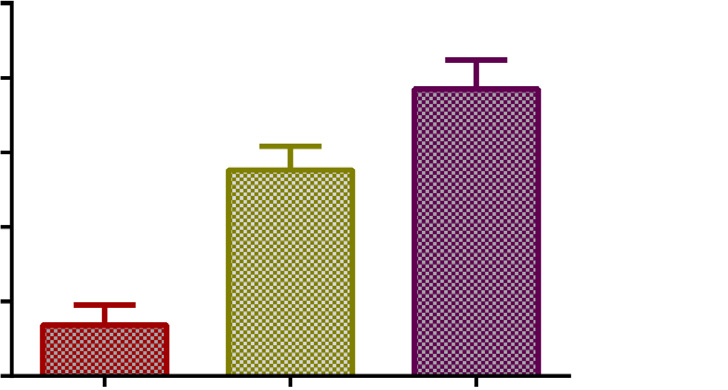Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
The systemic exposure to inhaled beclometasone/formoterol pmdi with valved holding chamber is independent of age and body size


YPUPT1369_proof ■ 18 April 2014 ■ 1/8
Contents lists available at
Pulmonary Pharmacology & Therapeutics
The systemic exposure to inhaled beclometasone/formoterol pMDI
with valved holding chamber is independent of age and body size
Mirco Govoni ,, Annalisa Piccinno Germano Lucci , Gianluigi Poli Daniela Acerbi ,
Roberta Baronio , Dave Singh Piotr Kuna Bo L.K. Chawes Hans Bisgaard
a Department of Clinical Pharmacology, Chiesi Farmaceutici, Parma 43122, Italy
b Department of Statistics and Data Management, Chiesi Farmaceutici, Parma 43122, Italy
c University of Manchester, The Medicines Evaluation Unit, Manchester M23 9QZ, United Kingdom
d Division of Internal Medicine, Asthma and Allergy, Barlicki University Hospital, Medical University of Lodz, 90-153, Poland
Copenhagen Prospective Studies on Asthma in Childhood, Health Sciences, University of Copenhagen & Danish Pediatric Asthma Center,
Copenhagen University Hospital, Gentofte, Denmark
Background: Asthma guidelines recommend prescription of inhaled corticosteroids at a reduced dosage
Received 28 January 2014
in children compared to older patients in order to minimize the systemic exposure and risk of unwanted
Received in revised form
side effects. In children, pressurized metered dose inhalers (pMDI) are recommended in combination
with a valved holding chamber (VHC) to overcome the problem of coordinating inhalation with actua-
Accepted 5 April 2014
tion. However, the in
fluence of age and body size on the systemic exposure of drugs to be administered
via a pMDI with VHC is still not fully elucidated. Therefore, we aimed to compare the systemic exposure
to the active ingredients of a
fixed combination of beclometasone-dipropionate/formoterol-fumarate
administered via pMDI with VHC in children, adolescents and adults.
Methods: The pharmacokinetics of formoterol and beclometasone-17-monopropionate (active metabo-
lite of beclometasone-dipropionate) were evaluated over 8 h from three studies, each performed in a
different age and body size group. Children (7e11 years, n ¼ 20), adolescents (12e17 years, n ¼ 29) and
adults ("18 years, n ¼ 24) received a single dose of beclometasone/formoterol (children: 200 mg/24 mg,
adolescents and adults: 400 mg/24 mg) via pMDI with AeroChamber Plus!.
Results: The systemic exposure in children in comparison to adolescents was equivalent for formoterol
while it was halved for beclometasone-17-monopropionate in accordance with the halved dose of
beclometasone administered in children (90% CIs within 0.8e1.25 for formoterol and 0.4e0.625 for
beclometasone-17-monopropionate, respectively). The systemic exposure to beclometasone-17-
monopropionate and formoterol was equivalent between adolescents and adults.
Conclusions: The systemic exposure to the active ingredients of a fixed dose combination of beclome-
tasone/formoterol administered via pMDI with AeroChamber Plus! correlates with the nominal dose
independently of patient age and body size. Thus, dose reduction in relation to age when using a pMDI
with VHC may be unnecessary for reducing the systemic exposure in children.
" 2014 Published by Elsevier Ltd.
Abbreviations: AUC, area under the plasma drug concentration-time curve; BDP,
International guidelines for treatment of childhood asthma such
beclometasone-dipropionate; B17MP, beclometasone-17-monopropionate; Cmax,
as GINA recommend prescription of inhaled corticosteroids
maximum plasma concentration; FEV1, forced expiratory volume in 1 s; FF, for-
moterol fumarate; ICS, inhaled corticosteroid; PK, pharmacokinetic; pMDI, pres-
adjusted to age or body size The deposition of drug in the
surized metered dose inhaler; t1/2, half-life; tmax, time to maximum plasma
lungs determines the clinical response, whereas systemic exposure
concentration; VHC, valved holding chamber.
in terms of drug concentration in the bloodstream determines the
* Corresponding author. Department of Clinical Pharmacology, Chiesi Farm-
risk of systemic side effects. Children in comparison to adolescents
aceutici, Largo Francesco Belloli 11A, 43122 Parma, Italy. Tel.: þ39 0521 279 780;
and adults under the same anti-asthmatics dose regimen may be
þ39 0521 279 333.
E-mail addresses: , (M. Govoni).
exposed to significantly higher systemic concentrations due to their
1094-5539/" 2014 Published by Elsevier Ltd.
