-2 inhibitors. However, these
(COXs) [1]. PLA2s catalyze the hydrolysis of cell-membrane
inhibitors also have severe side effects such as myocardial
glycerophospholipids at the sn-2 position leading to the generation
infarction [5,6]. Overcoming this problem could involve the
of free fatty acids such as arachidonic acid. The later is
development of novel anti-inflammatory agents to efficiently
subsequently metabolized into potent pro-inflammatory mediators
inhibit the PLA2-dependent production of COX substrates
such as eicosanoids (e.g. prostaglandin E2 [PGE2]) through a
without impairing the balance between COX-1 and COX-2.
pathway involving COX-1 and COX-2 in part [2]. PGE2 is the
PLA2s represent a growing family of enzymes of two main
main eicosanoid contributing to pain and inflammation in
categories, intracellular and secreted. Among the 10 human
rheumatic diseases [3,4]. Nonsteroidal anti-inflammatory drugs
secreted PLA2s (sPLA2s) known to date, the most studied is the
(NSAIDs) reduce the production of PGE2, which leads to a
non-pancreatic Group IIA, denoted GIIAPLA2, because of its
significant improvement in rheumatic symptoms. However, these
involvement in the pathogenesis of many inflammatory diseases
drugs exhibit gastrointestinal toxicity mainly because of a marked
(for a review, see [7]). GIIAPLA2 was originally purified from the
decrease in COX-1 activity [4] and renal and blood pressure
synovial fluid of patients with rheumatoid arthritis [8,9,10]. The
toxicities mainly because of a decrease in COX-2 activity. COX-1
number of rheumatoid arthritis-affected joints and the presence of
is constitutively expressed in most tissues and appears to be
destructive erosion correlate with the amount of GIIAPLA2 in the
responsible for maintaining normal physiological function. How-
serum of patients [11]. Moreover, GIIAPLA2 induces an
PLoS ONE www.plosone.org
June 2010 Volume 5 Issue 6 e10914
PLA2 Inhibitors in Chondrocyte
inflammatory response when injected in rabbit joints [12] and
inhibitory potencies of C2 to C8 against GIIAPLA2 was analyzed
exacerbates rat adjuvant arthitis after intradermal injection [13].
by enzymatic assay, and the anti-inflammatory activity of the most
The systemic implication of sPLA2s in inflammation has
potent compound, C8, was evaluated in IL-1b-treated articular
prompted a number of research groups to develop selective
inhibitors of different types of these enzymes. Some potentcandidates have been evaluated in phase II clinical trials.
Surprisingly, no effect was observed when such inhibitors wereused to treat patients with sepsis or rheumatoid arthritis [14,15].
Molecular modeling
This failure could be due to the complexity of the inflammation
We previously reported that one of the essential interactions
process and the existence of compensatory pathways. However,
between C1 and the target GIIAPLA2 is Ca(II) bidentate chelation
these molecules have been tested only in high-level systemic
by the oxadiazolone moiety in its anionic form [20]. Because C2-
inflammatory diseases, not in low-level inflammatory diseases such
C8 are structurally similar to C1 (Fig. 1), docking was performed
as atherosclerosis, diabetes, Alzheimer's, and osteoarthritis.
upon first anchoring the oxadiazolone ring in the same position as
Varespladib, a sPLA
compound C1, followed by energy minimization and molecular
2 inhibitor, was recently found to reduce
atherosclerosis in apolipoprotein-E-null mice [16]. Thus, the
dynamics. As was observed for compound C1, the lowest-energy
frames of C2-C8/enzyme complexes are stabilized by p–p and
inhibitors in these low-level inflammatory
diseases should be re-examined.
cation-p interactions involving His6, Phe23, and Phe63 on the one
We have developed various selective inhibitors of sPLA
hand and Arg7 and Arg33 of GIIAPLA
2 on the other. Table 1 lists
[17,18,19,20,21]. Previously, we reported on the computer-
the energy values corresponding to the lowest-energy frames from
assisted design and synthesis of a series of novel oxadiazolone
molecular dynamics.
derivatives that were shown to exhibit potent inhibitory propertiesagainst GIIAPLA
2 [20]. In this series, a Ca(II)-binding oxadiazo-
lone ring was connected through a polymethylene chain of varying
As outlined in Figure 1, 4-(5-bromopent-1-yloxy)benzyl cyanide
lengths to an indole ring, which has been shown to be involved in
1 is prepared according to Dehaen and Hassner [22] by mono-
apolar and cation-p interactions with GIIAPLA2 residues. The
substitution of 1,5-dibromopentane with 4-hydroxybenzyl cyanide
optimal length of the linker was found to encompass 5 methylenes,
in moderate yield. Compound 1 is then condensed in 25% to 50%
and the corresponding compound, (3-{4-[5-(indol-1-yl)pentoxy]-
yields, with 5-substituted indole derivatives or different aromatic
benzyl}-4H-1,2,4-oxadiazol-5-one), is denoted C1 in the present
alcohols, through their sodium salts prepared prior to use, to give
study. In the current work, the indole moiety was replaced by
2a–g. The nitrile function of 2a–g is converted into amidoxime,
other aromatic groups, which gave rise to compounds C2 to C8.
by use of hydroxylamine released in situ from its HCl salt, to
Using molecular modeling, we computed and ranked energy
provide 3a–g in 35% to 80% yields. The action of phenyl
balances for the binding of these inhibitors to GIIAPLA2. The
chloroformate to the amidoximes 3a–g leads to the corresponding
Figure 1. Synthesis scheme. Reagents and conditions: (a) Br(CH2)5Br, K2CO3, DMF, RT, 10 days; (b) Ar-NH, K2CO3, CH3CN, reflux; (c) NaOH, AbsEtOH; (d) 1, DMF, RT, 24 h; (e) NH2OH-HCl, K2CO3, Abs EtOH; (f) PhOCOCl, Et3N, CH2Cl2; (g) toluene, reflux. The terms 1, 2a-g and 3a-g written in boldrefer to the C2-C8 precursors. The terms a to g written in bold in the bottom of the figure refer to the radicals (R) of the C2 to C8 compounds,respectively. The radical of the C1 compound is also shown.
doi:10.1371/journal.pone.0010914.g001
PLoS ONE www.plosone.org
June 2010 Volume 5 Issue 6 e10914
PLA2 Inhibitors in Chondrocyte
Rekker's fragmental data [24] (Table 2). The molecules C1-C8
Table 1. Energy balances (e = 4) from performing single-point
are specific inhibitors of hGIIAPLA2 because none inhibited
Poisson-Boltzmann calculations of continuum solvation
pGIBPLA2 at the highest concentration tested (100 mM). Such
selectivity implies that C1-C8 should not interfere with thedigestion process.
