Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
The profile of mephedrone on human monoamine transporters differs from 3,4-methylenedioxymethamphetamine primarily by lower potency at the vesicular monoamine transporter


Contents lists available at
European Journal of Pharmacology
journal homepage:
Neuropharmacology and analgesia
The profile of mephedrone on human monoamine transporters differsfrom 3,4-methylenedioxymethamphetamine primarily by lowerpotency at the vesicular monoamine transporter
Christian Pifl Harald Reither, Oleh Hornykiewicz
Center for Brain Research, Medical University of Vienna, Spitalgasse 4, A-1090 Vienna, Austria
Mephedrone (4-methylmethcathinone, MMC) and 3,4-methylenedioxymethamphetamine (MDMA) are
Received 8 January 2015
constituents of popular party drugs with psychoactive effects. Structurally they are amphetamine-like
Received in revised form
substances with monoamine neurotransmitter enhancing actions. We therefore compared their effects
on the human monoamine transporters using human cell lines stably expressing the human noradrena-
Accepted 4 March 2015
line, dopamine and serotonin transporter (NET, DAT and SERT); preparations of synaptic vesicles from
Available online 11 March 2015
human striatum in uptake experiments; and a superfusion system where releasing effects can be reliably
measured. MMC and MDMA were equally potent in inhibiting noradrenaline uptake at NET, with IC50
values of 1.9 and 2.1 mM, respectively. Compared to their NET inhibition potency, both drugs were weaker
Dopamine transporter
uptake inhibitors at DAT and SERT, with MMC being more potent than MDMA at DAT (IC
12.6 mM) and less potent than MDMA at SERT (IC
Serotonin transporter
50: 19.3 vs 7.6 mM). MMC and MDMA both induced
Vesicular monoamine transporter
[3H]1-methyl-4-phenylpyridinium-release from NET-, DAT or
Synaptic vesicles
expressing cells which was clearly transporter-mediated release as demonstrated by the selectiveinhibitory effects of nmolar to low mmolar concentrations of desipramine, GBR 12909 and fluoxetine,respectively. MMC and MDMA differed most in their inhibition of [3H]dopamine uptake by synapticvesicles from human striatum with MDMA being 10-fold more potent than MMC (IC50: 20 vs 223 mM)and their ability to release [3H]dopamine from human vesicular monoamine transporter expressingSH-SY5Y neuroblastoma cells in which MDMA seems to have a stronger effect. Our findings give amolecular explanation to the lower long-term neurotoxicity of MMC compared to MDMA.
& 2015 Elsevier B.V. All rights reserved.
nerve endings which is well supported by studies using in vivomicrodialysis (
Mephedrone (4-Methylmethcathinone, MMC) and ecstasy (3,4-
Similar to high doses of other releasing drugs repeated MDMA
methylenedioxymethamphetamine, MDMA) are both designer
administration induced a selective neurotoxic loss of 5-HT in
drugs used for illicit recreational consumption due to their
forebrain regions of the rat and damage to dopamine nerve
psychoactive effects. They are structurally related to amphetamine
terminals of the mouse (for review, see By
and thus act as psychostimulants with a risk of addiction. In fact,
contrast, the majority of studies did not find neurotoxic loss of
MMC and MDMA inhibited the uptake of tritiated dopamine,
parameters of serotoninergic or dopaminergic nerve terminals
after binge-type dosing schedule of MMC in rats (
and induced release from rat brain
synaptosomes preloaded with tritiated substrates indicating their
status as substrates of the plasmalemmal monoamine transporters
Finally, MMC did not activate glia or increase glial fibrillary
These findings suggest a strong mono-
acidic protein (),
amine releasing effect of MMC and MDMA on monoaminergic
whereas the latter marker of neurodegeneration was increased byMDMA
A connection between longterm neurotoxicity of amphetamine-
related drugs and interaction with mechanisms regulating the
intraneuronal neurotransmitter concentrations has been established.
Correspondence to: Center for Brain Research, Medical University of Vienna,
Amphetamine-induced redistribution of dopamine from synaptic
Spitalgasse 4, A-1090 Vienna, Austria. Tel.: þ43 1 4016034080; fax: þ43 1 40160934053.
E-mail address: Pifl).
vesicles to the cytosol followed by metabolism accompanied by the
0014-2999/& 2015 Elsevier B.V. All rights reserved.
C. Pifl et al. / European Journal of Pharmacology 755 (2015) 119–126
production of radical oxygen species has been hypothesized to
Nonspecific uptake was determined in the presence of 10 mM
trigger nerve terminal loss
mazindol (DAT- and NET cells) or 3 mM fluoxetine (SERT-cells).
. The vesicular monoamine transporter 2/
The radioactivity remaining in each well was determined by
SLC18A2 (VMAT2) is a significant regulator of intraneuronal mono-
incubating with 0.4 ml 1% sodium dodecyl sulfate and transferring
amine concentrations and its impairment has recently been impli-
this solution into scintillation vials containing 3 ml scintillation
cated in the dopaminergic degeneration in idiopathic Parkinson's
cocktail (Ultima Gold MV, Packard, Dovners Grove, IL). The uptake
disease ). Since meth/amphetamine users have been
buffer consisted of (mmol/l): 4 Tris–HCl; 6.25 4-(2-hydroxyethyl)-
shown to have an above-normal risk of developing Parkinson's
1-piperazineethanesulfonic acid (HEPES); 120 NaCl; 5 KCl;
disease ) and even for human MDMA users
1.2 CaCl2; 1.2 MgSO4; 5.6 D-glucose; 0.5 ascorbic acid; pH 7.1.
there are hints for a neurotoxic potential (for review, see ), we reasoned that it might be interesting to compare thepharmacology of MMC and MDMA at the human monoamine
2.4. Superfusion experiments
transporters in uptake and superfusion experiments using trans-fected cells loaded with the metabolically inert transporter substrate
Cells were seeded onto poly-D-lysine-coated 5-mm-diameter
[3H]1-methyl-4-phenylpyridinium (MPPþ ) which allows a clear
glass cover slips in 96-well tissue culture plates (7 � 104 SK-N-MC
distinction between transport-inhibiting and carrier-mediated out-
cells/well, 3 � 104 HEK cells/well and 5 � 104 SH-SY5Y cells/well).