Please cite this article in press as: Govoni M, et al., The systemic exposure to inhaled beclometasone/formoterol pMDI with valved holdingchamber is independent of age and body size, Pulmonary Pharmacology & Therapeutics (2014), http://dx.doi.org/10.1016/j.pupt.2014.04.003
YPUPT1369_proof ■ 18 April 2014 ■ 2/8
M. Govoni et al. / Pulmonary Pharmacology & Therapeutics xxx (2014) 1e8
lower body size. Thus the rationale behind age and body size dose
<4.7 mm), the fine particle fraction (fine particle dose expressed as
adjustment is to reduce the systemic exposure and minimize the
% of the delivered dose), the coarse dose (induction port plus ACI
risk of unwanted side effects in children. However, the influence of
stages S0eS2; particles >4.7 mm), the mass median aerodynamic
age and body size on the systemic exposure is not yet fully
diameter (MMAD) and the geometric standard deviation (GSD)
were also evaluated.
An extra-fine hydrofluoroalkane (HFA) fixed pressurized
metered-dose inhaler (pMDI) combination of beclometasone
dipropionate (BDP)/formoterol fumarate (FF) is licensed for use in
asthmatic adults and currently under development in the pediat-
Study data was collected from three independent clinical trials
rics population. In children, pMDIs are recommended to be used in
each performed on a different age group population, namely chil-
combination with valved holding chambers (VHCs) to avoid the
dren (7e11 years) [ClinicalTrials.gov ID: NCT01848769] ado-
problem of coordinating inspiration with actuation. VHCs maxi-
lescents (12e17 years) [ClinicalTrials.gov ID: and
mize lung deposition and minimize the extra-thoracic delivery of
adults ("18 years) [ClinicalTrials.gov ID: ]. Patients
drug. In a recent scintigraphic study in which a FF pMDI
with a documented clinical history of asthma diagnosed by the
formulation with the same composition of BDP/FF pMDI , was
responsible physician of each trial according to the GINA guidelines
used in conjunction with AeroChamber Plus!, the extra-thoracic
and under regular treatment with ICS or ICS/LABA or using
drug delivery was minimized to less than 10% of the total nomi-
short-acting inhaled b2-agonist as reliever to control asthma
nal dose. Considering that only a fraction (oral systemic availability
symptoms, were considered for inclusion in the study. Eligible
) of the extra-thoracic component contributes to the total
patients were all those able to properly use a pMDI with VHC and
blood levels, the systemic exposure of B17MP and formoterol after
with a pre-bronchodilator forced expiratory volume in 1 s (FEV1)
inhalation via BDP/FF pMDI with AeroChamber Plus! can be
>70% ("60% for adults) of predicted values (% pred). Main exclusion
considered a reasonable indicator of lung deposition.
criteria were exacerbation of asthma symptoms or lower respira-
Debate remains regarding the safety of long-term use of inhaled
tory tract infection within the previous 4 weeks and past or present
corticosteroids/long acting b2-agonists (ICS/LABA) in children e
diagnoses of cardiovascular, renal or liver disease.
Concerns primarily arose from short-term studies showing
reduced lower leg growth rate and impact on the hypothalamic-
pituitary-adrenal axis after using ICS . The clinical relevance
of these findings is still unknown, but a recent large randomized
Trials were conducted in accordance with the Declaration of
controlled study of 943 children with asthma demonstrated a lower
Helsinki (1996) and written informed consent was obtained from
mean adult height of 1.2 cm in the budesonide treated children
all patients.
compared to placebo Therefore, ICS in children have been
The trial in children (EudraCT 2009-010434-22) was approved
recommended at lower nominal doses of that recommended in
by the Laegemiddelstyrelsen Danish Medicines Agency; approval
number 2612-4085 on the 13 August 2009 and by the Region-
In a previous study we found similar systemic exposure to a
shuset, Viborg, The Health Secretariat, The Research Ethics Com-
fixed nominal dose of budesonide delivered via a pMDI with VHC in
mittees for the Region Midtjylland; Approval number 20090106.
children and adults This suggests that dose-reduction in
The trial in adolescents (EudraCT 2011-005108-14) was
relation to age may lead to significantly lower systemic exposures
approved by the Office for Registration of Medicinal Products,
in young patients, possibly reflecting a lower and sub-therapeutic
Medical Devices and Biocidal Products 41 zabkowska str. 03-736
lung dose. In the present study, we have again investigated the
Warsaw; approval: ur.dbl.ble.4500.430.2011 dated 30 Jan 2012 and
influence of age and body size on the systemic exposure from an
by the Bioethics Committee at the Medical University in Lodz 4
ICS/LABA fixed combination inhaled via pMDI with VHC. We
kosciuszki av., 90-419 Lodz approval: rnn/221/11/ke dated 13
studied the systemic exposures to formoterol and beclometasone-
December 2011.
17-monopropionate (B17MP; active metabolite of BDP) after a
The trial in adults (EudraCT 2010-024384-40) was approved by
single dose administration of BDP/FF pMDI used with AeroChamber
the NRES e Committee North West e Greater Manchester Central
Plus!. In order to disentangle the effects of age and body size, we
M1 3DZ e ManchestereUK Approval: 11/NW/0160 15 Apr 2011 and
studied three different asthma populations: (1) children aged 7e11
by the MHRA Approval: 06607/0243/001-0001 28 Apr 2011.