The experimentally measured IC
50s for hGIIAPLA2 (Table 2)
Esolvlig Esolvprot dEsolv dE2
are associated with the final energy balances, denoted dE2 in
2115.9 2344.3 222.5 2385.1
Table 1. The ranking of C1-C8 in terms of IC50 is the same as
2113.4 2343.6 223.9 2385.1
that of the dE2 magnitudes. In C2, the second phenyl ring is
2114.9 2344.1 222.7 2385.1
substituted with the ether O in the ortho position and in C6 in thepara position. Both IC
2116.5 2343.7 221.7 2385.1
50 and dE2 values show C2 to have a
significantly enhanced affinity for PLA2 as compared with C6,
2116.2 2350.0 221.8 2385.1
even though both are iso-lipophilic (Tables 1 and 2). In C2, the
2111.3 2350.1 221.7 2385.1
biphenyl group has favorable van der Waals interactions with both
2109.0 2340.2 221.8 2385.1
Phe23 and Val30 of the enzyme, but in C6, the interactions are
2118.2 2349.7 221.8 2385.1
limited to Phe23. Such interactions could be further optimized, aswhen the biphenyl ring was replaced by phenantrene in C8. The
All energies are given in kcal/mol. Eint denotes the inhibitor (compounds C1 to
lowest-energy complex is now stabilized by an enhanced overlap of
C8)-protein interaction energy, and dElig and dEprot the costs of conformational
this ring with Phe63 (Fig. 2). However, the lipophilicity increases in
energy rearrangements of the inhibitor and the protein, respectively, onpassing from their free to complexed states, and dE
parallel, which could possibly limit the bioavailability of C8. We
1 is the sum: Eint+ dElig +
dEprot. dE1 corresponds to a gas-phase complexation energy. Esolvprot and Esolvlig
found C8 indeed endowed with the most favorable dE2 value
denote the continuum solvation energies of the isolated protein and the ligand,
(Table 1), which was experimentally associated with the lowest
respectively, following gas-phase energy minimization in the absence of
IC50 value (0.62 mM vs. 5 mM for C1).
complexation. They represent the energy cost necessary to dehydrate bothentities prior to complex formation. E
At the other extreme, replacing the C1 indole ring by the
solvtot denotes the continuum solvation
energy of the complex. Thus, dEsolv = Esolvtot – Esolvlig – Esolvprot, which
smaller and less electron-rich phenyl ring, as in C7, resulted in a
represents the resulting solvation energy balance. The overall energy balance
reduction of 10.3 kcal/mol in dE2 value. Thus, C7 can be
including solvation is denoted as dE2 = dE1 + dEsolv.
predicted to have the least inhibitory potency in the series. This
finding was confirmed by experimentation showing C7 to have thehighest IC
carbonate intermediates, which, when heated to reflux of toluene,
50 value (35 mM vs. 5 mM for C1).
Similar to C1, compounds C3-C5 have a bicyclic ring, whereas
cyclizes intra-molecularly to generate the substituted oxadiazo-
C3 possesses a benzo-1,3-thiazole instead of an indole ring. C4
lones C2-C8 in 34% to 50% yields.
and C5 have a chlorine and a methoxy substituent, respectively, inposition 5 of the indole. In C3-C5, the aromatic rings interact
In vitro inhibition of enzymatic activity of sPLA2s by C1-
simultaneously with His6, Arg7, and Val3, as was previously
observed for C1 [20]. The difference in activity between C4 and
The compounds C1-C8 were submitted to fluorimetric assay to
C5 could be explained by additional electrostatic and/or van der
determine their inhibitory potencies and selectivity towards human
Waals interactions contributed by methoxy substitution. C3 has
GIIAPLA2 (hGIIAPLA2) versus porcine group IB PLA2 (pGIB-
anti-hGIIAPLA2 activity close to that of C1, along with
PLA2) (Table 2). GIBPLA2 is an enzyme of the same family as
substantially reduced lipophilicity (2.88 vs. 3.81 for C1).
GIIAPLA2 (sPLA2) but is mainly involved in digestion of dietary
Thus, in the C1-C8 series, C8 has the most favorable dE2 value
phospholipids and is secreted by the pancreas [23]. Lipophilicity
and the lowest IC50 on human GIIAPLA2 activity, as evaluated by
parameters, log P, of these products are calculated by use of
enzymatic assay. On the bases of the IC50 values we focused ourcellular assays on the most potent compound C8, the solecompound with a sub-micromolar activity. We thus chose to
Table 2. Inhibition of enzymatic activities of porcine
evaluate the cytotoxicity and anti-inflammatory activity of C8 in
pancreatic group IB (pGIB) and human group IIA (hGIIA) PLA2s
primary cultured rabbit articular chondrocytes treated with the
by compounds C1 to C8 and their corresponding log P
pro-inflammatory cytokine IL-1b, which is known to play a key
role in rheumatic diseases such as osteoarthritis (for reviews see[25,26]). Chondrocyte is the unique cell type in joints, and the cellmodel we chose is widely used to study the effect of inflammatory
stress on joint cells.
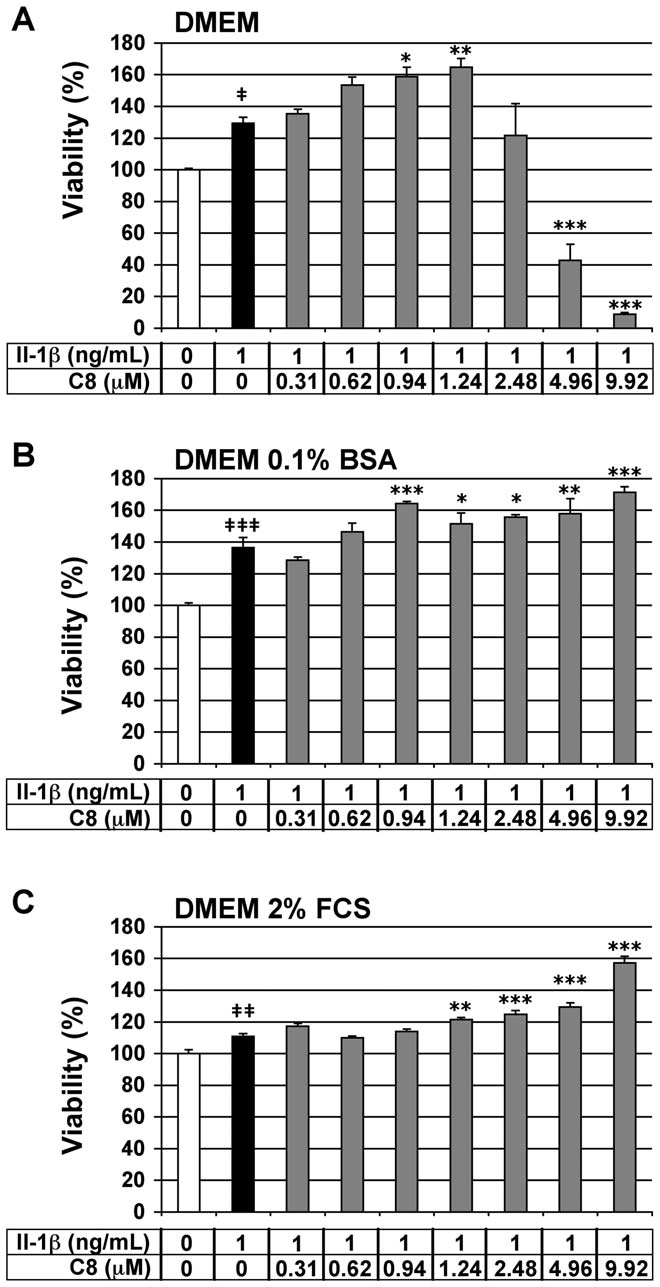
Evaluation of the cytotoxicity of C8 on articular
We assessed the viability of the chondrocytes by MTT assay to
evaluate the cytotoxic effects of C8 on these cells. Chondrocytes
were treated for 20 h with 1 ng/mL IL-1b alone or 1 h after the
addition of C8 at 0.31 to 9.92 mM, which corresponded to 0.5- to
5-fold the IC50 of C8 on human GIIAPLA2 activity (Table 2).
