Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Doi:10.1016/s0920-9964(02)00213-x
Schizophrenia Research 63 (2003) 63 – 71
Source distribution of neuromagnetic slow-wave activity
in schizophrenic patients—effects of activation
Thorsten Fehr, Johanna Kissler, Christian Wienbruch, Stephan Moratti,
Thomas Elbert, Hans Watzl, Brigitte Rockstroh *
Department of Psychology, University of Konstanz, P.O. Box D23, D-78457 Konstanz, Germany
Center for Psychiatry Reichenau, D-78467 Reichenau, Germany
When slow waves in the EEG delta and theta frequency range appear in the waking state, they may indicate pathological
conditions including psychopathology. The generators of focal slow waves can be mapped using magnetic source imaging. Theresulting brain maps may possibly characterize dysfunctional brain areas. The present study examined the stability of thedensity and distribution of MEG slow waves during three conditions—rest, mental arithmetic and imagery—in 30schizophrenic patients and 17 healthy controls. Schizophrenic patients displayed a higher density of delta and theta generatorsprimarily in temporal and parietal areas. The group difference was not affected by the particular conditions. The focalconcentration of delta and theta slow waves did not differ between patients with and without neuroleptic medication, whereasthe prominence of theta dipoles in the temporal area correlated with neuroleptic dosage. The relative amount of temporal slowwaves was correlated with the negative symptoms score (PANSS-N) suggesting that temporal dysfunction may be related tonegative symptomatology. Results suggest that the distribution of slow-wave activity, measured in a standardized setting, mightadd diagnostic information about brain abnormalities in schizophrenia.
D 2002 Elsevier Science B.V. All rights reserved.
Keywords: Schizophrenia; Magnetoencephalography; Delta; Theta; Dipole density; Negative symptoms
Lopes da Silva, 1987; Lewine and Orrison, 1995;Matsuoka, 1990; Vieth et al., 2000; De Jongh et al.,
If prominent during the waking state, slow waves
2001). Since enhanced activity in lower EEG-fre-
generated in a circumscribed brain region typically
quency bands has also been reported in a variety of
characterize pathological or dysfunctional neural tis-
psychopathological conditions (for schizophrenia, see,
sue. Focal slow waves appear in the vicinity of struc-
tural lesions like cerebral infarcts, contusions, local
1991; Rockstroh et al., 1997), we have previously
infections, tumors, developmental defects, degenera-
suggested that dysfunctional brain areas in psychiatric
tive defects or subdural hematomas
patients might be indicated by the concentration of focalmagnetic slow waves Inschizophrenic patients, we found slow-wave generators
Corresponding author. Department of Psychology, University
in the magnetoencephalogram (MEG) to be more
of Konstanz, P.O. Box D23, D-78457 Konstanz, Germany. Tel.:+49-7531-882085; fax: +49-7531-882891.
frequent in association cortices, mostly in temporal
0920-9964/02/$ - see front matter D 2002 Elsevier Science B.V. All rights reserved.
doi:10.1016/S0920-9964(02)00213-X
T. Fehr et al. / Schizophrenia Research 63 (2003) 63–71
and parietal regions Given that focal
The data reported here result from a larger long-
slow-wave concentration is related to neuro-
term project that attempts to examine the diagnostic
psychological dysfunctions in schizophrenic patients,
usefulness of focal slow-wave mapping. Further out-
brain mapping of focal slow-wave generators may add
come will be reported elsewhere. For the resting state
information about possible neurophysiological corre-
only, a fraction of the presently reported data from a
lates of psychopathology. However, the dependency of
subsample of 16 patients was included in
this phenomenon on the particular context, state of
(2001). As there were no differences between the
activation or mental task activity of a patient and its
original 16 and the presently added 14 patients in
interaction with psychoactive medication needs to be
the outcome measures, they are pooled here in one
thoroughly explored before further consideration from
the perspective of diagnostic usefulness.