years, (2) adolescents aged 12e17 years, and (3) adults aged "18
2.4. Study design
2. Materials and methods
Each age group population was dosed with an hydro-
fluoroalkane (HFA)-propelled extra-fine fixed combination of BDP/
2.1. In vitro study data
FF pMDI (Chiesi Farmaceutici, Parma, Italy) in combination with
AeroChamber Plus! (Trudell Medical International, Ontario, Can-
An Andersen Cascade Impactor (ACI) (Copley Instruments,
ada) in one of the treatment periods. In the present study the
Nottingham, UK) operated at 28.3 l/min was used to determine the
pharmacokinetic (PK) profiles of formoterol and B17MP in adults,
particle size distributions of the pMDI with VHC used in the study
adolescents and children obtained after administration of 4 puffs of
at two different dose strengths of BDP/FF (100/6 mg and 50/6 mg per
BDP/FF pMDI with AeroChamber Plus! was studied. In adults and
actuation). Devices were actuated directly into the induction port of
adolescents each actuation contained 100/6 mg (BDP/FF), giving a
the impactor and the amount of drug collected at each stage was
total dose of 400/24 mg. In children the BDP/FF dose was 50/6 mg per
determined using a high performance liquid chromatography with
actuation, giving a total dose of 200/24 mg pMDIs were primed prior
U.V. detector fully validated method. The delivered dose was the
to administration and the AeroChamber Plus! were cleaned in
amount of drug deposited in the induction port as well as in all
accordance with the instructions leaflet. The use of short acting b2-
stages of the impactor (S0-Filter). The total emitted dose was the
agonists, LABA and BDP, had to be avoided for at least 4, 24 and 48 h,
delivered dose plus the amount of drug recovered in the actuator
respectively, prior to study drug administration. At the end of
and spacer. The fine particle dose (ACI stages S3-Filter; particles
inhalation treatment, adult patients were given orally 5 g of
Please cite this article in press as: Govoni M, et al., The systemic exposure to inhaled beclometasone/formoterol pMDI with valved holdingchamber is independent of age and body size, Pulmonary Pharmacology & Therapeutics (2014), http://dx.doi.org/10.1016/j.pupt.2014.04.003
YPUPT1369_proof ■ 18 April 2014 ■ 3/8
M. Govoni et al. / Pulmonary Pharmacology & Therapeutics xxx (2014) 1e8
activated charcoal suspended in 50 mL of water; 1, 2 and 4 h after
model and equivalence was demonstrated if the 90% CI range was
the treatment, patients were given another dose of charcoal. Pa-
within the 0.8e1.25 acceptance region An ANCOVA model
tients practiced the inhalations with placebo pMDIs with Aero-
with body surface area (BSA) or weight as covariate was used to
Chamber Plus! until the investigator judged the technique to be
assess the effect of these covariates on the systemic exposure
optimal and were instructed to hold their breath for at least 5 s
among different age-group populations Correlation assessments
following each inhalation and to wait about 30 s before the next
were performed using Pearson's analysis test. Statistical compari-
inhalation. Evaluable patients were all those receiving BDP/FF pMDI
sons of demography data were performed by means of one-way
with AeroChamber Plus! excluding subjects without any valid
ANOVA followed by post-hoc Dunnett's test for multiple
pharmacokinetic (PK) measurement over 8 h post-dose or with
major protocol deviations significantly affecting the PK, e.g. incor-
rect inhalation, change in patient condition (worsening of asthma,
cold), failure in delivery of the device, use of non-permitted med-
ications. Study patients were not excluded on the basis of statistical
3.1. In vitro results
analysis or for PK reasons.
The in vitro deposition parameters of BDP and FF for the
2.5. Pharmacokinetic assessments
different strengths of BDP/FF pMDI used in combination with
AeroChamber Plus! are shown in The total emitted dose
Patients attended the clinics in the morning of the administra-
matched with the nominal dose of BDP and FF for both strengths.
tion day after having fasted overnight and remained fasted until 2 h
According to the nominal doses, the delivered dose (total emitted
post-dosing. At each administration period, the patients were not
dose subtracted by the amount of drug recovered in the actuator
allowed to lie down or sleep for 2 h after administration, except
fine particle dose (particles of the delivered dose
when undergoing clinical assessments. They remained seated as
<4.7 mm) of BDP was halved for BDP/FF 50/6 pMDI in comparison to
much as possible and avoided strenuous activities and remained
BDP/FF 100/6 pMDI while both these parameters were similar be-
under constant surveillance of the nursing staff. No alcohol or
tween the different strengths for FF. With the use of the Aero-
xanthine (tea, chocolate, cola, etc.) containing beverages or foods,
Chamber Plus! the
fine particle fractions (fine particle dose
or grapefruits were taken from 48 h before each drug administra-
expressed as % of the delivered dose) approached 100% of the
tion until 24 h after administration. No food or drink, except water,
delivered dose and, accordingly, the coarse doses (particles of the
was allowed for 2 h after drug administration.
>4.7 mm) were virtually completely abolished.
Blood samples were collected pre-dose and at 0.5, 1, 2, 4, 6, 8 h
post dose. Samples for B17MP and formoterol determination in
plasma were collected into vacuum tubes containing EDTA and
3.2. Study population
lithium heparin, respectively. All samples were immediately chilled
(ice bath) and plasma preparation was done within 15 min after
Patient disposition is depicted in and demography data are
blood collection. The plasma was separated in a refrigerated
presented in .
centrifuge at þ4 $C and at 2500 rpm for 15 min and transferred into
prelabelled polypropylene tubes. For stabilizing formoterol, the
polypropylene tubes were pre-filled with 50 mL of citric acid and
26 patients were randomized in the children trial. Three were
centrifuged before use in order to ensure that the citric acid was at
withdrawn before receiving any treatment and one child was
the bottom of each tube. Samples for B17MP and formoterol anal-
withdrawn after treatment due to difficulties with blood sampling.