Three different culture medium compositions were used: DMEM
alone, or supplemented with 0.1% BSA or 2% FCS. IL-1b had no
cytotoxic effects as compared with the untreated control condition
*: calculated using the Rekker's hydrophobic fragmental constants
for the three culture media tested (Fig. 3). In chondrocytes cultured
in DMEM alone but with IL-1b, C8 had no cytotoxic effects at
PLoS ONE www.plosone.org
June 2010 Volume 5 Issue 6 e10914
PLA2 Inhibitors in Chondrocyte
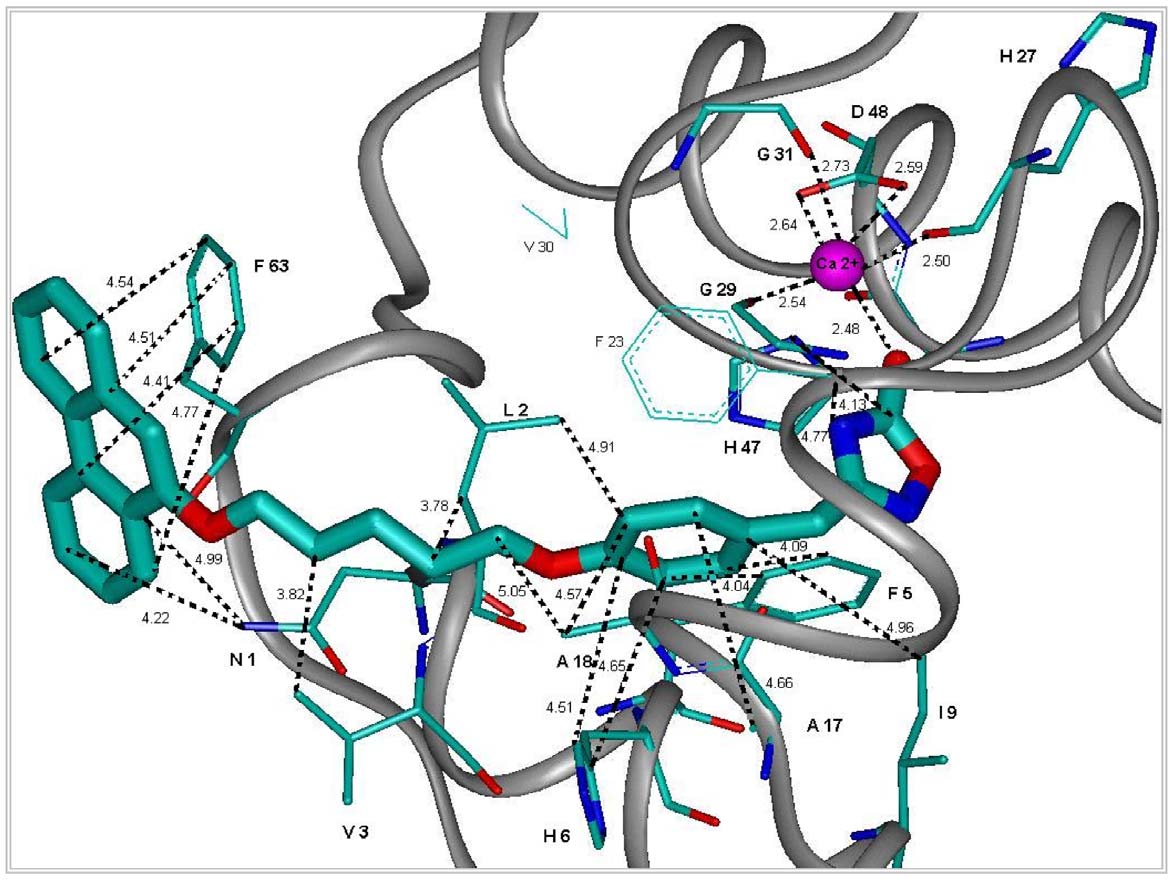
Figure 2. Representation of the most important interactions between C8 and the binding site of hGIIAPLA2 found from modeling.
The structure presented in the figure was derived from molecular dynamics using the Accelrys software and the cff91 force field (see SupportingInformation S1). In this presentation are shown in particular: a) the chelating of Ca(II) by the oxadiazolone moiety of C8, as well as Gly29 (G29), Gly31(G31) and Asp48 (D48) of hGIIAPLA2 with their main-chain or side-chain carbonyls; b) the phenoxy binding site [(Leu2 (L2), Phe5 (F5), His6 (H6) andAla18 (A18)]; and c) the binding site of 5-(phenanthren-9-yloxy)pentyl [Asn1 (N1), Val3 (V3) and Phe63 (F63)].
doi:10.1371/journal.pone.0010914.g002
0.31 to 2.48 mM (Fig. 3A). In chondrocytes cultured in DMEM
concentrations of 0.31-, 0.62-, 0.94-, and 1.24-mM, C8 decreased
with 0.1% BSA or 2% FCS and IL-1b, C8 had no cytotoxic effects
the production of PGE2 induced by IL-1b by 59-, 58-, 74-, and 80-%,
at 0.31 to 9.92 mM (Fig. 3B and 3C). Thus, we evaluated the anti-
respectively (Fig. 4A). In DMEM supplemented with 0.1% BSA, at
inflammatory activity of C8 in culture conditions from 0.31 to
concentrations of 0.31-, 0.62-, 0.94-, 1.24-, 2.48-, and 4.96-mM, C8
1.24 mM in DMEM alone and from 0.31 to 4.96 mM in DMEM
decreased the production of PGE2 induced by IL-1b by 31-, 30-, 45-,
supplemented with 0.1% BSA or 2% FCS.
43-, 81-, and 92-%, respectively (Fig. 4B). In DMEM supplementedwith 2% FCS, at concentrations of 0.31-, 0.62-, 0.94-, 1.24-, 2.48-,
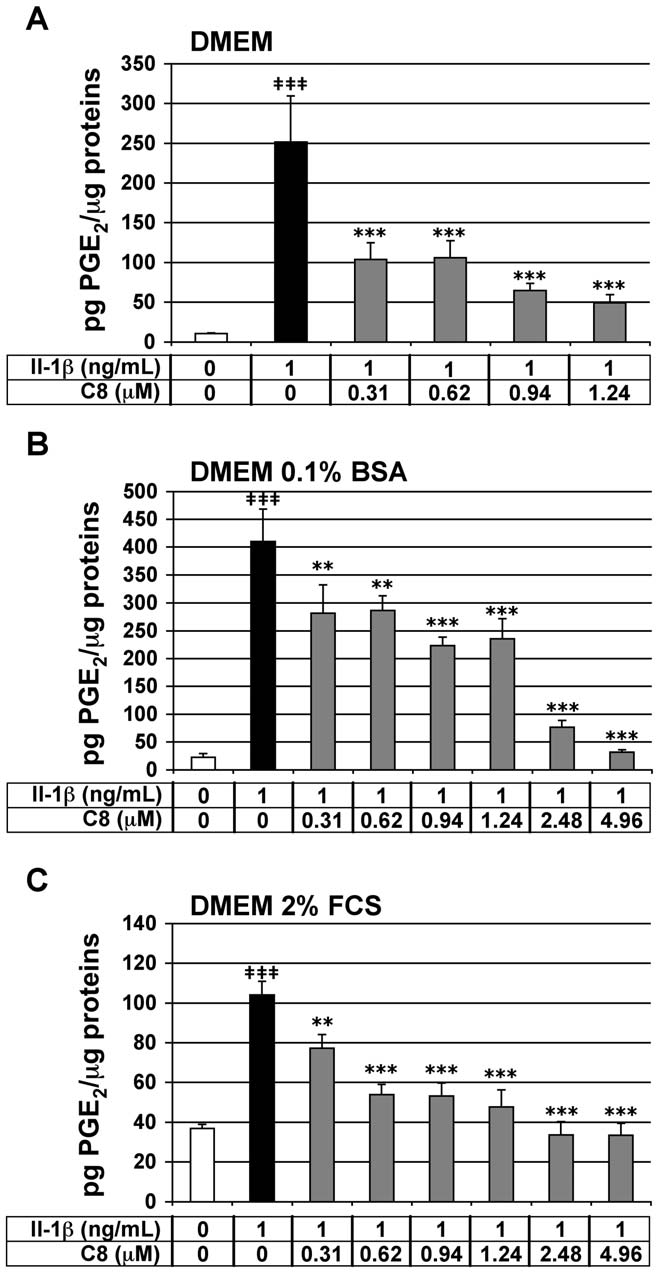
Effect of C8 on IL-1b-stimulated PGE2 secretion in
and 4.96-mM, C8 decreased the production of PGE2 induced by IL-
articular chondrocytes
1b by 26-, 48-, 49-, 54-, 68-, and 68-%, respectively (Fig. 4C). It is
We tested the effect of C8 on the IL-1b-stimulated secretion of
important to note that C8 down-regulated the IL-1b-stimulated
2 in chondrocytes. PGE2 synthesis takes place mainly in response
2 to the level of the control untreated condition at
to cell activation by IL-1b, and its generation accounts for many of
4.96 mM in DMEM supplemented with 0.1% BSA and at 2.48 and
the actions induced by this cytokine [27]. In vitro, IL-1b induces the
4.96 mM in DMEM supplemented with 2% FCS. The effect of C8
expression of COX-2 by chondrocytes, which results in increased
was then evaluated at the extreme concentrations (0.31- and 4.96-
mM) in DMEM supplemented with 2% FCS and containing
2 production [28]. PGE2 release thus represents a powerful IL-
decreasing (1-, 0.5-, and 0.25-ng/mL) IL-1b concentrations
2-dependent inflammatory marker in our cell model.