Previous reports of enhanced electric slow waves
in psychiatric patients over posterior, frontal-midline
2. Methods and materials
and frontal-temporal regions Fernandez et al., 1995; Iramina et al., 1996; Naka-
shima and Sato, 1992) did not distinguish focalgenerators from more widespread slow activity, i.e.,
Thirty patients (12 females) aged 31.6 F 8.9 years
they did not differentiate likely indices of patholog-
with the DSM-IV diagnosis of a schizophrenic dis-
ical brain activity from those that appear in the intact
order were compared to 18 healthy subjects (2
brain under various conditions of activation and
females). After one control subject had to be excluded
mental load. A first indication that the slow waves
because of artifact-contaminated data, the mean age of
in schizophrenic patients may be of a particular
the group of 17 controls was 32.4 F 11.2 years.
nature came from a study by
All patients, inpatients of the university research
which showed not only enhanced delta power in
ward at the local Center of Psychiatry, were asked by
schizophrenic patients, but also a different form of
the psychiatrist or psychologist in charge whether they
these slow waves and the embedding EEG time
would be willing to participate in the study. Diagnoses
course between groups.
were given by the psychiatrist/psychologist in charge
On this background, the present study explored the
on the bases of ICD-10 diagnostic criteria. As sum-
stability of focal magnetic slow waves across three
marized in the majority of patients met the
different conditions of mental activation, designed to
diagnosis of paranoid – hallucinatory subtype (N = 22;
enhance either left (mental arithmetic) or right parieto-
three patients were diagnosed as undifferentiated, two
temporal (spatial imagery) brain hemispheric activa-
as disorganised; two patients met the criteria of a
tion. We wanted to know to what extent the topo-
schizophreniform disorder and one of a schizoaffec-
graphical pattern of slow-wave activity may serve as a
tive disorder. Additional diagnoses—mostly of drug
marker of cortical dysfunction during the waking state
dependence—were given to six patients.
irrespectively of the type of the particular mental
The psychopathological status of each patient was
activity during which the measurement is obtained.
assessed on the day of the experiment by the psychol-
Effects of task or activation would make it difficult to
ogist/psychiatrist in charge by means of the Positive
establish a diagnostic tool based on focal slow-wave
and Negative Syndrome Scale (PANSS)
mapping. Another possible complication may come
1987) (average scores PANSS-P: 15.8 F 4.9, range 8 –
from the impact of medication. General slowing of
26; PANSS-N: 21.2 F 6.7, range 9 – 33; PANSS-G:
EEG frequencies has been reported as a consequence
37.8 F 12.0, range 25 – 87) (see for individual
of neuroleptic medication
symptom scores). Twenty-one patients were under
Malow et al., 1994), but also normalizing' effects
neuroleptic medication at the time of the assessment,
12 receiving typical neuroleptics only, 3 atypical
fore, the possible relationship between the focal
neuroleptics only and 5 a combination of typical and
clustering of slow waves and neuroleptic medication
atypical neuroleptics (see also The patient
was also addressed.
with schizoaffective disorder received a combination
T. Fehr et al. / Schizophrenia Research 63 (2003) 63–71
Table 1ICD-10 diagnoses, type of medication (INN) and PANSS scores for the 30 schizophrenic patients
clozapin, flupentixol
clozapin, melperon
lithium, chlorprothixen
haloperidol, clozapin
chlorprothixen, atypical
fluphenazin, perphenazin
F20.0, F10.1, F19.1
haloperidol perphenazin
Each PANSS scale comprises seven items with scores ranging from 1 (not apparent) to 7 (maximal strength of symptom). Thus, sum scores canvary between 7 (asymptomatic) to 49.
of lithium and chlorprothixene. Two patients received
did not report any history of psychiatric illness for
additional anticholinergics. The average daily dosage
themselves or first degree relatives, if they did not
was 144.99 F 163.6 mg CPZ eq (CPZ eq were deter-
report head injury or other neurological disorders
mined after the atypical
affecting the brain, and if they did not report to be
neuroleptics used, clozapine and risperidone, were
under current psychoactive medication or regular
adjusted for the CPZ eq according to the same table
drug use or abuse. In all subjects, handedness was
with 0.9 and 8, respectively). Nine patients were
assessed by a modified version of the Edinburgh
unmedicated at the time of the measurement, because
Handedness Questionnaire asking
they had refused neuroleptic treatment until their
subjects to demonstrate hand use on various actions
admission. For all patients, it was ascertained that
(like using a broom, brushing teeth, writing, etc.).
patients did not use any psychoactive substances other
Five patients proved to be left-handed, while all
than nicotine during their inpatient treatment. Dura-
controls were determined as right-handed. Prior to
tion of illness varied between 1 and 107 months
the experiment, subjects were familiarized with the
(mean 22.04 F 29.37 months).
recording environment, informed about the procedure
Control subjects, recruited by announcements in
and gave written consent to participate in the experi-
the hospital and the university, were interviewed by a
ment, for which they received a financial bonus of
trained psychologist and were only accepted if they
about US$10.