ysis were stored below %20 $C and %65 $C, respectively, before
Two out of the remaining 22 children had no quantifiable study
shipment on cold dry ice to the laboratory (SGS Life Sciences Ser-
drug levels leaving 20 children evaluable for this study. Among
vices, Belgium). The pharmacokinetic assays were performed using
these patients, 15 were males (75%) and 5 were females (25%) and
validated liquid chromatography-mass spectrometry (LC-MS/MS)
their median (range) age was 9.5 (7e12 years).
methods with lower limit of quantification of 2 pg/ml for for-
moterol and 50 pg/ml for B17MP
The following PK parameters were calculated from the indi-
vidual plasma concentrations versus time profiles: maximum
In vitro characterization of beclometasone dipropionate (BDP) and formoterol
fumarate (FF) for the different strengths of BDP/FF pMDI used in combination with
max), time to maximum concentration (tmax), area
AeroChamber Plus!.
under the concentration-time curve from 0 to last measurable
point over 8 h and extrapolated to infinity (AUC
BDP/FF 50/6 pMDI with
BDP/FF 100/6 pMDI with
respectively), half-life (t
AeroChamber Plus!
AeroChamber Plus!
1/2) calculated as 0.693/lz, where lz is the
first-order terminal rate constant.
Total emitted dose (mg)
2.6. Data analysis
Delivered dose(mg)
Fine particle dose(mg)
PK study calculations were performed according to a non-
Fine particle fraction(%) 95.3 (2.3)
compartmental kinetic model using Phoenix! WinNonlin#
version 6.2.1 (Pharsight Corporation, Cary, USA). Statistical analysis
of PK data were performed with SAS/STAT software, Version 9.2 of
Results are expressed as the mean (SD), n
the SAS System for Windows (Cary, USA) and GraphPad Prism#
a Total emitted dose subtracted by the amount of drug recovered in the actuator
version 6.0 (GraphPad Software, La Jolla, USA). All PK variables were
and valved holding chamber.
log transformed before analysis. Comparisons of PK variables be-
b Particles of the delivered dose <4.7 mm.
tween the different age-group populations were analyzed with an
Fine particle fraction expressed as % of the delivered dose.
Particles of the delivered dose >4.7 mm.
analysis of variance (ANOVA) model. The 90% confidence intervals
¼ Mass Median Aerodynamic Diameter.
(CIs) for the ratio of the geometric means were derived from the
f GSD ¼ Geometric Standard Deviation.
Please cite this article in press as: Govoni M, et al., The systemic exposure to inhaled beclometasone/formoterol pMDI with valved holdingchamber is independent of age and body size, Pulmonary Pharmacology & Therapeutics (2014), http://dx.doi.org/10.1016/j.pupt.2014.04.003
YPUPT1369_proof ■ 18 April 2014 ■ 4/8
M. Govoni et al. / Pulmonary Pharmacology & Therapeutics xxx (2014) 1e8
Assessed for eligibility (n=27)
Assessed for eligibility (n=30)
Assessed for eligibility (n=36)
-Not mee ng inclusion criteria (n=9)
- Mee ng exclusion criteria
- Mee ng exclusion criteria (n=3)
Randomized (n=26)
Randomized (n=30)
Randomized (n=24)
Allocated to interven on:
Allocated to interven on:
Allocated to interven on:
(BDP/FF 50/6 pMDI with AeroChamber Plus™) (n=26)
(BDP/FF 100/6 pMDI with AeroChamber Plus™) (n=30)
(BDP/FF 100/6 pMDI with AeroChamber Plus™) (n=24)
Receive interven on: (n=23)
Receive interven on: (n=30)
Receive interven on: (n=24)
Did not receive interven on: (n=3)
Did not receive interven on: (n=0)
Did not receive interven on: (n=0)
Discon nued interven on: (n=1)
Discon nued interven on: (n=1)
Discon nued interven on: (n=0)
Excluded from analysis: (n=2)
Excluded from analysis: (n=1)
Excluded from analysis: (n=0)
Fig. 1. Patient disposition.
3.2.2. Adolescents
the bioequivalence reference range of 0.8
e1.25 for formoterol
30 patients were randomized in the adolescent trial. One subject
(0.96 with 90% CI of 0.83
e1.12 for AUC0et and 0.94 with 90% CI of
was discontinued due to insuf
ficient number of samples taken after
0.80e1.12 for AUC0einf) ).