ward transport activity of drugs ) and include
On the following morning SK-N-MC and HEK cells were loaded
experiments on the human vesicular monoamine transporter by
with [3H] MPP þ in uptake buffer at 37 1C for 20 min: DAT-cells,
taking advantage of our recently reported preparation of function-
6 mM with 0.2 Ci/mmol; NET-cells, 0.1 mM with 29 Ci/mmol; SERT-
ally active synaptic vesicles from autopsied human striatum
cells, 10 mM with 0.4 Ci/mmol. SH-SY5Y cells were washed and
incubated with serum-free medium one day after seeding and onefurther day later loaded in uptake buffer with 0.2 mM [3H]dopa-mine with 40 Ci/mmol at 37 1C for 45 min. After loading, coverslips
2. Materials and methods
were transferred to small chambers and superfused (25 1C, 1.0 ml/min) with the uptake buffer mentioned above in a setup as
described previously (Aftera washout period of 45 min to establish a stable efflux of radio-
Media, sera and other tissue culture reagents were obtained
activity the experiment was started with the collection of 4-min
from Life Technologies (Vienna, Austria). [7-3H]dopamine (22 Ci/
fractions. At the end of the experiment cells were lysed by
mmol), levo-[7-3H]noradrenaline (15 Ci/mmol), 5-[1,2-3H[N])-
superfusion with 4 ml 1% SDS. The radioactivity in the superfusate
hydroxytryptamine (21 Ci/mmol) were obtained from New Eng-
fractions and the SDS-lysates was determined by liquid scintilla-
land Nuclear GmbH (Vienna, Austria). [3H]1-Methyl-4-phenylpyr-
tion counting. Release of tritium was expressed as fractional rate, i.
idinium (MPP þ ; 85 Ci/mmol) was supplied by American Radio-
e. the radioactivity released during a fraction was expressed as
labeled Chemicals (St. Louis, MO), desipramine from Ciba-Geigy
percentage of the total radioactivity present in the cells at the
Limited (Stein, Switzerland), fluoxetine from Eli Lilly and Company
beginning of that fraction.
Limited (Dublin, Ireland) and mazindol from Sandoz GmbH(Vienna, Austria). The other chemicals were purchased fromSigma-Aldrich or Merck.
2.5. Preparation of synaptic vesicles
2.2. Cell culture
Samples of about 600 mg of human striatal tissue from autop-
SK-N-MC, SH-SY5Y (human neuroblastoma) and human embryo-
sied frozen half brains of control subjects without evidence in
nic kidney (HEK) 293 cells were grown in minimum essential medium
their records of any neurological or psychiatric disorder derived
with Earle's salts and L-glutamine, 10% heat inactivated fetal bovine
from our recent study on vesicular dopamine storage in Parkin-
serum and 50 mg/l gentamicin. Cells were grown in 100 or 60 mm
son's disease ) were homogenized in ice-cold 0.3 M
diameter tissue culture dishes (polystyrene, Falcon) at 37 1C under an
sucrose containing 25 mM Tris (pH 7.4) and 10 mM pargyline in a
atmosphere of 5% CO
glass Teflon Potter-type homogenizer and vesicles in the super-
2/95% air. The human dopamine transporter/
SLC6A3 (DAT) or noradrenaline transporter/SLC6A2 (NET) cDNA was
natants of a P2-pellets of a crude synaptosomal preparation and in
stably expressed in SK-N-MC cells using methods as described
H2O-lysates of P2-pellets were combined as described recently
previously (The human serotonin transporter/SLC6A4
and stored at �80 1C until uptake analysis.
(SERT) was similarly expressed in HEK 293 cells using the vector pRc/CMV and selection by 1 g/l G418 in the medium and the human DATin SH-SY5Y cells also using the vector pRc/CMV and selection by 0.6 g/
2.6. Vesicular uptake
Uptake was performed in a total volume of 1.5 ml 0.13 M
2.3. Cellular uptake experiments
potassium phosphate buffer pH 7.4 containing 2 mM MgATP,0.1 mM of [3H]dopamine and various concentrations of the uptake
The cells were seeded in poly-D-lysine-coated 24-well plates
interfering drugs. Unspecific uptake was determined in the pre-
(2 � 105 SK-N-MC or 1 � 105 HEK cells/well) and, one day later,
sence of 1 mM reserpine. Transport was initiated by placing the
each well was washed with 0.5 ml uptake buffer and incubated
tubes in a 30 1C water bath and adding 0.5 ml vesicle suspension
with 0.5 ml buffer containing various concentrations of the drugs.
(obtained from about 20 to 25 mg human tissue) for 4 min. Uptake
Uptake was started by addition of [3H]dopamine, [3H]noradrena-
was terminated by the addition of 2.5 ml ice-cold potassium
line or [3H]serotonin at a final concentration of 1 mM (specific
phosphate buffer, immediate filtration under vacuum onto What-
activity 0.14 Ci/mmol) after 2 min of preincubation. After incuba-
man GF/B filter paper pre-soaked in 1% polyethylenimine, using a
tion for 2.5 min at 25 1C, it was stopped by aspirating the uptake
Brandel harvester. The filters were washed twice with additional
buffer and washing each well twice with 1 ml icecold buffer.
3 ml of cold potassium phosphate buffer.
C. Pifl et al. / European Journal of Pharmacology 755 (2015) 119–126
2.7. Data analysis
Uptake data of each separate experiment were fitted to the
equation f¼minþ(max�min)/(1þx/IC50), "min" being non-specificuptake, "max" the uptake in the absence of inhibiting drug, x themolar concentration of the inhibiting drug, and IC50 the drugconcentration that inhibits 50% of specific uptake by the non-linear curve-fitting computer program SigmaPlot (Systat Software,Inc., CA, U.S.A). min was constrained to nonspecific uptake. Allresults were expressed as means7S.E.M.