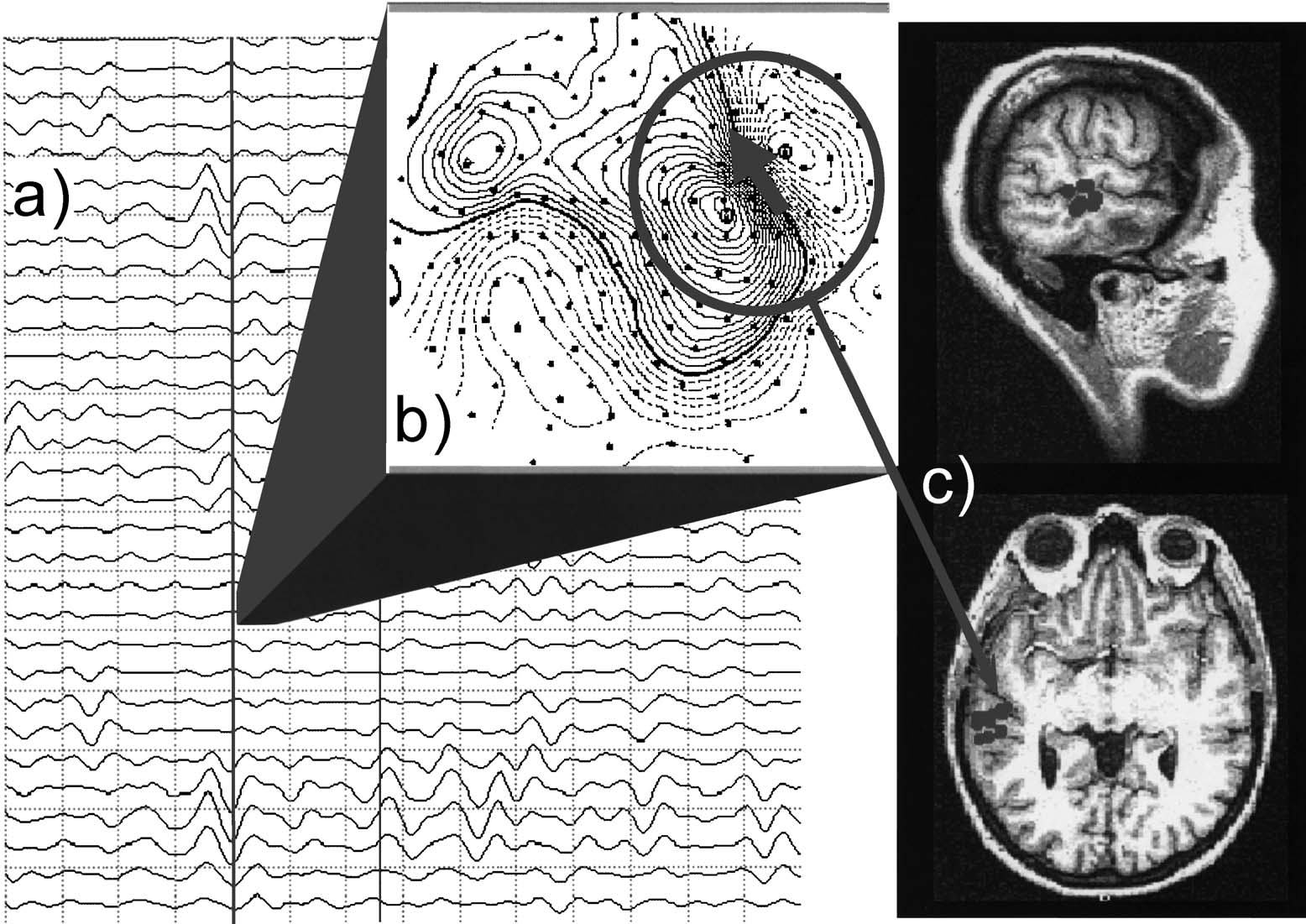
Fig. 1. Determination of dipole density. (a) Selection of MEG traces. Large-amplitude slow waves can be observed in a subset of channels. (b) Contour plot. The algorithm detects aregional dipolar source which can be fitted with an equivalent current dipole model (GOF > 0.90 required). (c) Localization of the dipolar activity and determination of number ofdipoles located within predefined brain regions per unit time. Note that a high goodness for the dipole fit is required only for a subset of 37 channels (e.g., within the circle indicated in(b)) and that activity may or may not appear simultaneously at other sensors.