Chondrocytes were treated for 20 h with IL-1b alone or 1 h after the
(Table 3). The anti-IL-1b inhibitory effect of C8 at 0.31 mM
addition of C8. As expected, IL-1b significantly stimulated PGE
increases when IL-1b concentration decreases. The inhibitory effect
secretion by chondrocytes in the three different culture media: 23.3-,
of C8 at 4.96 mM does not change when IL-1b concentration
18.3- and 2.8-fold induction as compared with untreated control
decreases. This is probably due to the fact that at 4.96 mM, the
conditions, in DMEM alone or supplemented with 0.1% BSA or 2%
inhibitory effect of C8 on IL-1b-induced PGE2 production is
FCS, respectively (Fig. 4). In chondrocytes treated with IL-1b, C8
maximal. A parallel cellular test was performed on the compound C1
had a strong and statistically significant inhibitory effect on PGE2
whose IC50 is 5 mM (Table 2) and we observed that a 8 mM dose of
secretion at all concentrations tested: from 0.31 to 1.24 mM in
C1, corresponding to 1.6-fold the IC50 of C1 on human GIIAPLA2
DMEM alone or from 0.31 to 4.96 mM in DMEM supplemented
activity, does not decrease the stimulated PGE2 secretion by IL-1b at
with 0.1% BSA or 2% FCS (Fig. 4). In DMEM alone, at
1 ng/mL (data not shown). Thus, C8, but not C1, decreases the IL-
PLoS ONE www.plosone.org
June 2010 Volume 5 Issue 6 e10914
PLA2 Inhibitors in Chondrocyte
Figure 4. Effect of IL-1b and C8 on PGE
Figure 3. Effect of IL-1b and C8 on viability of articular
2 secretion by articular
chondrocytes. Chondrocytes were untreated (white bars) or treated
chondrocytes. Chondrocytes were untreated (white bars) or treated
for 20 h with IL-1b alone (black bars) or 1 h after the addition of C8
for 20 h with IL-1b alone (black bars) or 1 h after the addition of C8
(grey bars) in DMEM alone (A) or with 0.1% BSA (B) or with 2% FCS (C).
(grey bars) in DMEM alone (A) or supplemented with 0.1% BSA (B) or 2%
FCS (C). Cell viability was evaluated by an MTT-based test. Data
2 concentration was determined in conditioned culture medium,
and protein concentration was determined in whole-cell protein
represent the absorbance570nm - absorbance690nm and are expressed as
extracts. Data represent the ratio of PGE
relative arbitrary units, where the IL-1b-untreated group represents
2 concentration relative to
whole cell protein concentration (pg PGE
100%. Values are means 6 SEM (n = 3 to 8 independent determina-
2/mg proteins). Values are
means 6 SEM (n = 3 to 7 independent determinations). { P,0.05,
tions). { P,0.05, {{ P,0.01, {{{ P,0.001 between untreated and IL-1b-
{{ P,0.01, {{{ P,0.001 between untreated and IL-1b-treated groups;
treated groups; * P,0.05, ** P,0.01, *** P,0.001 between IL-1b- and
* P,0.05, ** P,0.01, *** P,0.001 between IL-1b- and IL-1b+C8-treated
1b-stimulated PGE2 secretion in a dose-dependent manner in the
inflammatory responses. In vitro, IL-1b induces the expression of
three culture medium compositions used.
inducible NO synthase (iNOS) by chondrocytes, and consequentlyan increase in NO production [29]. NO secretion, evaluated by
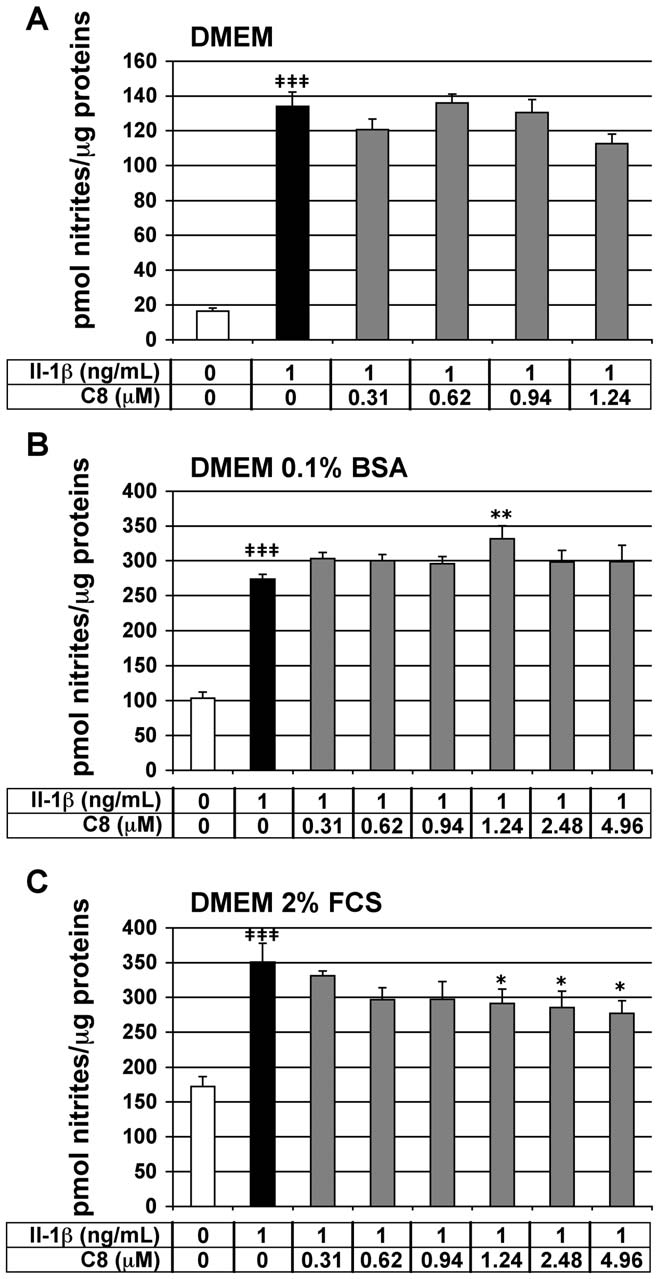
Effect of C8 on IL-1b-stimulated NO secretion in articular
nitrite concentration in the cell culture medium, represents a
reliable IL-1b-dependent and PLA2-independent inflammatory
We tested the effect of C8 on the IL-1b-stimulated secretion of
marker in our cell model. Chondrocytes were treated for 20 h with
NO in chondrocytes. NO is a mediator of immune and
IL-1b alone or 1 h after the addition of C8. As expected, IL-1b
PLoS ONE www.plosone.org
June 2010 Volume 5 Issue 6 e10914
PLA2 Inhibitors in Chondrocyte
Table 3. Effect of C8 on PGE2 secretion by articularchondrocytes incubated with different IL-1b concentrations.