inhalation. This resulted in 29 adolescents evaluable for the present
The mean plasma concentration-time pro
files of B17MP and
study. Among these patients 14 were males (48%) and 15 were fe-
formoterol in adolescents compared to adults were similar over the
males (52%) and their median (range) age was 16.0 (12
8-h sampling period (). Accordingly, the overall systemic
exposure to both B17MP and formoterol was equivalent in adoles-
cents compared to adults. The PE and 90% CI of the geometric means
24 patients were randomized in the adult trial; all of them were
ratio adolescents/adults for AUC
0et and AUC0einf were within the
evaluable for this study. Among these patients, 15 were males (63%)
bioequivalence reference range; the PE of AUC
0et was 1.07 (90% CI of
and 9 females (37%). The adults
' median (range) age was 45.5 (22e
0.93e1.22) for B17MP and 0.93 (90% CI of 0.82e1.05) for formoterol,
while the PE of AUC
0einf was 1.07 (90% CI of 0.94e1.21) for B17MP
In the three age group populations the mean body surface area
and 0.93 (90% CI of 0.81
e1.08) for formoterol (
(BSA), calculated according to the Mosteller formula and the
The terminal half-lives (t
1/2) for both B17MP and formoterol
mean weight increased signi
ficantly from children to adolescents
were similar among the three age group populations. Mean (SD) t1/2
and from adolescents to adults (p
for children, adolescents and adults were 2.1 (1.0) h, 3.0 (0.7) h and
3.2 (1.0) h for B17MP and 4.3 (1.7) h, 4.6 (1.4) h and 4.7 (1.2) h for
3.3. Pharmacokinetics
Upon normalization for the BDP/FF dose in the three age group
The overall summary of pharmacokinetic parameters is repre-
populations (normalization of the BDP component in children to
sented in . Children in comparison to adolescents and adults
mg), the total systemic exposure to B17MP and formoterol
were treated with a halved dose of BDP but with the same dose of
0einf did not correlate with the BSA (r ¼ %0.2,
FF. Accordingly, the mean plasma concentration-time pro
p ¼ 0.08 for B17MP and r ¼ 0.01, p ¼ 0.96 for formoterol), age
pediatric population in comparison to older patients were
¼ %0.10, p ¼ 0.38 for B17MP and r ¼ 0.08, p ¼ 0.49 for formoterol)
approximately halved for B17MP but comparable for formoterol
¼ %0.19, p ¼ 0.10 for B17MP and r ¼ 0.03, p ¼ 0.79 for
(According to the dose given in the different age
formoterol) of patients ). BSA or weight inserted as covariate
group populations, the point estimate (PE) of the geometric means
in an ANCOVA model for the analysis of the total systemic exposure
ratio children/adolescents for AUC
0et and AUC0einf was 0.49 (90% CI
to formoterol (compound given at the same dose among the three
e0.58) and 0.48 (90% CI of 0.41e0.57) for B17MP, and within
age-group populations) were not found significant for the
Adolescents (n ¼ 29)
14.9 (1.7; 12e17)
42.4 (11.3; 22e65)
141 (10; 123e168)
168 (12; 139e191)
173 (10; 150e192)
17.7 (2.1; 14.9e23.4)
21.8 (2.8; 18.5e30.1)
26.9 (2.8; 22.4e31.6)
1.17 (0.14; 0.98e1.49)
1.69 (0.21; 1.24e2.10)
1.96 (0.23; 1.51e2.41)
2.09 (0.46; 1.27e3.03)
3.22 (0.82; 1.79e4.79)
2.74 (0.62; 1.71e4.09)
Results are presented as the mean (SD; range). BMI: Body Mass Index; BSA: Body Surface Area calculated according to the Mosteller formula; FEV
1: Pre-bronchodilator forced
expiratory volume in 1 s; FEV
1 % pred: FEV1 % of predicted normal value.
Please cite this article in press as: Govoni M, et al., The systemic exposure to inhaled beclometasone/formoterol pMDI with valved holdingchamber is independent of age and body size, Pulmonary Pharmacology & Therapeutics (2014), http://dx.doi.org/10.1016/j.pupt.2014.04.003
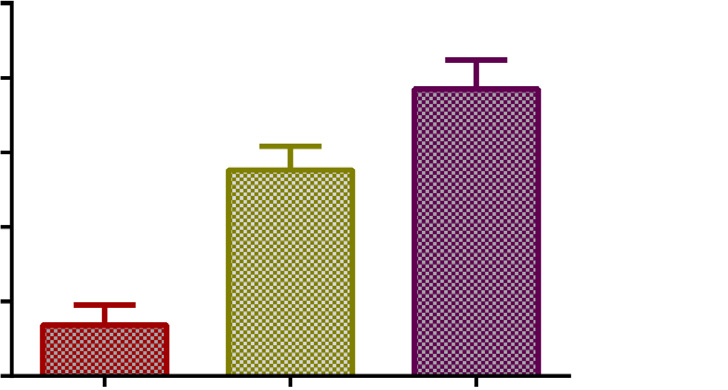
YPUPT1369_proof ■ 18 April 2014 ■ 5/8
M. Govoni et al. / Pulmonary Pharmacology & Therapeutics xxx (2014) 1e8
Fig. 2. Mean body surface area and mean body weight (95% confidence intervals) among the different age group populations (****p < 0.0001).
comparisons between children and adolescents or adults and ad-
to age or body size when prescribing a pMDI with VHC may be
olescents (p > 0.1).
unnecessary to minimize the risk of systemic side effects in chil-
dren and may cause sub-therapeutic lung dose.
4.2. Interpretation
4.1. Main finding
The pharmacokinetic properties of a drug assuming mono-
The systemic exposure to the active metabolite of BDP (B17MP)
compartmental distribution in the body after absorption to the
and formoterol after pMDI administration with AeroChamber
systemic circulation can be analyzed by the following pharmaco-
Plus! was independent of age and body size. The same dose of FF
kinetic equation :
Volume of distribution of the drug in the body compartment
ðVÞ ¼ Amount of drug absorbed into the body compartment=ðAUC ( lzÞ
administered through the pMDI with VHC in children, adolescents
and adults led to comparable systemic exposures in the three
The equation relates the volume of distribution of the drug in
populations. For BDP, children received half the dosage of adoles-
the body after systemic absorption, with the total amount of drug
cents and adults which led to a corresponding reduction in the
absorbed systemically, the total systemic exposure (AUC) and the
systemic levels of B17MP to half of that recorded in adolescents and
elimination constant (lz) calculated as 0.693/t1/2 . Reasonably,
adults. Since dose proportionality can be demonstrated between
the volume of drug distribution in the body, for the same drug, is
the different strengths of BDP/FF pMDI and the systemic exposure
proportional to the body surface area of individual patients. In our
to B17MP and formoterol it follows that dosing children with
specific study the BSA increased significantly from children to ad-
the same amount of BDP as adolescents and adults would lead to
olescents and from adolescents to adults while the half-lives and
comparable systemic exposures among the three populations. This
the systemic exposures were similar. It follows that the amount of
is in agreement with our previous study of age-dependent dosing
drug that reaches the systemic circulation after administration of
showing similar PK-profiles in young children and adults receiving
BDP/FF pMDI with AeroChamber Plus! is proportional to the BSA
the same nominal dose of budesonide pMDI via the Nebuchamber
(and therefore lower for young patients) with the net effect of
spacer device These two studies together argue against a dose
maintaining the same systemic exposure among the different age
regimen adjusted to age and suggest that dose-reduction in relation
group populations.