T. Fehr et al. / Schizophrenia Research 63 (2003) 63–71
2.2. Data collection
segments were determined by visual inspection. Sin-gle-equivalent current dipoles in a homogeneous
Using a 148-channel whole-head neuromagnetom-
sphere were fitted for each time point in the selected
eter (MAGNESk 2500 WH, 4D Neuroimaging, San
epochs. Only dipole fit solutions at time points with a
Diego, USA), the MEG was measured during three
root mean square 100 fT < (RMS=(
periods of 5 min each. In the resting condition,
< 300 fT and a goodness of fit (GOF) greater than 0.90
subjects were asked to relax but to stay awake and
were accepted for further analysis. These restrictions
not to engage in any specific mental activity. In the
should ensure that neither artifacts nor small ampli-
mental arithmetic condition, subjects were asked to
tude biological noise would affect the results, and that
translate the words of a common German folksong
only dipolar fields that were generated by focal
letter by letter into numbers (a corresponding to 1, b to
sources were analyzed. Since artifact-free epochs
2, c to 3, etc.) and total them up. In the mental
varied in length, the percentage of data time points
imagery condition, subjects were asked to imagine
per second that could be fitted by the dipole model in
as vividly as possible walking a well-known and
a particular area was submitted to the statistical
recently strolled footpath, e.g., through the hospital
analyses (see for an illustration of steps in data
area. Breaks separated the three recording periods and
allowed subjects to move. MEG recordings were
The distribution of dipole density was assessed by
obtained in a supine position, and subjects were asked
dividing the total brain volume into 10 regions, five in
to fixate upon a colored fixation mark on the ceiling
each hemisphere: prefrontal, frontal, temporal, parietal
of the magnetically shielded room throughout the
and occipital. Effects of conditions and diagnosis on
recording in order to avoid eye- and head-movement.
the pattern of dipole densities in the delta and theta
A video camera installed inside the chamber allowedmonitoring the subject's behavior and compliance atany time throughout the experiment. For the mentalarithmetic condition, compliance was assessed bycomparing the result of totaling up the word sumsand the position in the song that was reached by thesubject at the end of the 5-min period. For the mentalimagery condition compliance was assessed by askingthe subject to describe the imagined tour in detail.
The MEG was recorded with a 678.17-Hz sam-
pling rate, using a band-pass filter of 0.1 – 200 Hz. Forartifact control, eye movements (EOG) were recordedfrom four electrodes attached to the left and rightouter canthus and above and below the right eye. Theelectrocardiogram (EKG) was monitored via electro-des attached to the right collarbone and the lowest leftrib. A Synamps amplifier (NEUROSCAN) served forthe recording of EOG and EKG.
2.3. Data reduction and analysis
For each of the three 5-min recording epochs the
data were band-pass filtered by a second order filter in
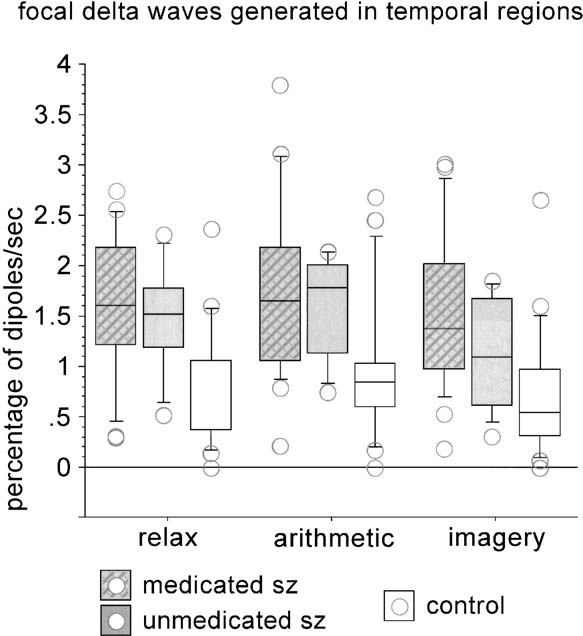
Fig. 2. Boxplot for the percentage of delta dipoles per second
the delta (1.5 – 4.0 Hz) and theta (4.0 – 8.0 Hz) band.