C8 inhibitory effect (%inhibition)
Chondrocytes were untreated or treated for 20 h with IL-1b (1-, 0.5-, or 0.25-ng/mL) alone or 1 h after the addition of C8 (0.31- or 4.96-mM) in DMEM with 2%FCS. PGE2 concentration was determined in conditioned culture medium, andprotein concentration was determined in whole-cell protein extracts. The PGE2concentration was normalized relatively to whole cell protein concentration (pgPGE2/mg proteins). The means of PGE2 concentrations from 3 independentdeterminations were calculated and the anti-IL-1b inhibitory effect of C8 wasdetermined with the formula %inhibition = 10021006[(mean PGE2 in IL-1b+C8condition)/(mean PGE2 in IL-1b condition)]).
*P,0.05,***P,0.001 between IL-1b- and IL-1b+C8-treated groups.
doi:10.1371/journal.pone.0010914.t003
significantly stimulated nitrite secretion by chondrocytes in thethree different culture media: 8.1-, 2.6-, and 2.0-fold induction ascompared with the control conditions, in DMEM medium aloneor supplemented with 0.1% BSA or 2% FCS, respectively (Fig. 5).
C8 did not significantly inhibit the IL-1b-stimulated nitritesecretion in chondrocytes cultured in DMEM medium alone orsupplemented with 0.1% BSA (Fig. 5A, B). In DMEMsupplemented with 2% FCS, C8 did not inhibit the IL-1b-stimulated nitrite secretion at 0.31-, 0.62-, and 0.94-mM andslightly decreased by 17-, 19-, 21-% the IL-1b-induced nitriteproduction at 1.24-, 2.48-, and 4.96-mM, respectively (Fig. 5C).
Thus, C8 did not inhibit the IL-1b-stimulated NO secretion inDMEM alone or supplemented with 0.1% BSA and slightlyinhibited IL-1b-stimulated NO secretion in DMEM supplementedwith 2% FCS.
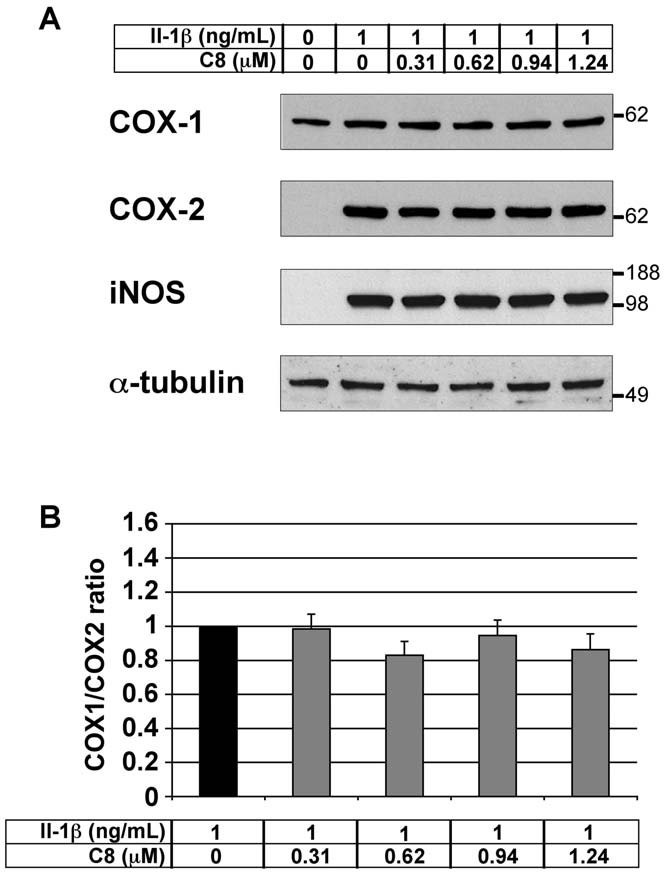
Effect of C8 on COX-1, COX-2, and iNOS protein levels inarticular chondrocytes
We evaluated the effect of C8 on COX-1, COX-2 and iNOS
protein levels in chondrocytes treated with IL-1b. Chondrocyteswere treated for 20 h with IL-1b alone or 1 h after the addition ofC8 (0.31-1.24 mM) in DMEM, and protein extracts wereexamined by western blot analysis. As expected, COX-1 protein
Figure 5. Effect of IL-1b and C8 on nitrite secretion by articular
was detectable but COX-2 and iNOS proteins were undetectable
chondrocytes. Chondrocytes were untreated (white bars) or treated
in untreated control conditions (Fig. 6A). Moreover, IL-1b
for 20 h with IL-1b alone (black bars) or 1 h after the addition of C8
treatment induced the expression of COX-2 and iNOS proteins
(grey bars) in DMEM alone (A) or supplemented with 0.1% BSA (B) or 2%
without affecting the level of COX-1 protein (Fig. 6A). In the
FCS (C). NO secretion was indirectly evaluated by determination of
presence of IL-1b, C8 did not alter the COX-1, COX-2 and
nitrite concentration in conditioned culture medium. Protein concen-tration was determined in whole-cell protein extracts. Data represent
iNOS protein levels (Fig. 6A). Consequently, the protein ratio of
the ratio of nitrite concentrations relative to whole-cell protein
COX-1 to COX-2 was not modified by C8 (Fig. 6B).
concentration (pmol nitrites/mg protein). Values are means 6 SEM(n = 4 to 7 independent determinations). { P,0.05, {{ P,0.01,
{{{ P,0.001 between untreated and IL-1b-treated groups; * P,0.05,** P,0.01, *** P,0.001 between IL-1b- and IL-1b+C8-treated groups.
Nonsteroidal anti-inflammatory drugs (NSAIDs), which inhibit
COX-1 and COX-2, and selective COX-2 inhibitors are currentlyused to reduce rheumatic symptoms. However, these drugs exhibit
ranked energy balances for the binding of these inhibitors to
gastrointestinal, renal, blood pressure and cardiovascular toxici-
GIIAPLA2. The energy balances (Table 1) taking into account
ties. To overcome this problem, GIIAPLA2 inhibitors could be
solvation effects show a correlation between dE2, the overall
developed to inhibit the production of COX substrates without
energy balance for binding, and the experimentally measured IC50
impairing the balance between COX-1 and COX-2. We designed
for our novel compounds C1-C8. This finding should lend
and synthesized 7 new oxadiazolone derivatives (C2 to C8)
additional credence to our previous results [20], despite the
derived from C1. Using molecular modeling, we computed and
approximations of the computational approach used in that study,
PLoS ONE www.plosone.org
June 2010 Volume 5 Issue 6 e10914
PLA2 Inhibitors in Chondrocyte
passing from compound C1 with an indole ring to C3 with abenzothiazole. Nevertheless, the high lipophilicity of C8 should bean interesting option for its prospective clinical development,considering the possibility of local administration (intra-articularinfiltration).