Beclometasone 17-monopropionate (B17MP) and formoterol pharmacokinetic parameters in children, adolescents and adults.
AUC0einf (pg*h/mL)
Children (N ¼ 20)
Adolescents (N ¼ 29)
3003.47 (1016.02)
Children (N ¼ 20)
Please cite this article in press as: Govoni M, et al., The systemic exposure to inhaled beclometasone/formoterol pMDI with valved holdingchamber is independent of age and body size, Pulmonary Pharmacology & Therapeutics (2014), http://dx.doi.org/10.1016/j.pupt.2014.04.003
YPUPT1369_proof ■ 18 April 2014 ■ 6/8
M. Govoni et al. / Pulmonary Pharmacology & Therapeutics xxx (2014) 1e8
Pharmacokinetic analysis of beclometasone 17-monopropionate (B17MP) and for-
moterol after administration of BDP/FF pMDI with AeroChamber Plus! in children
and adults versus adolescents.
PK variable B17MP
Children/Adolescents AUC
The point estimate is calculated as ratio of the geometric means for the different PK
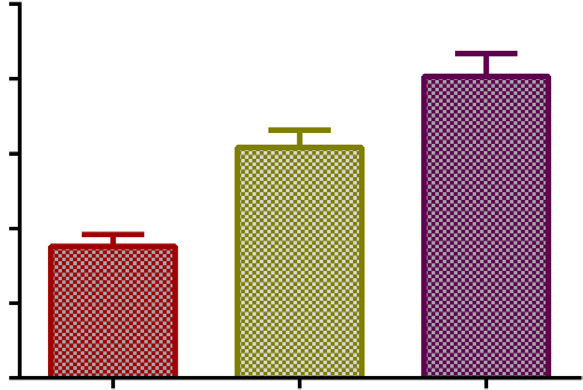
Fig. 3. B17MP average plasma concentrations (95% con
fidence intervals) after four
inhalation of BDP/FF pMDI with AeroChamber Plus! (BDP/FF dose per actuation of
50 mg/6 mg in children and 100 mg/6 mg in adolescents and adults) for a total single BDP
dose of 200 mg in children and 400 mg in adolescents and adults.
systemic absorption from the gastrointestinal tract. However, we
have shown here and elsewhere that the use of AeroChamber
As discussed above when BDP/FF pMDI is used in combination
Plus! virtually abolishes the coarse particle fraction higher than
with the AeroChamber Plus!, the entire dose inhaled via the VHC
4.7 mm that is potentially suitable for ingestion and GI absorption
comprises the "respirable" fraction (<4.7 mm) with a limited
This "spacer effect" was also demonstrated in vivo in healthy
amount of drug reaching the extra-thoracic compartment This
volunteers Indeed, the use of charcoal block did not affect the
indicates that the overall systemic exposure observed in the study
systemic availability of budesonide (oral bioavailability similar to
has a predominant pulmonary origin and thereby largely reflects
formoterol w10e20% after pMDI administration with
lung exposure. Based on this consideration, the lower amount of
Nebuhaler# spacer device In addition, we recently demon-
drug reaching the systemic circulation in young patients could be
strated in a scintigraphic study that after pMDI administration with
explained by a reduced amount of the inhaled drug reaching the
AeroChamber Plus! the extra-thoracic delivery of drug accounts
lungs Indeed, the possibility of a reduced amount of drug
for less than 10% of the total nominal dose in contrast to more the
delivered to the lung in young patients in comparison to older
50% when the spacer is not used . Based on a delivered dose of
patients may be explained by the decreased upper airway geometry
approximately 60% of the total nominal dose () the extra-
, the lower inspiratory capacity and/or the shorter inhalation
thoracic and thoracic drug delivery would account for 16.6% (10/
times of the pediatric population
60*100) and 83.4% (100-16.6) of the delivered dose, respectively.
Taken together, the present results suggest that the appropriate
Considering a thoracic systemic availability flung ¼ 1 and an extra-
dosage of drug to be administered via a pMDI with VHC in children
thoracic systemic availability foral ¼ 0.4 and 0.15, for B17MP and
cannot be simply extrapolated from data in older patients upon
formoterol, respectively , the systemic exposure could be
correction for the age or body size. Indeed, for a normalized dose of
derived by Eq. as follow:
BDP/FF, the total systemic exposure to B17MP and formoterol did
not correlate with the age BSA or weight of the patients. In other
AUC ¼ AUClung þAUCoral ¼ delivereddose*0:834*flung ðV*lzÞ
words, children cannot be regarded as little adults for this specific
study drug, which is presumably a general
finding for drugs inhaled
via pMDI in combination with a VHC.
groupingdelivereddose=ðV*lzÞ ¼ K :
¼ K*0:834*flung þ K*0:166*foral;
It is a limitation that adults, differently from adolescents and
children, took a concomitant dose of charcoal block to prevent the
*0:834*flung ¼ AUClung :
¼ AUClung þ AUClung*0:199* foral flung
¼ AUClung 1 þ 0:199 foral flung
AUC (B17MP) ¼ AUClung*1.08 and AUC (formoterol) ¼ AUClung*1.03,
i.e. the oral contribution to the systemic exposure would account
for less than 8% and 3% for B17MP and formoterol, respectively.