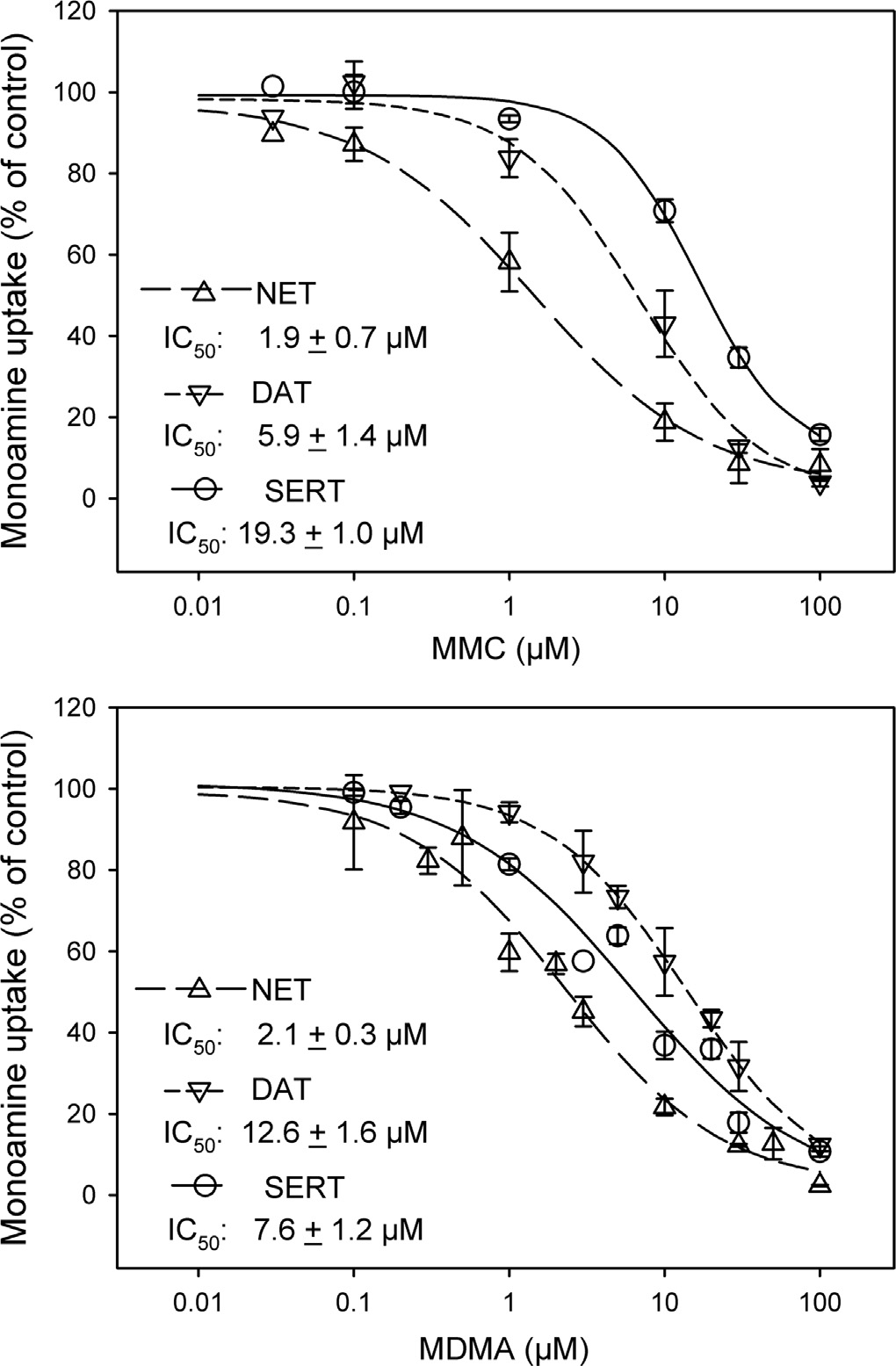
3.1. Inhibition of NET-, DAT- and SERT-mediated uptake by MMCand MDMA
In cells stably expressing the human NET, DAT or SERT, MMC
and MDMA concentration-dependently inhibited transport oftritiated noradrenaline, dopamine or serotonin, respectively). Whereas both MMC and MDMA displayed the highestand about equal potency at the NET (IC50, MMC: 1.970.7 mM,n ¼5; MDMA: 2.170.3 mM, n¼5), the rank order of potency wasdifferent at the DAT and SERT with MMC being clearly more potentthan MDMA at the DAT (IC50, MMC: 5.971.4 mM, MDMA:12.6 71.6 mM, n¼4) the opposite rank order of potency at theSERT (IC50, MMC: 19.371.0 mM, n¼5; MDMA: 7.671.2 mM, n¼5).
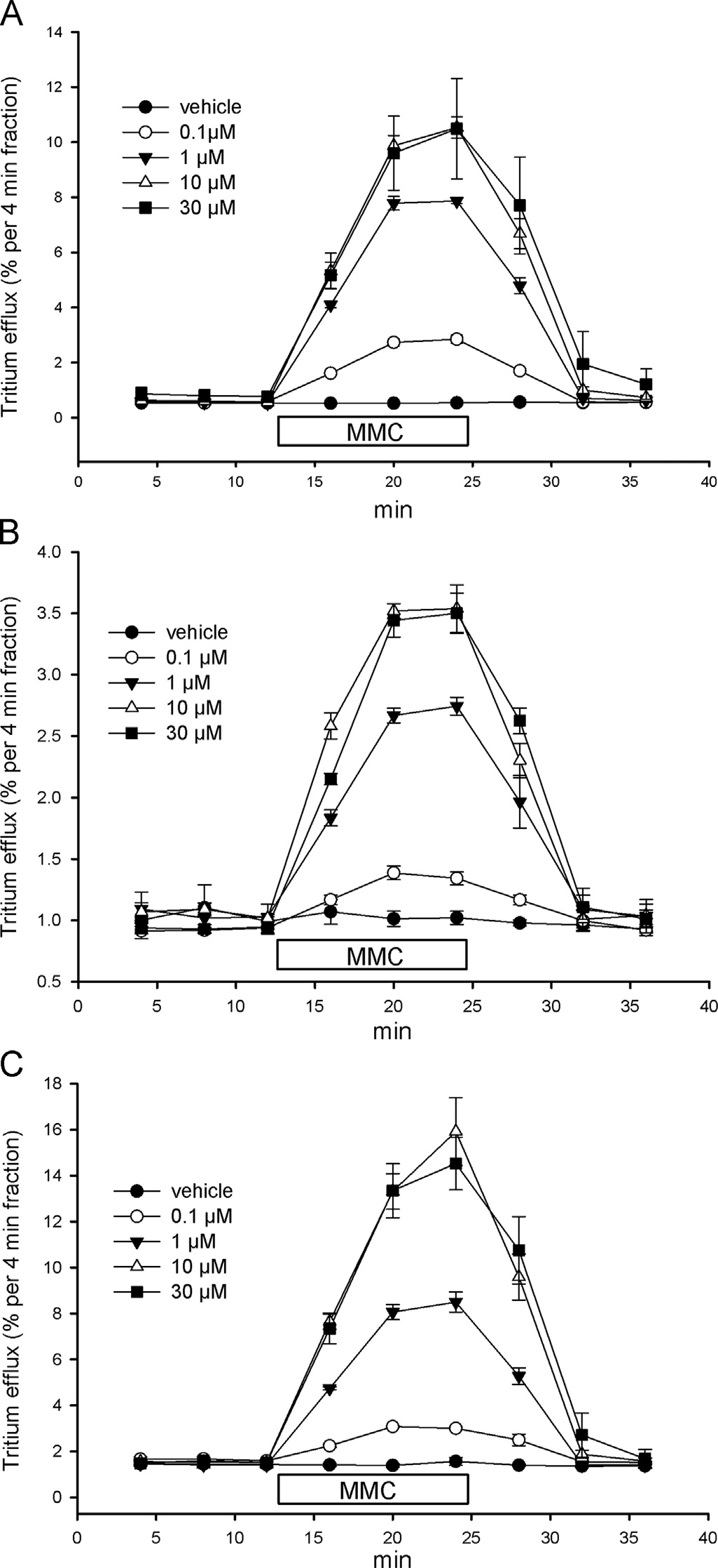
3.2. Stimulation of NET, DAT and SERT-mediated release by MMCand MDMA
In cells expressing the human NET, DAT and SERT preloaded
with the metabolically inert transporter substrate [3H]MPP þ ,grown on cover-slips and superfused in microchambers, MMCand MDMA added to the superfusion buffer concentration-dependently increased radioactivity in the fractionated perfusatesin a completely reversible manner (respectively). At
Fig. 1. Effects of MMC and MDMA on uptake by the human NET, DAT and SERT.