The toxicity and anti-inflammatory activity of C8 were
evaluated in rabbit articular chondrocytes in primary culture.
The toxicity of C8 was assessed by MTT, which allows anevaluation of the cell number and/or metabolic activity in cells.
C8 (from 0.31 to 9.92 mM) did not decrease cell viability in culturemedium supplemented with 0.1% BSA or 2% FCS but did (at 4.96and 9.92 mM) in culture medium alone. This observation isprobably due to the cells being weakened in the absence of BSA orFCS. We also observed, as expected, an increase in cell numberand/or metabolic activity in response to IL-1b. This effectincreases in the presence of C8, at non toxic doses, whatever theculture conditions. Thus, depending on the culture conditions orC8 doses, C8 increases or decreases cell number and/or metabolicactivity. Moreover, C8 from 0.31 mM inhibited IL-1b-inducedsecretion of PGE2 by chondrocytes, corresponding to half of theIC50 on human GIIAPLA2 activity evaluated in vitro by enzymaticassay. Therefore, C8 could be a potent anti-inflammatory drug invivo. However, C8 did not inhibit IL-1b-induced NO secretion bychondrocytes cultured in DMEM alone or supplemented with0.1% BSA and slightly inhibited IL-1b-stimulated NO secretion inDMEM supplemented with 2% FCS. These data suggest that theanti-inflammatory property of C8 in chondrocytes mainly dependson its capacity to inhibit PLA2 activity.
COX-1 is involved in normal physiological functions, whereas
COX-2 is involved in the inflammatory response. Anti-inflammatory drugs such as NSAIDs and selective COX-2
Figure 6. Effect of IL-1b and C8 on the COX-1, COX-2, and iNOS
inhibitors, used to treat rheumatic disease, have severe side
protein levels in articular chondrocytes. (A) Chondrocytes were
effects owing to impairment in the balance between COX-1 and
untreated or treated for 20 h with IL-1b alone or 1 h after the addition
COX-2 [4,5,6]. Interestingly, the present work shows that the
of C8 in DMEM. 20 mg aliquots of whole-cell protein extracts were
potent PLA2 inhibitor C8 decreases PGE2 production without
examined by western blot analysis with antibodies against COX-1, COX-2, and iNOS. a-tubulin immunodetection is shown as a control for
impairing this balance. Consequently, C8 could be a useful
protein loading and transfer. Results from one representative experi-
candidate in developing new anti-inflammatory drugs lacking
ment in five are shown. (B) Intensities of the COX-1 and COX-2
the side effects observed with NSAIDs and selective COX-2
immunoreactive bands evaluated by semi-quantitative scanning
densitometry. Data represent the COX-1/COX-2 protein ratio and are
In summary, we report on the design, synthesis and testing of 7
expressed as relative arbitrary units, where the IL-1b-treated group
C1 analogs that differ from C1 by indole substitution or by indole
represents 1. Values are means 6 SEM (n = 5 independent determina-tions). No significant differences were found between the groups.
replacement by other aromatic rings, the largest being phenan-
threne. Compounds C2-C8 show both inhibitory activity onsecreted/synovial GIIAPLA2 and selectivity as compared withGIBPLA2, a pancreatic enzyme involved in the digestion of dietary
which allows for only single-point computations of Poisson-
phospholipids. The order of interaction energies predicted by
Boltzmann solvation energies for the most stable minima of the
molecular modeling of these compounds is associated with their
molecular dynamics procedure. Our useful predictions made with
experimental IC50 values with GIIAPLA2 used as a target. The
the present simplified energy potential may be due to the very local
most promising compound is C8 in terms of computed energy
changes we made in the C1-C8 series. These bear on the series'
balance for binding GIIAPLA2 and experimental potency towards
sole terminal aromatic group and target a limited number of
GIIAPLA2, namely one order of magnitude larger than that of
amino acids, so that the accuracy of the energy potential may be
C1. In addition, C8 is endowed with anti-inflammatory activity in
sufficient. We plan to study such energy balances with the
articular chondrocytes by inhibiting IL-1b-stimulated PGE2
polarizable molecular mechanics procedure SIBFA [30], which,
secretion in these cells. Furthermore, it does not modify the ratio
along with the Langlet-Claverie methodology for Continuum
between the COX-1 and COX-2 isoenzymes. C8 is therefore an
solvation [31], was recently used to investigate the binding of
attractive candidate for anti-inflammatory therapy in joints.
inhibitors to metalloenzymes [32]. This study should also allow for
Experiments in animal models of rheumatic diseases are in
considering changes on other parts of the drugs as well.
progress in our laboratory.
One possible unfavorable feature of C8 is its enhanced
lipophilicity as compared with the other compounds. Nevertheless,
Materials and Methods
this feature did not prevent the pharmacological efficiency of C8in chondrocytes. Reduction in Log P could be anticipated by
Ethics Statements
replacing phenantrene with heterocyclic analogs and/or substitu-
Experimental protocols using rabbits complied with French
tion with hydrophilic groups. Such reductions were seen on
legislation on animal experimentation and were approved by
PLoS ONE www.plosone.org
June 2010 Volume 5 Issue 6 e10914
PLA2 Inhibitors in Chondrocyte
INSERM (Intitut National de la Sante´ et de la Recherche
Determination of PGE2 and nitrite concentrations in
Me´dicale)'s Committee for Animal Studies.
20 h after the addition of IL-1b to the chondrocytes, culture
Molecular modeling
media were collected, and aliquots were stored at 280uC until
Molecular modeling is described in Supporting Information S1.
PGE2 and nitrite quantification. PGE2 concentration in culturemedia was determined by use of an enzyme immunoassay (EIA) kit
Synthesis of oxadiazolone derivatives
(PGE2 EIA Kit-monoclonal; Cayman Chemical). Nitrite concen-
Synthesis of compounds C1-C8 is described in Supporting
tration was determined by a spectrophotometry method based on
Information S1.
the Griess reaction [36]. Briefly, 200 mL of culture medium orsodium nitrite (NaNO2, Merck) standard dilutions were mixed
In vitro PLA2 assay
with 100 mL Griess reagent [0.5% (w/v) sulphanilic acid (Merck),
Fatty-acid free BSA and pancreatic PLA
0.05% (w/v) N(1-naphtyl)ethylenediamine (Merck), 30% (v/v)
2 were from Sigma.
hGIIAPLA2 was prepared as previously described [33]. The
acetic acid, 1.5 N HCl] and incubated for 10 min at 50uC. The
fluorescent substrate for PLA2 assay, 1-hexadecanoyl-2-(10-
absorbance was measured at 540 nm.
pyrenedecanoyl)-sn-glycero-3-phosphoglycerol, ammonium salt(b-py-C10-PG) was from Molecular Probes (Eugene). PLA2
Preparation of whole-cell protein extracts, protein
activity was evaluated as previously described [34] with b-py-
quantification and western blot analysis
C10-PG used as a substrate (2 mM final concentration). In a
Proteins were extracted from the cultured cells by addition of
total volume of 1 mL, the standard reaction medium contained
lysis buffer [10 mM Tris (pH 7.4), 0.5% (v/v) NP40, 150 mM
50 mM Tris-HCl (pH 7.5), 500 mM NaCl, 1 mM EGTA,
NaCl, 1 mM PMSF, 0.1 mM Na3VO3, complete-EDTA-free
2 mM b-py-C10-PG, 0.1% fatty-acid free BSA and 6 ng/mL
protease inhibitor cocktail (Roche)]. Cell lysates were centrifuged
pancreatic PLA2 or 1 ng/mL hGIIAPLA2. The fluorescence
for 15 min at 14000 rpm at 4uC and supernatants were collected.