Correcting the individual systemic exposures in adults for these
values, the 90% CIs for the AUC0-t ratio adolescents/adults still fall
within the 0.8e1.25 bioequivalence region (corrected 90%
¼ 0.86e1.11 for B17MP and 0.80e1.02 for formoterol).
Fig. 4. Formoterol average plasma concentrations (95% confidence intervals) after four
inhalation of BDP/FF pMDI with AeroChamber Plus! (BDP/FF dose per actuation of
The results of the present study demonstrate that the exposure
mg/6 mg in children and 100 mg/6 mg in adolescents and adults) for a total single FF
dose of 24 mg in each age group population.
to B17MP and formoterol after BDP/FF pMDI with AeroChamber
Please cite this article in press as: Govoni M, et al., The systemic exposure to inhaled beclometasone/formoterol pMDI with valved holdingchamber is independent of age and body size, Pulmonary Pharmacology & Therapeutics (2014), http://dx.doi.org/10.1016/j.pupt.2014.04.003
YPUPT1369_proof ■ 18 April 2014 ■ 7/8
M. Govoni et al. / Pulmonary Pharmacology & Therapeutics xxx (2014) 1e8
r = -019, p = 0.10
pg*h/ml 8.0
pg*h/ml 8.0
pg*h/ml 8.0
Age (years)
Weigth (kg)
r = 0.03, p = 0.79
r = 0.01, p = 0.96
r = 0.08, p = 0.49
pg*h/ml 4.5
pg*h/ml 4.5
Age (years)
Weight (kg)
Fig. 5. Correlation between the total systemic exposure to B17MP and formoterol with the body surface area (BSA), age and weight of individual patients. Correlations were
considered significant at p < 0.05 (Pearson's analysis test). In children the systemic exposure to B17MP was normalized to a BDP dose of 400 mg.
Plus! correlates with the dose independently of patient age and
Global strategy for asthma management and prevention. Workshop Report,
body size. Thus, dose reduction in relation to age when using a
updated 2012. Available on: ; 2012.
[2] Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al.
pMDI with VHC may be unnecessary for reducing the systemic
Global strategy for asthma management and prevention: GINA executive
exposure in children with asthma and runs the risk that sub-
summary. Eur Respir J 2008;31(1):143
therapeutic doses reach the lungs.
[3] Pedersen SE, Hurd SS, Lemanske Jr RF, Becker A, Zar HJ, Sly PD, et al. Global
strategy for the diagnosis and management of asthma in children 5 years and
younger. Pediatr Pulmonol 2011;46(1):1e17.
[4] National Asthma Education and Prevention Programme. Expert Panel:
guidelines for the diagnosis and management of asthma
All authors contributed to study design conceptualization,
[5] Brand PL, Baraldi E, Bisgaard H, Boner AL, Castro-Rodriguez JA, Custovic A,
analysis and interpretation, and manuscript preparation and/or
et al. Definition, assessment and treatment of wheezing disorders in pre-
critical revision.
school children: an evidence-based approach. Eur Respir J 2008;32(4):
[6] Mariotti F, Poli G, Acerbi D, Herpich C, Brand P, Gleske J, et al. Lung deposition
Competing interests
of formoterol in COPD patients: comparison of formoterol HFA pMDI, and
formoterol dry powder inhaler [abstract] ERS Conf 2007;P721:100s.
[7] Häussermann S, Acerbi D, Brand P, Herpich C, Poli G, Sommerer K, et al. Lung
This study was financially supported by Chiesi Farmaceutici
deposition of formoterol HFA (Atimos/Forair) in healthy volunteers, asthmatic
S.p.A; Mirco Govoni, Annalisa Piccinno, Germano Lucci, Gianluigi
and COPD patients. J Aerosol Med 2007;20(3):331e41.
Poli, Roberta Baronio and Daniela Acerbi are employed at Chiesi
[8] Singh D, Collarini S, Poli G, Acerbi D, Amadasi A, Rusca A. Effect of Aero-
Farmaceutici S.p.A that sponsored the clinical trials in children,
Chamber Plus! on the lung and systemic bioavailability of beclometasone
dipropionate/formoterol pMDI. Br J Clin Pharmacol 2011;72(6):932e9.
adolescents and adults. Dave Singh was the principal investigator of
[9] Pedersen S. Clinical safety of inhaled corticosteroids for asthma in children: an
the trial in adults and received sponsorship to attend international
update of long-term trials. Drug Saf 2006;29(7):599e612.
meetings, honoraria for lecturing or attending advisory boards and
[10] Allen DB. Safety of inhaled corticosteroids in children. Pediatr Pulmonol
research grants from Chiesi. Piotr Kuna was the principal investi-
[11] Allen DB. Effects of inhaled steroids on growth, bone metabolism and adrenal
gator of the trial in adolescents and received lecture fee and cost of
function. Expert Rev Respir Med 2007;1(1):65e74.
participation for international congresses from Chiesi. Bo L. K.