all transporters, a releasing effect of MMC was clearly discernable
Concentration-inhibition curves on [3H]noradrenaline, [3H]dopamine and [3H]serotonin
at 0.1 mM and the maximum effect leveled off at concentrations
uptake in SK-N-MC or HEK293 cells stably expressing the NET (triangles up, long dash),
between 10 and 30 mM. A releasing effect of MDMA was obvious at
DAT (triangles down, short dash) or SERT (circles, solid). The cells were incubated in 24-
0.1 mM at the NET and SERT, but the effect of 0.1 mM MDMA was
well plates for 2.5 min at 25 1C with 1 mM of the tritiated monoamines in the absence(control) or presence of MMC (A) or MDMA (B) at the concentrations indicated, and
not different from vehicle at the DAT; again there was no further
uptake was determined as described in Materials and Methods. Symbols represent
increasing effect between 10 and 30 mM at all transporters with
means7S.E.M. of three to five independent experiments, each in duplicates. The data of
even a slightly lower maximum of MDMA at 30 mM than at 10 mM
each experiment were fitted by nonlinear regression and the mean of the IC50
in SERT expressing cells.
values7S.E.M. are inserted into the panels.
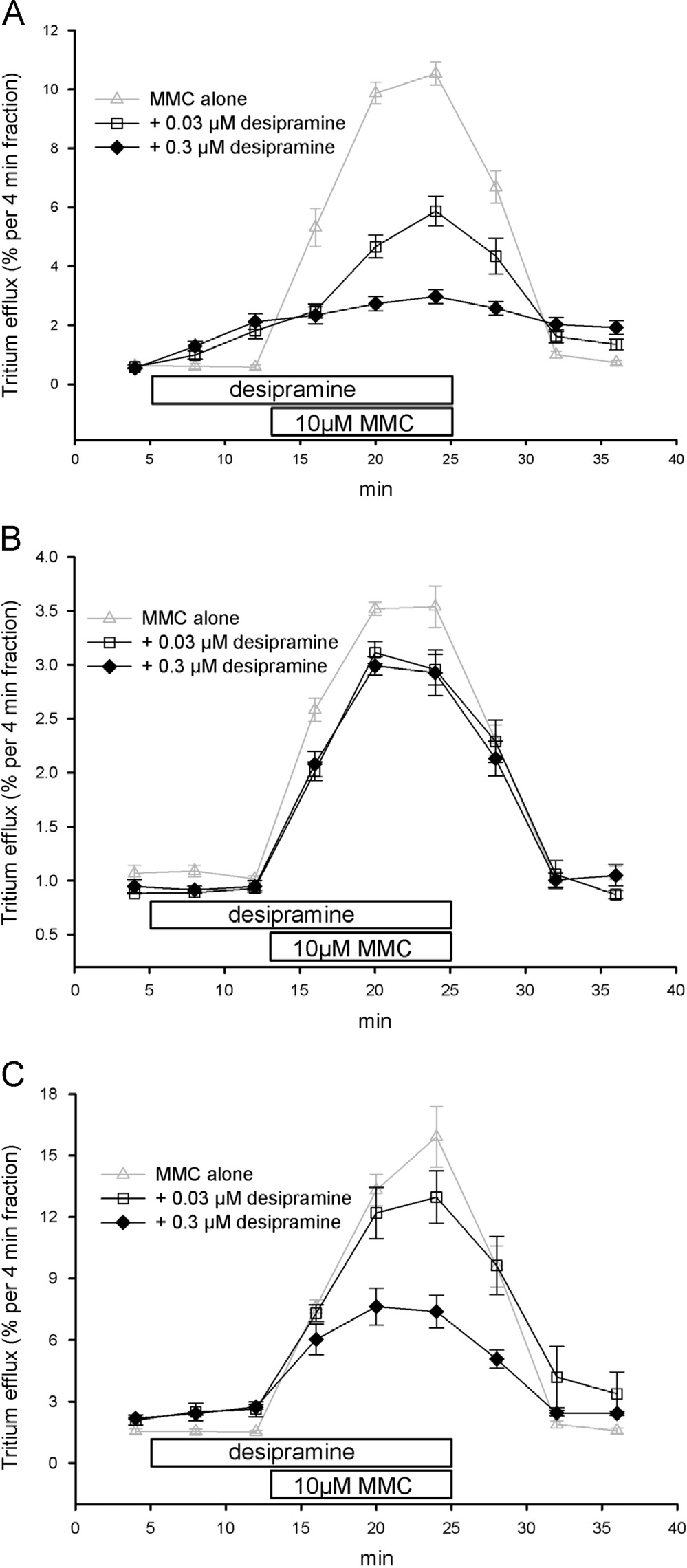
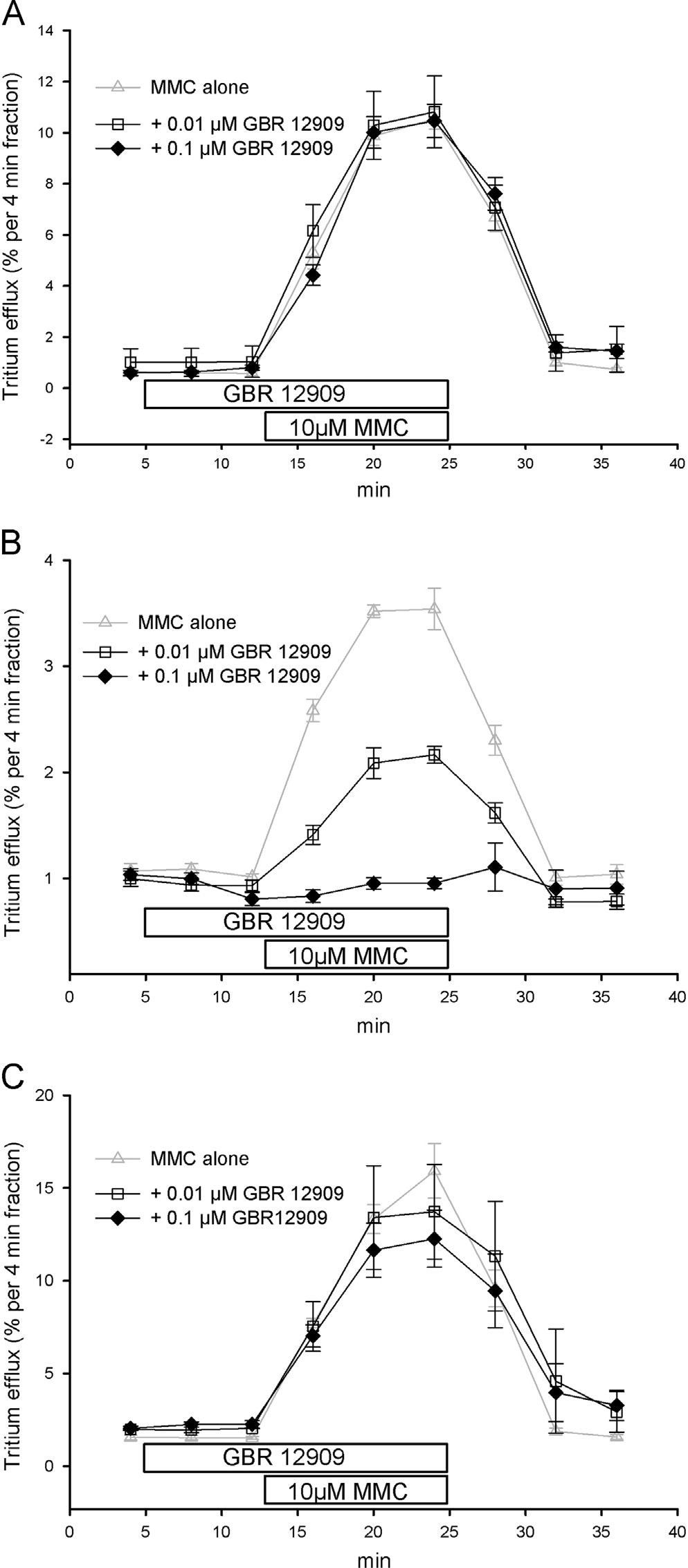
3.3. Blocking action of selective inhibitors of monoamine
0.1 or 1 mM, was without effect on the efflux stimulatory effect
transporters on MMC-induced release
of 10 mM MMC in NET cells had a minor but notconcentration-dependent inhibitory effect in DAT cells (B),
The highly potent NET blocker desipramine, added at the
but completely blocked the releasing effect of MMC at both