(lex = 342 nm and lem = 398 nm) of the enzymatic reaction
Protein concentrations were determined by the Bradford method
medium was recorded for 3 min with use of a spectrofluorimeter
[37] by use of the Protein Assay dye reagent (Bio-Rad). Protein
LS 50 (Perkin-Elmer) equipped with a Xenon lamp. The
extracts (20 mg) were size-separated by SDS-PAGE in a 10% (w/v)
reaction was initiated by the addition of CaCl2 (10 mM, final
polyacrylamide gel and electroblotted to a nitrocellulose mem-
concentration). The increase in fluorescence was continuously
brane. Equal protein loading and transfer was confirmed by
recorded for 1 min, and PLA2 activity was calculated as
staining the membrane with Ponceau Red [0.2% (w/v) in
previously described [34]. When used, the inhibitor was added
to the reaction medium after introduction of BSA. The activity
2O:acetic acid 99:1]. The membrane was sequentially incubated
with antibodies against COX-1 (1:200, Santa Cruz Biotechnolo-
is expressed in micromoles of fluorescent b-py-C10-PG hydro-
gy), COX-2 (1:500, Santa Cruz Biotechnology), iNOS (1:400, BD
lyzed per min. The standard error of the mean of three
Biosciences) or a-tubulin (1:100, Santa Cruz Biotechnology) and
independent experiments was less than 10%, which allows for
then with peroxidase-conjugated donkey anti-goat IgG (1:20000)
the determination of the IC50 values (concentration of inhibitors
or donkey anti-rabbit IgG (1:200, both Santa Cruz Biotechnology).
producing 50% inhibition) of each compound.
Immunocomplexes were detected by an enhanced chemilumines-cence kit (Amersham Bioscience). The membrane was stripped by
Isolation and culture of chondrocytes from rabbit
incubation in 0.2 M NaOH between successive immunodetec-
articular cartilage
tions. Semi-quantitative scanning densitometry involved use of the
Articular chondrocytes were isolated from 5-week-old Fauve de
ImageJ program (NIH, USA).
Bourgogne female rabbits (CPA, Orleans, France) and cultured atthe first passage in conditions avoiding cell dedifferentiation as
Statistical analysis
previously described [35]. Cells were cultured at 37uC in 12-well
Results are expressed as means 6 SEM for the number of
plates in Ham's F-12 medium containing 10% FCS, 20 IU/mL
experiments indicated. Statistical analysis involved use of the
penicillin, and 20 mg/mL streptomycin (all from Invitrogen) until
Kruskal-Wallis test, then the ANOVA Fisher's test. A P,0.05 was
nearly confluent. Then medium was replaced with DMEM
considered statistically significant.
(Invitrogen) containing 20 IU/mL penicillin, and 20 mg/mLstreptomycin and, if necessary, 0.1% fatty acid free BSA (Sigma)or 2% FCS. At this time the C8 compound dissolved in DMSO
Supporting Information
(Sigma) was added to the medium (the amount of DMSO was kept
Supporting Information S1
Materials and Methods in chem-
at 1% (v/v) in all the wells). 1 h after the addition of C8, IL-1b
istry and molecular modeling.
(PeproTech) was added to the medium. Consequently, chondro-
Found at: doi:10.1371/journal.pone.0010914.s001 (0.12 MB
cytes were incubated for 20 h with IL-1b and for 21 h with C8.
Evaluation of cell viability
At 18 h after the addition of IL-1b, 3-[4,5-dimethylthiazol-2-
yl]-2,5-diphenyl tetrazolium bromide (MTT; Sigma) was added to
We thank the computer center CINES-Montpellier for computer time and
the cell culture medium at 0.5 mg/mL. Cells were incubated 2
technical support.
more hours at 37uC. The medium was then removed, and DMSOwas added to dissolve the formazan crystals. The absorbance of
Author Contributions
the resulting solution was spectrophotometrically measured at 570
Conceived and designed the experiments: JEO CZD FPR FH NG CC.
and 690 nm (background). The value corresponding to absorban-
Performed the experiments: JEO NT SP LT FM AD EEH YS CC.
ce570nm - absorbance690nm was directly proportional to the number
Analyzed the data: JEO NT CZD SP FPR FM NG CC. Wrote the paper:
and activity of the viable cells.
JEO NT CZD FPR FM NG CC.
PLoS ONE www.plosone.org
June 2010 Volume 5 Issue 6 e10914
PLA2 Inhibitors in Chondrocyte
1. Murakami M, Kudo I (2004) Recent advances in molecular biology and
physiology of the prostaglandin E2-biosynthetic pathway. Prog Lipid Res 43:
zol-5-one (PMS1062) derivatives specific for group II enzyme. Bioorg Med
Chem 13: 1989–2007.
2. Dennis EA (1994) Diversity of group types, regulation, and function of
20. Plocki S, Aoun D, Ahamada-Himidi A, Tavare s-Camarinha F, Dong CZ, et al.
phospholipase A2. J Biol Chem 269: 13057–13060.
(2005) Molecular modeling, design, and synthesis of less lipophilic derivatives of
3. McCoy JM, Wicks JR, Audoly LP (2002) The role of prostaglandin E2 receptors
3-(4-tetradecyloxybenzyl)-4H-1,2,4-oxadiazol-5-one (PMS1062) specific for
in the pathogenesis of rheumatoid arthritis. J Clin Invest 110: 651–658.
group II enzyme. Eur J Org Chem 2005: 2747–2757.
4. Martel-Pelletier J, Pelletier JP, Fahmi H (2003) Cyclooxygenase-2 and
21. Touaibia M, Djimde A, Cao F, Boilard E, Bezzine S, et al. (2007) Inhibition of
prostaglandins in articular tissues. Semin Arthritis Rheum 33: 155–167.
secreted phospholipase A2. 4-glycerol derivatives of 4,5-dihydro-3-(4-tetradecy-
5. Chen YF, Jobanputra P, Barton P, Bryan S, Fry-Smith A, et al. (2008)
loxybenzyl)-1,2,4-4H-oxadiazol-5-one with broad activities. J Med Chem 50:
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac,
meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for
22. Dehaen W, Hassner A (1991) Cycloadditions. 45. Annulation of heterocycles via
osteoarthritis and rheumatoid arthritis: a systematic review and economic
intramolecular nitrile oxide-heterocycle cycloaddition reaction. J Org Chem 56:
evaluation. Health Technol Assess 12: 1–278, iii.
6. Solomon DH, Avorn J, Sturmer T, Glynn RJ, Mogun H, et al. (2006)
23. Carey MC, Small DM, Bliss CM (1983) Lipid digestion and absorption. Annu
Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflam-
Rev Physiol 45: 651–677.
matory drugs: high-risk subgroups and time course of risk. Arthritis Rheum 54:
24. Rekker RF, De Kort HM (1979) The hydrophobic fragmental constant; an
estimation to a 1000 data point set. Eur J Med Chem 14: 479–488.