(ordinate) for the group of controls and the groups of schizophrenicswith and without medication. The three different conditions
The number of sample points was reduced by factor
(abscissa: resting, arithmetic, imagery) produce similar differences
16 prior to further analysis, each sample point repre-
between groups in temporal regions, with many schizophrenic
senting an epoch of about 20.8 ms. Artifact-free time
subjects exhibiting values outside the range of controls.
T. Fehr et al. / Schizophrenia Research 63 (2003) 63–71
range were evaluated by analyses of variance with thebetween-subjects factor GROUP and the within-sub-jects factors CONDITION, AREA (comparing pre-frontal, frontal, temporal, parietal, and occipital dipoledensities) and HEMISPHERE (comparing the left-and right-hemispheric areas). For interactions withdegrees of freedom larger than 1, the degrees offreedom were corrected using the Greenhouse –Geisser procedure to account for possible violationsof the sphericity assumption.
Schizophrenic patients exhibited more focal delta
activity (i.e., a higher percentage of delta dipoles persecond) than controls, group differences being most
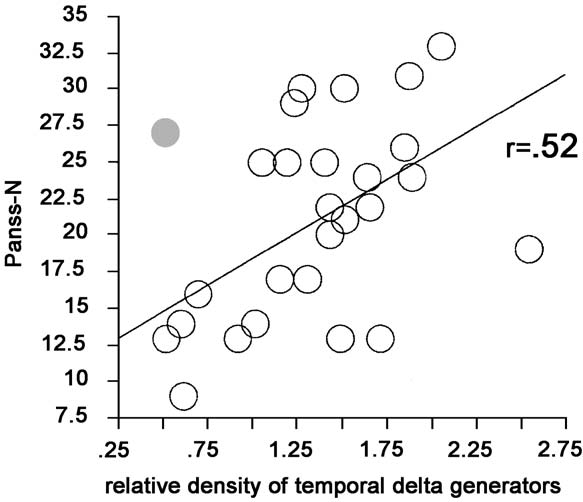
Fig. 3. Correlation between the relative density of temporal delta
pronounced in temporal and parietal areas [GROUP
dipoles (percentage temporal dipoles per second divided by the
average percentage of dipoles per second across all brain regions)
and the amount of negative symptoms as measured by the PANSS-
p < 0.05; GROUP, F(1,45) = 6.41, p < 0.05; for tem-
N scale. The correlation is significant even if one subject with a high
poral areas, GROUP, F(1,45) = 10.75, p < 0.01; for
dipole density in all areas (indicated by the dark dot) is included.
parietal areas, GROUP, F(1,45) = 5.47, p < 0.05]. This
result was equally prominent for all conditions, i.e.,
there was no interaction of group with task (and
Mean ( F S.D. in brackets) percentage of delta dipoles per second of
Temporal delta was equally prominent in
artifact-free epochs in the two groups (p = schizophrenic patients,
medicated (N = 21) and nonmedicated (N = 9) patients
c = controls) for the three conditions (resting, mental arithmetic and
The temporal percentage of delta dipoles per sec-
Mental arithmetic Mental imagery
ond relative to the total amount (averaged across all
areas) correlated positively with the negative symp-
toms (r = 0.42, P < 0.05; excluding one subject with a
(1.00) (1.25) (1.36)
relative large amount of delta dipoles in all other
Right prefrontal 1.27
regions and, therefore, a lower fraction of temporal
(0.98) (0.59) (1.55)
activity would increase the correlation coefficient to
(0.61) (0.48) (0.75)
In both groups, mental arithmetic produced a
(1.00) (0.45) (1.22)
somewhat higher amount of focal slow waves than
rest and imagery in temporal [CONDITION,
(0.92) (0.77) (0.98)
F(2,90) = 4.51, p < 0.05] and prefrontal [CONDI-
(0.68) (0.55) (0.73)
TION, F(2,90) = 4.83, p < 0.05] areas.