concentration of 0.03 or 0.3 mM to the superfusion buffer at min
concentrations on SERT cells C).
8, concentration-dependently blocked the releasing action of10 mM MMC in NET-cells with a clear inhibitory action already to
3.4. Inhibition of dopamine uptake into synaptic vesicles
be seen at 0.03 mM A). There was no effect of 0.03 or 0.3 mM
from human striatum by MMC and MDMA
desipramine on the releasing effect of 10 mM MMC in DAT cellsB) whereas in SERT cells 0.03 mM desipramine was without
Uptake of [3H]dopamine into synaptic vesicles prepared from
effect and 0.3 mM desipramine partially prevented the effect of
human caudate or putamen shows all features of uptake driven by
the VMAT2 in terms of ATP-dependence and activity in completely
The experimental DAT blocker GBR12909, added at the con-
sodium-free potassium phosphate buffer which rules out interfer-
centration of 0.01 or 0.1 mM to the superfusion buffer at min 8, was
ing effects on sodium- and chloride-dependent DAT, SERT or NET
without effect on the efflux stimulatory effect of 10 mM MMC in
(Reserpine concentration-dependently blocked
NET (and SERT cells (but GBR12909 blocked the
uptake with its well established high potency in the nanomolar
MMC effect in DAT cells partially at the concentration of 0.01 mM
range (IC50: 5.571.5 nM, n¼3; ). MMC and MDMA inhibited
and completely at 0.1 mM C).
dopamine uptake with a much lower potency which differed
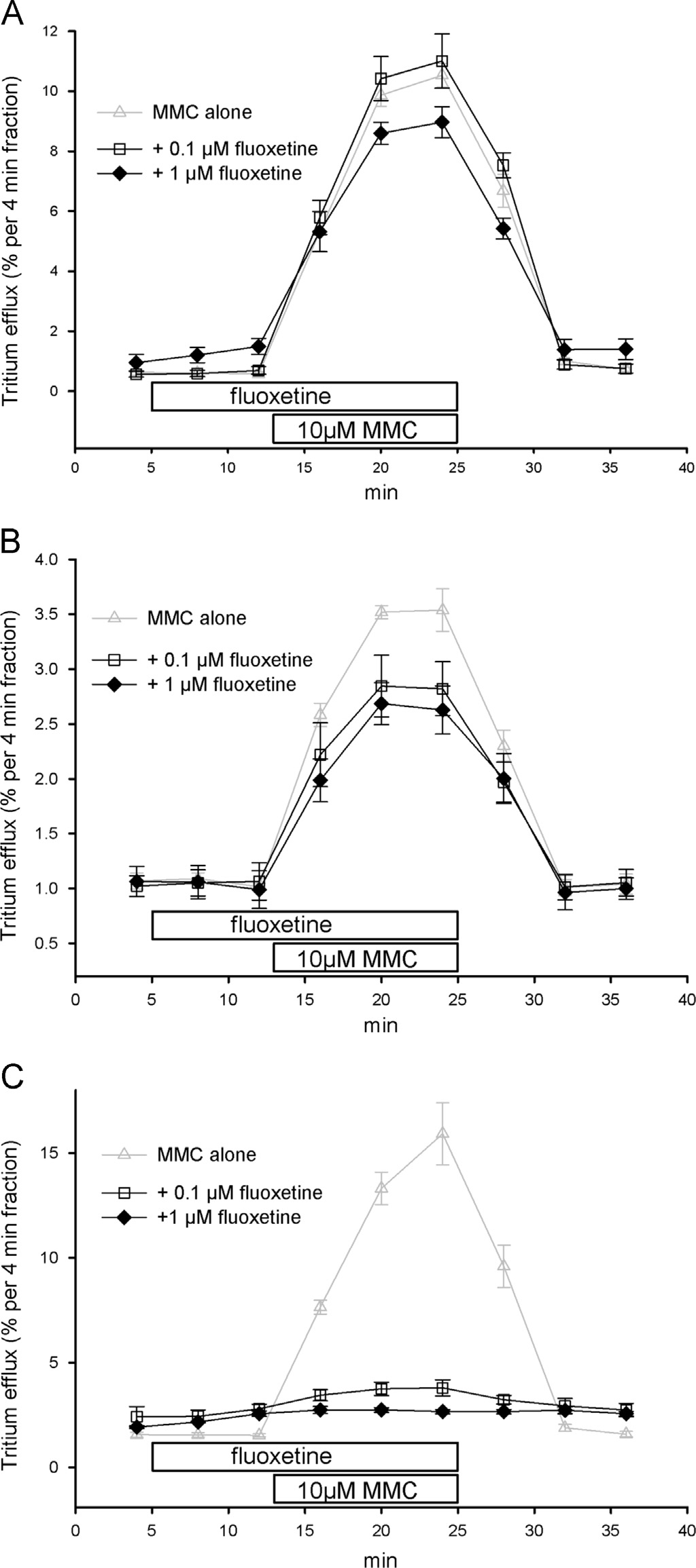
The first selectively serotonin transport inhibiting and widely
between MMC and MDMA by a factor of 10 (IC50, MMC:
used antidepressant fluoxetine, added at the concentration of
223 755 mM, n¼5; MDMA: 2076 mM, n¼5; ).