[12] Volovitz B, Amir J, Malik H, Kauschansky A, Varsano I. Growth and pituitary-
adrenal function in children with severe asthma treated with inhaled bude-
Chawes was the coordinator of the trial in children and received
sonide. N Engl J Med 1993;329(23):1703e8.
honoraria for consulting and participation to international con-
[13] Kelly HW, Sternberg AL, Lescher R, Fuhlbrigge AL, Williams P, Zeiger RS, et al.
gresses from Chiesi. Hans Bisgaard was the principal investigator of
Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med
the trial in children and received honorarium from Chiesi for
[14] Onhøj J, Thorsson L, Bisgaard H. Lung deposition of inhaled drugs increases
consulting. The relationship between the Sponsor and the authors'
with age. Am J Respir Crit Care Med 2000;162(5):1819e22.
affiliations was regulated by financial agreements. There are no
[15] Chawes BL, Piccinno A, Kreiner-Møller E, Vissing NH, Poorisrisak P,
Mortensen L, et al. Pharmacokinetic comparison of inhaled
other relationships or activities that could appear have in
fixed combination
vs. the Free combination of Beclomethasone and formoterol pMDIs in asth-
submitted work.
matic children. Br J Clin Pharmacol 2013;75:1081e8.
[16] EMEA CHMP. Guideline on the investigation of bioequivalence (CPMP/EWP/
QWP/1401/98 Rev. 1/Corr); 2010.
[17] Lam TK, Leung DT. More on simplified calculation of body-surface area. N Engl
J Med 1988;318(17):1130.
[1] Global Initiative for Asthma (GINA), National Institute of Health, National
[18] Fantini M, Collarini S, Usberti F, Poli G. In vitro and in vivo dose linearity of
Heart Lung and Blood Institute (NHLBI)/World Health Organisation (WHO).
beclometasone dipropionate (BDP) in a pMDI BDP plus formoterol fixed
Please cite this article in press as: Govoni M, et al., The systemic exposure to inhaled beclometasone/formoterol pMDI with valved holdingchamber is independent of age and body size, Pulmonary Pharmacology & Therapeutics (2014), http://dx.doi.org/10.1016/j.pupt.2014.04.003
YPUPT1369_proof ■ 18 April 2014 ■ 8/8
M. Govoni et al. / Pulmonary Pharmacology & Therapeutics xxx (2014) 1e8
CombinationDose linearity of beclometasone dipropionate (BDP) in a pMDI
[24] Van Schayck CP, Donnell D. The efficacy and safety of QVAR (hydro-
BDP plus formoterol fixed combination [abstract] RDD Eur 2009;2:279e84.
fluoroalkane-beclometasone diproprionate extrafine aerosol) in asthma (Part
[19] Rowland M, Tozer TN. Clinical pharmacokinetics and pharmacodynamics:
2): clinical experience in children. Int J Clin Pract 2004;58(8):786e94.
concepts and applications. 4th ed. Philadelphia, PA: Lippincott Williams &
[25] Thorsson L, Edsbäcker S. Lung deposition of budesonide from a pressur-
Wilkins; 2010.
ized metered-dose inhaler attached to a spacer. Eur Respir J 1998;12(6):
[20] Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors
affecting therapeutic effectiveness of aerosolized medications. Br J Clin
[26] Nadarassan DK, Chrystyn H, Clark BJ, Assi KH. Validation of high-performance
Pharmacol 2003;56(6):588
liquid chromatography assay for quantification of formoterol in urine samples
[21] Wildhaber JH, Dore ND, Wilson JM, Devadason SG, LeSouëf PN. Inhalation
after inhalation using UV detection technique. J Chromatogr B Analyt Technol
therapy in asthma: nebulizer or pressurized metered-dose inhaler with
Biomed Life Sci 2007;850(1e2):31e7.
holding chamber? In vivo comparison of lung deposition in children. J Pediatr
[27] McKeage K, Goa KL. Budesonide (Entocort EC Capsules): a review of its
therapeutic use in the management of active Crohn's disease in adults. Drugs
[22] Tal A, Golan H, Grauer N, Aviram M, Albin D, Quastel MR. Deposition pattern
of radiolabeled salbutamol inhaled from a metered-dose inhaler by means of a
[28] Daley-Yates PT, Price AC, Sisson JR, Pereira A, Dallow N. Beclomethasone
spacer with mask in young children with airway obstruction. J Pediatr
dipropionate: absolute bioavailability, pharmacokinetics and metabolism
following intravenous, oral, intranasal and inhaled administration in man. Br J
[23] Berg E. In vitro properties of pressurized metered dose inhalers with and
Clin Pharmacol 2001;51(5):400e9.
without spacer devices. J Aerosol Med 1995;8(Suppl. 3):S3e10 [discussion S1].
Please cite this article in press as: Govoni M, et al., The systemic exposure to inhaled beclometasone/formoterol pMDI with valved holdingchamber is independent of age and body size, Pulmonary Pharmacology & Therapeutics (2014), http://dx.doi.org/10.1016/j.pupt.2014.04.003
Source: http://asthmaandchildren.org/userfiles/2014_Govoni_Pulm%20Pharmacol%20Ther.pdf
Newsletter Issue 2, April 2016 In this Edition: From thin section to bulk sample * Lithium in Ostrobothnia * FAMEly homes: Porto * Interview Peter Robinson Thoughts from FAMErs From thin section to bulk sample – the FAME resource base between outcrop and microscope The FAME project had a very productive first year with field campaigns documenting and sampling the greisens, skarns and pegmatites of the six reference deposits of Cinovec, Tabuaço, Gonçalo, Pöhla and Keliber, followed by mineral and geochemical studies (see scheme below) to characterise the mineralogical variables significant for process flow design. In parallel, the economic potential of skarn, pegmatite and greisen ore deposits of Europe was assessed and requirements for profitable mining defined.
MATERIAL SAFETY DATA SHEET SECTION: 1.1 PRODUCT IDENTIFICATION Product Name: Melt & Pour Soap Base Suspending Product Use: CAS #: n/a Country of Origin: SECTION: 1.2 COMPANY IDENTIFICATION Company Name: Saffire Blue Inc. Address: 1444 Bell Mill Road, Tillsonburg, ON N4G4G9 Canada