Patients also produced a significantly greater per-
(1.32) (0.52) (0.90)
centage of theta dipoles per second than controls, group
differences being again most pronounced in temporal
(1.20) (0.43) (0.85)
and parietal areas compared to prefrontal, frontal and
(0.86) (0.40) (0.73)
occipital areas [GROUP �AREA � HEMISPHERE,
F(4,180) = 5.11, p < 0.01; GROUP, F(1,45) = 5.73,
(0.71) (0.62) (0.44)
p < 0.05; for temporal areas, GROUP, F(1,45) = 7.97,
T. Fehr et al. / Schizophrenia Research 63 (2003) 63–71
p < 0.01; for parietal areas, GROUP, F(1,45) = 4.27,
2001) in addition to the equally often reported frontal
p < 0.05]. While the prominence of temporal theta
dysfunction. Indications of temporal dysfunctions
activity did not differ significantly between medicated
have been obtained from imaging
and nonmedicated patients ( F < 1), the patients with
1995; Hirayasu et al., 1999) and event-related poten-
higher daily medication displayed more pronounced
temporal theta prominence (r = 0.51, p < 0.01).
If we consider volume reduction in the temporal lobe
Effects of mental activation on theta dipole density
were similar in both groups (interactions n.s.) with
indication of dysfunction comparable to a lesion,
higher density of theta dipoles during spatial imagery
enhanced slow-wave activity arising from these
than during the other two conditions [CONDITION,
regions is a conceivable finding.
F(2,90) = 3.41, p < 0.05]. Imagery reduced and mental
Patients with and without neuroleptic medication
arithmetic increased parietal theta activity, while both
did not differ in the temporal enhancement of slow-
activation conditions reduced frontal theta activity rel-
wave (delta and theta) activity, whereas temporal theta
ative to rest [CONDITION � AREA, F(8.360) =
prominence correlated with daily dosage of neuro-
4.92, p < 0.01].
leptics. This does not seem to support findings of ageneral slowing of EEG frequencies as a consequenceof neuroleptic medication
et al., 1994), but might be considered in line with thefinding of increased EEG theta activity concomitant
The present findings underscore the previous
with an increase in haloperidol plasma levels in patients
report of more frequent generators
who responded to the treatment
of focal slow waves (in the delta and theta bands) in
1991). However, pharmaco-EEG studies have mostly
schizophrenic patients. This activity prevails in tem-
reported evenly distributed activity
poral and parietal areas. A generator within the
Morikawa et al., 1997) or a ‘‘flat table distribution'' of
temporal lobe may be oriented such that its volume
theta activity sometimes
currents project to frontal scalp regions (like, e.g., the
with anterior predominance of theta bursts
generators of the auditory evoked N100). In this case
and Herrmann, 1996), but no relationship between
EEG recordings would pick up activity over frontal
temporal enhancement of slow-wave generators and
regions. The group-specific distribution of focal slow
medication. Therefore, we assume a rather weak impact
waves appeared under all conditions of mental acti-
of neuroleptic medication on the diagnosis-specific
vation. Mental activation changed the pattern of focal
pattern of slow-wave generators. Moreover, an indirect
slow-wave activity relatively little, only in prefrontal
relationship between focal slow waves and severity of
areas, and to a similar degree, whereas the prominent
illness is indicated by the negative symptom score,
difference between groups in temporal brain regions
while the impact of neuroleptic medication was not
was not affected at all. Thus, a disease-specific pattern
supported by a significant correlation between the two
of focal slow-wave activity and the effect of activation
latter measures.
on slow waves seem to add together. It might be
Group differences were also statistically mean-
possible that characteristics in the course of brain
ingful for the theta band. However, the magnitude of
activity over time might allow to tease apart these
effects was less pronounced, possibly, because focal
two different types of slow-wave activity, given that
theta activity was affected by medication and the
nonlinear measures add to the differentiation between
type of task. Increased power in the EEG theta
frequency band has been found with different mental
The accentuation of focal slow wave in the tem-
tasks including, for instance, the encoding of mate-
poral regions correlated with the negative symptoms
rial that was later successfully retrieved
score. If these focal waves do indicate dysfunctional
al., 1996, 1997, 2001; Doppelmayr et al., 1998;
brain tissue, then the present results add to structural
Yamamoto and Matsuoka, 1990). Although the type
and functional evidence of temporal abnormalities in
of task differs between those studies and the present
one, a common element in memory encoding and
T. Fehr et al. / Schizophrenia Research 63 (2003) 63–71
retrieval and the imagination of a well-known path
R.D., Harsh, J.R. (Eds.), Sleep Onset. APA Press, Washington,
from memory might be associated with enhanced
pp. 201 – 218.