C. Pifl et al. / European Journal of Pharmacology 755 (2015) 119–126
Fig. 2. Concentration-dependent effect of MMC on release by the human NET, DAT andSERT. SK-N-MC or HEK293 cells stably expressing the NET (A), DAT (B) or SERT (C) wereloaded with [3H]MPPþ, superfused and 4-min fractions were collected. After threefractions (12 min) of basal efflux, cells were exposed for three fractions (bar) to buffers
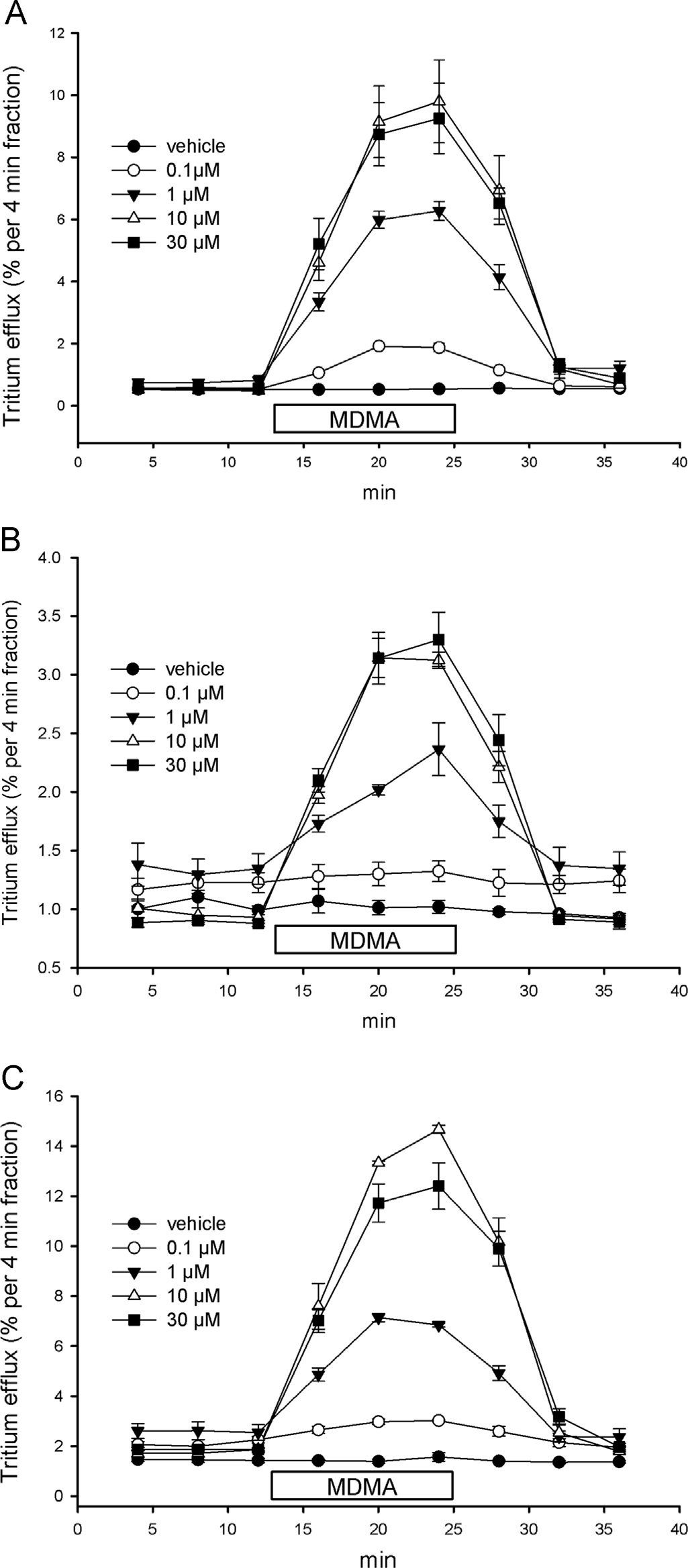
Fig. 3. Concentration-dependent effect of MDMA on release by the human NET,
containing vehicle (filled circle) or different concentrations of MMC (0.1 mM, open circle;
DAT and SERT. SK-N-MC or HEK293 cells stably expressing the NET (A), DAT (B) or
1 mM, filled triangle down; 10 mM, open triangle up; 30 mM, filled square). Data are
SERT (C) were loaded with [3H]MPP þ , superfused and 4-min fractions were
presented as fractional efflux, i.e., each fraction is expressed as the percentage of
collected. After three fractions (12 min) of basal efflux, cells were exposed for
radioactivity present in the cells at the beginning of that fraction. Symbols represent
three fractions (bar) to buffers containing vehicle (filled circle) or different
means7S.E.M. of three to four independent experiments.
concentrations of MDMA (0.1 mM, open circle; 1 mM, filled triangle down; 10 mM,open triangle up; 30 mM, 30 mM, filled square). Data are presented as fractionalefflux, i.e., each fraction is expressed as the percentage of radioactivity present in
3.5. Stimulation of DAT-mediated release in [3H]dopamine loaded
the cells at the beginning of that fraction. Symbols represent means 7S.E.M. ofthree to four independent experiments.
SH-SY5Y neuroblastoma cells by MMC and MDMA
veratridine-evoked monoamine release and the VMAT2 (
The human neuroblastoma SH-SY5Y express many pro-
We stably transfected them
perties of monoaminergic neurons including potassium and
with the human DAT, grew them on cover-slips, loaded them with
C. Pifl et al. / European Journal of Pharmacology 755 (2015) 119–126
Fig. 5. Effects of GBR 12909 on MMC-induced release by the human NET, DAT and
Fig. 4. Effects of desipramine on MMC-induced release by the human NET, DAT and
SERT. SK-N-MC or HEK293 cells stably expressing the NET (A), DAT (B) or SERT
SERT. SK-N-MC or HEK293 cells stably expressing the NET (A), DAT (B) or SERT
(C) were loaded with [3H]MPP þ , superfused and 4-min fractions were collected.