7. Nevalainen TJ, Haapamaki MM, Gronroos JM (2000) Roles of secretory
phospholipases A(2) in inflammatory diseases and trauma. Biochim Biophys Acta
25. Fernandes JC, Martel-Pelletier J, Pelletier JP (2002) The role of cytokines in
1488: 83–90.
osteoarthritis pathophysiology. Biorheology 39: 237–246.
8. Hara S, Kudo I, Chang HW, Matsuta K, Miyamoto T, et al. (1989) Purification
26. Choy EH, Panayi GS (2001) Cytokine pathways and joint inflammation in
and characterization of extracellular phospholipase A2 from human synovial
rheumatoid arthritis. N Engl J Med 344: 907–916.
fluid in rheumatoid arthritis. J Biochem 105: 395–399.
27. Goetzl EJ, An S, Smith WL (1995) Specificity of expression and effects of
9. Kramer RM, Hession C, Johansen B, Hayes G, McGray P, et al. (1989)
eicosanoid mediators in normal physiology and human diseases. FASEB J 9:
Structure and properties of a human non-pancreatic phospholipase A2. J Biol
Chem 264: 5768–5775.
28. Amin AR, Attur M, Patel RN, Thakker GD, Marshall PJ, et al. (1997)
10. Seilhamer JJ, Plant S, Pruzanski W, Schilling J, Stefanski E, et al. (1989) Multiple
Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected
forms of phospholipase A2 in arthritic synovial fluid. J Biochem 106: 38–42.
cartilage. Influence of nitric oxide. J Clin Invest 99: 1231–1237.
11. Lin MK, Farewell V, Vadas P, Bookman AA, Keystone EC, et al. (1996)
29. Sakurai H, Kohsaka H, Liu MF, Higashiyama H, Hirata Y, et al. (1995) Nitric
Secretory phospholipase A2 as an index of disease activity in rheumatoid
oxide production and inducible nitric oxide synthase expression in inflammatory
arthritis. Prospective double blind study of 212 patients. J Rheumatol 23:
arthritides. J Clin Invest 96: 2357–2363.
30. Gresh N, Cisneros GA, Darden TA, Piquemal JP (2007) Anisotropic, Polarizable
12. Bomalaski JS, Lawton P, Browning JL (1991) Human extracellular recombinant
Molecular Mechanics Studies of Inter- and Intramolecular Interactions and
phospholipase A2 induces an inflammatory response in rabbit joints. J Immunol
Ligand-Macromolecule Complexes. A Bottom-Up Strategy. J Chem Theory
146: 3904–3910.
Comput 3: 1960–1986.
13. Murakami M, Kudo I, Nakamura H, Yokoyama Y, Mori H, et al. (1990)
31. Langlet J, Claverie P, Caillet J, Pullman A (1988) Improvements of the
Exacerbation of rat adjuvant arthritis by intradermal injection of purified
continuum model. 1. Application to the calculation of the vaporization
mammalian 14-kDa group II phospholipase A2. FEBS Lett 268: 113–116.
thermodynamic quantities of nonassociated liquids. J Phys Chem 92:
14. Bradley JD, Dmitrienko AA, Kivitz AJ, Gluck OS, Weaver AL, et al. (2005) A
randomized, double-blinded, placebo-controlled clinical trial of LY333013, a
32. Roux C, Gresh N, Perera LE, Piquemal JP, Salmon L (2007) Binding of 5-
selective inhibitor of group II secretory phospholipase A2, in the treatment of
phospho-D-arabinonohydroxamate and 5-phospho-D-arabinonate inhibitors to
rheumatoid arthritis. J Rheumatol 32: 417–423.
zinc phosphomannose isomerase from Candida albicans studied by polarizable
15. Zeiher BG, Steingrub J, Laterre PF, Dmitrienko A, Fukiishi Y, et al. (2005)
molecular mechanics and quantum mechanics. J Comput Chem 28: 938–957.
LY315920NA/S-5920, a selective inhibitor of group IIA secretory phospholi-
33. Dong CZ, Romieu A, Mounier CM, Heymans F, Roques BP, et al. (2002) Total
pase A2, fails to improve clinical outcome for patients with severe sepsis. Crit
direct chemical synthesis and biological activities of human group IIA secretory
Care Med 33: 1741–1748.
phospholipase A2. Biochem J 365: 505–511.
16. Fraser H, Hislop C, Christie RM, Rick HL, Reidy CA, et al. (2009) Varespladib
34. Radvanyi F, Jordan L, Russo-Marie F, Bon C (1989) A sensitive and continuous
(A-002), a Secretory Phospholipase A2 Inhibitor, Reduces Atherosclerosis and
fluorometric assay for phospholipase A2 using pyrene-labeled phospholipids in
Aneurysm Formation in ApoE2/2 Mice. J Cardiovasc Pharmacol.
17. Assogba L, Ahamada-Himidi A, Habich NM, Aoun D, Boukli L, et al. (2005)
the presence of serum albumin. Anal Biochem 177: 103–109.
Inhibition of secretory phospholipase A2. 1-design, synthesis and structure-
35. Francois M, Richette P, Tsagris L, Raymondjean M, Fulchignoni-Lataud MC,
activity relationship studies starting from 4-tetradecyloxybenzamidine to obtain
et al. (2004) Peroxisome proliferator-activated receptor-gamma down-regulates
specific inhibitors of group II sPLA2s. Eur J Med Chem 40: 850–861.
chondrocyte matrix metalloproteinase-1 via a novel composite element. J Biol
18. Boukli L, Touaibia M, Meddad-Belhabich N, Djimde A, Park CH, et al. (2008)
Chem 279: 28411–28418.
Design of new potent and selective secretory phospholipase A2 inhibitors. Part 5:
36. Evans CH, Watkins SC, Stefanovic-Racic M (1996) Nitric oxide and cartilage
synthesis and biological activity of 1-alkyl-4-[4,5-dihydro-1,2,4-[4H]-oxadiazol-
metabolism. Methods Enzymol 269: 75–88.
5-one-3-ylmethylbenz-49-yl(oyl)] piperazines. Bioorg Med Chem 16: 1242–1253.
37. Bradford MM (1976) A rapid and sensitive method for the quantitation of
19. Dong CZ, Ahamada-Himidi A, Plocki S, Aoun D, Touaibia M, et al. (2005)
microgram quantities of protein utilizing the principle of protein-dye binding.
Inhibition of secretory phospholipase A2. 2-Synthesis and structure-activity
Anal Biochem 72: 248–254.
PLoS ONE www.plosone.org
June 2010 Volume 5 Issue 6 e10914
Source: http://seralpar.aphp.fr/sites/default/files/article2.pdf
Oncogene (2016), 1–11© 2016 Macmillan Publishers Limited All rights reserved 0950-9232/16 ORIGINAL ARTICLEEWS-FLI1-mediated suppression of the RAS-antagonistSprouty 1 (SPRY1) confers aggressiveness to Ewing sarcoma F Cidre-Aranaz1, TGP Grünewald2,3, D Surdez2, L García-García1, J Carlos Lázaro1, T Kirchner3, L González-González1, A Sastre4,P García-Miguel4, SE López-Pérez1, S Monzón1,5, O Delattre2 and J Alonso1
Chapter 10 Artificial Insemination in Poultry M.R. Bakst and J.S. Dymond Additional information is available at the end of the chapter Artificial insemination (AI) is the manual transfer of semen into the female's vagina. Basicallyit is a two step procedure: first, collecting semen from the male [1]; and second, inseminatingthe semen into the female [2]. In poultry, depending on the objectives and goals of the farm orlaboratory, there may be intervening steps such as semen dilution, storage, and evaluation.