(C) were loaded with [3H]MPP þ, superfused and 4-min fractions were collected.
After one fraction of basal efflux, cells were exposed to buffers containing either
After one fraction of basal efflux, cells were exposed to buffers containing either
0.01 mM (4 min, upper bar, open squares) or 0.1 mM GBR 12909 (4 min, upper bar,
0.03 mM (4 min, upper bar, open squares) or 0.3 mM desipramine (4 min, upper bar,fi
filled diamonds). Two fractions later (12 min, lower bar), the superfusion was
lled diamonds). Two fractions later (12 min, lower bar), the superfusion was
switched for three fractions to a buffer containing additional 10 mM MMC. Data on
switched for three fractions to a buffer containing additional 10 mM MMC. Data on
the release by 10 mM MMC (gray triangles up) are included for comparison from
the release by 10 mM MMC (gray triangles up) are included for comparison from
Data are presented as fractional efflux, i.e., each fraction is expressed as the
. Data are presented as fractional efflux, i.e., each fraction is expressed as the
percentage of radioactivity present in the cells at the beginning of that fraction.
percentage of radioactivity present in the cells at the beginning of that fraction.
Symbols represent means 7S.E.M. of three to four independent experiments.
Symbols represent means7S.E.M. of three to four independent experiments.
[3H]dopamine and superfused them in microchambers. MDMA and
radioactivity in the fractionated perfusates whereas MMC A)
MMC were added at a concentration of 10 or 30 mM to the super-
was without effect at 10 mM and stimulated efflux with significantly
fusion buffer; MDMA (concentration-dependently increased
weaker activity than MDMA at 30 mM (increase of tritium efflux at
C. Pifl et al. / European Journal of Pharmacology 755 (2015) 119–126
Fig. 7. Effects of reserpine, MDMA and MMC on the uptake by the human VMAT2.
Concentration-inhibition curves on dopamine uptake in synaptic vesicles from thehuman striatum. The vesicle preparations were incubated in potassium phosphatebuffer for 4 min at 30 1C with 0.1 mM [3H]dopamine in the absence (control) orpresence of reserpine (circles, solid line), MDMA (triangles up, long dash) or MMC(triangles down, short dash) at the concentrations indicated, and uptake wasdetermined as described in Symbols represent means7S.E.M. of three tofive independent experiments, each in duplicates. The data of each experimentwere fitted by nonlinear regression and the mean of the IC50 values7S.E.M. areinserted into the graph.
The main finding of our study is that with regard to their effects
on the human brain monoamine transporters, MMC and MDMAdiffer from each other primarily in their interaction with the humanbrain vesicular monoamine uptake, with MMC having a tenfoldlower transport inhibitory potency than MDMA. The differences ininhibition of uptake by plasmalemmal transporters are muchsmaller, with twofold higher potency of MMC at the DAT and aboutthreefold higher potency of MDMA at the SERT, a rank order inagreement with that in recent studies on human transporters inHEK cells ). The fact that the lower potency of MMC at the VMAT2 wenthand in hand with a significantly lower dopamine releasing actionof MMC vs. MDMA in a human neuroblastoma cell line shows thatdopamine release from [3H]dopamine loaded cells requires dualaction by the drug, i.e. dopamine mobilization from intracellularstores to the cytosol by VMAT2 inhibition and reverse transportinduction by plasmalemmal DAT interaction. The differences on DArelease in DAT expressing SH-SY5Y demonstrate differences ininteraction with VMAT2 in a cellular setting beyond that on isolatedsynaptic vesicles and are by no means relevant for differences inreleasing abilities on dopaminergic neurons in vivo which areclearly dominated by the high potency of MMC at the DAT; this study), which is again
Fig. 6. Effects of fluoxetine on MMC-induced release by the NET, DAT and SERT. SK-N-MC
consistent with the higher abuse liability of MMC (for review, see
or HEK293 cells stably expressing the NET (A), DAT (B) or SERT (C) were loaded with [3H]MPPþ , superfused and 4-min fractions were collected. After one fraction of basal ef
The weaker interaction with the vesicular
cells were exposed to buffers containing either 0.1 mM (4 min, upper bar, open squares) or
monoamine transporter of MMC however might be a potential
1 mM fluoxetine (4 min, upper bar, filled diamonds). Two fractions later (12 min, lower
explanation for the lack of long term depletion of brain mono-
bar), the superfusion was switched for three fractions to a buffer containing additional
amines and the lack of other signs of neurotoxicity in binge-type
10 mM MMC. Data on the release by 10 mM MMC (gray triangles up) are included for
repeated administration of MMC in rodent experiments. Our
comparison from . Data are presented as fractional ef
flux, i.e., each fraction is
expressed as the percentage of radioactivity present in the cells at the beginning of that
ings on human tissue and cultured cells appear significant con-
fraction. Symbols represent means7S.E.M. of three to four independent experiments.
sidering that chronic toxicity of amphetamine-related drugs cannotbe excluded even in human users (for review, see
20 min over the mean baseline values of the first three fractions:
and because increased risk of Parkinson's disease has been
30 mM MDMA, 0.21070.029%; 30 mM MMC, 0.09270.010%; n¼5,
related to the use of methamphetamine or other amphetamine-
type drugs ).
C. Pifl et al. / European Journal of Pharmacology 755 (2015) 119–126
methylone was also reported recently in bovine chromaffinegranules () and than MMC itself for inhibition ofreserpine sensitive uptake of [3H]serotonin into membranousstructures released by osmotic shock from HEK293 cells stablytransfected with the human VMAT2 cDNA ().
The EC50 value for MMC-induced release of [3H]noradrenaline atthis VAMT2 preparation was in fact insignificantly lower than thatof MDMA, however the maximum release was only half of it(A rather high potency of MMC wasreported for inhibition of [3H]dopamine uptake into synapticvesicles prepared from rat striatal synaptosomes, but no compara-tive data were shown for MDMA in this study Apart from this potential species difference, the rank orderof potency of MMC and MDMA was rather similar in DAT, NET andSERT assays obtained from rat tissue and in cells transfected withthe respective human transporter cDNA with similar potency ofMMC and MDMA at the NET, higher potency of MMC than MDMAat the DAT and the opposite rank order at the SERT (; ).
Our interaction experiments with specific uptake blockers
clearly demonstrate that MMC-induced release of [3H]MPP þ fromNET-, DAT- or SERT-expressing cells was solely due to carrier-mediated reverse transport via the plasmalemmal transporters: infact, specific concentrations of GBR12909 and fluoxetine werefound that completely suppressed MMC-induced release fromDAT- and SERT-cells, respectively, leaving release via the othertransporters unaffected. [3H]MPP þ is also a substrate of non-neuronal monoamine transporters ()which are found endogenously in kidney and neuroblastomatissue () and our interactionexperiments could rule out their potential contributions to releas-ing effects of MMC and MDMA studied in kidney and neuroblas-toma derived HEK293 or SK-N-MC cells.
The ability of a drug to induce transporter-mediated release can
Fig. 8. Effect of MMC and MDMA on the dopamine efflux of dopaminergic cells.
be detected most reliably in superfusion experiments on cells
Human SH-SY5Y neuroblastoma cells stably expressing the human DAT were
expressing the relevant transporters. Even in these experiments
loaded with [3H]dopamine, superfused and 4-min fractions were collected. After
interference of a pseudo-releasing action must be taken into
three fractions (12 min) of basal efflux, cells were exposed for three fractions (bar)
account. Such a pseudo-releasing action is due to inhibition by
to buffers containing MMC (A) or MDMA (B) at different concentrations (10 mM,open squares; 30
the drug of re-uptake of substrate released upstream by diffusion
mM, filled diamonds). Data are presented as fractional efflux, i.e.,
each fraction is expressed as the percentage of radioactivity present in the cells at
(or by another transporter such as an organic
the beginning of that fraction. Symbols represent means 7S.E.M. of three to four
cation transporter ). In our study this was
independent experiments. *P o0.05 10 mM MMC, #Po0.005 30 mM MMC or MDMA
minimized by superfusion of cells sparsely seeded on coverslips
vs mean value of fractions 1–3 by paired Student's t-test.
and loaded with a transporter substrate that is metabolically inert,hydrophilic due to a permanent charge and has a relatively low
The mechanism of long-term neurotoxicity of amphetamine-
affinity to the transporter on which reuptake blockade might
related drugs is still unclear. It was proposed that mobilization of
mimic transporter-mediated release. The metabolically inert and
monoamines from synaptic vesicles by altering vesicular pH or by
permanently charged MPP þ is an ideal transporter substrate on
direct VMAT2 interaction together with concurrent deficits of
the DAT and the SERT with KM values in uptake experiments above
plasmalemmal transporter function after repeated administration
7 mM, but not on the NET where the KM is around 0.8 mM (
of amphetamines might trap the neurotransmitter in the cytosol
). A pseudo-releasing action by inhibiting re-uptake of
because of increased efflux from vesicles into the cytosol parallel
released [3H]MPP þ at the NET may therefore be seen in
to reduced efflux from the cytosol to the extracellular space
as a small increase of tritium induced by the pure uptake inhibitor
Especially excessive cytosolic dopamine
desipramine in the fractions at min 8 and 12 and an efflux of
might damage nerve terminals by increased formation of
10 mM MMC even in the presence of 0.3 mM desipramine above
dopamine-associated oxygen radicals and reactive metabolites,
baseline. By contrast, because MPP þ has only low affinity to DAT
and serotonin oxidation by monoamine oxidase was also proposed
and SERT transporters, the DAT inhibitor GBR 12909 and the SERT
to cause free oxygen radical formation ). Such a
inhibitor fluoxetine did not affect basal efflux of [3H]MPPþ, while
mechanism is unlikely for MMC which inhibited uptake into
blocking the MMC-induced efflux to virtually baseline in DAT- and
synaptic vesicles only at high micromolar concentrations and only
SERT-cells, respectively B and C). MMC induced release of
weakly mobilized [3H]dopamine from neuroblastoma cells. As a
[3H]MPP þ from DAT expressing SK-N-MC cells equally well as
matter of fact, sustained release of preloaded [3H]dopamine from
MDMA, but was less effective than MDMA in inducing release of
superfused DAT expressing cells by amphetamine was shown to be
[3H]dopamine from DAT expressing SH-SY5Y cells. The reason lies
dependent on mobilization of a vesicular pool after additional
in the high cytosolic concentration of the metabolically inert [3H]
VMAT2 transfection (A more than tenfold higher
MPP þ which can be immediately released from the cell by MMC or
potency of MDMA than the MMC related methcathinone and
MDMA via the plasmalemmal transporter acting in reverse. By
C. Pifl et al. / European Journal of Pharmacology 755 (2015) 119–126
contrast, [3H]dopamine has low cytosolic concentrations due to
cytosolic degradation and has to be mobilized from storage site of
the SH-SY5Y cells to the cytosol by action on the VMAT2 before it
can be released by the DAT; as mentioned, MMC acts on the
VMAT2 much less effectively than MDMA. The greater extracel-
lular dopamine increase by MMC than MDMA reported in micro-
dialysed nucleus accumbens at equal dosage )
might be due to findings that amphetamine effects on release
in vivo are predominantly driven by DAT blockade (
In conclusion, MMC and MDMA are both able to reverse
translocation by human plasmalemmal monoamine transporters.
They act most potently at the NET as it was also reported for other
amphetamine-type stimulants in preparations from the rat
(and it can be expected that release of
noradrenaline contributes to the cardiovascular and stimulant-like
effects of MMC in humans as already shown for MDMA
and psychostimulants in general (
). MMC and MDMA differ from each other
particularly in their ability to mobilize intracellular monoamines
from synaptic vesicles, with a tenfold lower potency of MMC in
inhibiting the human VMAT2. This low potency of MMC on the
VMAT2 might make its long-term neurotoxic action, still not to be
ruled out for MDMA, less likely.
Source: https://www2.meduniwien.ac.at/neurocluster/db_files/pub_art_349.pdf
Cervical insulin-like growth factor binding protein-1(IGFBP-1) to predict spontaneous onset of labor andinduction to delivery interval in post-term pregnancy OGL1,2, EIRIK SKOGVOLL3,4 & RUNA HEIMSTAD2,5 1Department of Gynecology and Obstetrics, Levanger Hospital, Health Trust Nord-Trøndelag, 2Department of Obstetrics,3Anaesthesiology and Emergency Medicine, St. Olav University Hospital Trondheim, 4Institute of Cancer research andMolecular Medicine, Faculty of Medicine, and 5Institute of Laboratory Medicine, Children's and Women's Health, NorwegianUniversity of Science and Technology (NTNU), Trondheim, Norway
ORIGINAL CONTRIBUTION Health Outcomes After Stopping ConjugatedEquine Estrogens Among PostmenopausalWomen With Prior HysterectomyA Randomized Controlled Trial Andrea Z. LaCroix, PhD Context The Women's Health Initiative Estrogen-Alone Trial was stopped early af- Rowan T. Chlebowski, MD, PhD ter a mean of 7.1 years of follow-up because of an increased risk of stroke and little