Canive, J.M., Lewine, J.D., Edgar, J.C., Davis, J.T., Torres, F.,
theta activity. Thus, the present analysis confirms
Roberts, B., Graeber, D., Orrison, W.W., Tuason, V.B., 1996.
that mental imagery activates theta sources and
Magnetoencephalographic assessment of spontaneous brain ac-
indicates that the sources of this activity are con-
tivity in schizophrenia. Psychopharmacol. Bull. 32, 741 – 750.
fined to prefrontal brain areas.
Canive, J.M., Lewine, J.D., Edgar, J.C., Davis, J.T., Miller, G.A.,
Tasks had been selected to specifically activate
Torres, F., Tuason, V.B., 1998. Spontaneous brain magneticactivity in schizophrenic patients treated with aripiprazole. Psy-
either the left or the right hemisphere. Contrary to
chopharmacol. Bull. 34, 101 – 105.
our expectation though, the distribution of delta and
Czobor, P., Volavka, J., 1991. Level of haloperidol in plasma is
theta dipole densities was not asymmetrical and did
related to electroencephalo-graphic findings in patients who im-
not vary with the task in a hemisphere-specific man-
prove. Psychiatry Res. 42, 129 – 144.
ner. This suggests that delta and theta dipole densities
Deicken, R.F., Calabrese, G., Merrin, E.L., Vinogradov, S., Fein, G.,
Weiner, W., 1995. Asymmetry of temporal lobe phosphorous
are not task-related—like activity in the gamma band.
metabolism in schizophrenia: a phosphorous magnetic reso-
Thus, we have to conclude the tasks selected for
nance spectroscopy study. Biol. Psychiatry 38, 279 – 286.
hemisphere-specific mental activation and the meas-
De Jongh, A., de Munck, J.C., Baayen, J.C., Jonkman, E.J., Hee-
ure selected as indication of (dys)function did not fit
thaar, R.M., van Dijk, B.W., 2001. The localization of sponta-
most favorably.
neous brain activity: first results in patients with cerebraltumors. Clin. Neurophysiol. 112, 378 – 385.
In sum, the present results support previous EEG
Doppelmayr, M., Klimesch, W., Schwaiger, J., Auinger, P., Winkler,
and MEG studies regarding the evidence of an acti-
T., 1998. Theta synchronization in the human EEG and episodic
vation-invariant pattern of prominent temporal slow-
retrieval. Neurosci. Lett. 20, 41 – 44.
wave activity in schizophrenics. The distribution of
Elbert, T., Lutzenberger, W., Rockstroh, B., Berg, P., Cohen, R.,
focal slow-wave clusters might add diagnostic infor-
1992. Physical aspects of the EEG in schizophrenics. Biol. Psy-chiatry 32, 595 – 606.
mation, the more so as recent results from our labo-
Faux, S.F., Nestor, P.G., McCarley, R.W., Shenton, M.E., Horvath,
ratory indicate a diagnostic specificity of the presently
T., Davis, K., 1990. P300 asymmetries in unmedicated schizo-
reported pattern for schizophrenia when contrasted
phrenics. In: Brunia, C.H.M., Gaillard, A.W.K. (Eds.), Psycho-
physiological Brain Research. Tilburg Univ. Press, Tilburg, pp.
publication). An abnormal activity in the temporal
209 – 212.
Fehr, T., Kissler, J., Elbert, T., Wienbruch, C., Moratti, S., Rock-
association cortices seems to vary with negative
stroh, B., 2001. Source distribution of neuromagnetic focal slow
symptoms, while it seems possible that abnormal
waves and MEG-delta activity in schizophrenic patients. Biol.
activity in other areas could represent other aspects
Psychiatry 50, 108 – 116.
of the schizophrenic symptomatology. It might be
Fernandez, T., Harmony, T., Rodroguez, M., Bernal, J., 1995. EEG
worthwhile to examine the clinical usefulness of this
activation patterns during the performance of tasks involvingdifferent components of mental calculation. Electroencephalogr.
Clin. Neurophysiol. 94, 175 – 182.
Hasan, J., Broughton, R., 1994. Quantitative topographic EEG map-
ping during drowsiness and sleep onset. In: Ogilvie, R.D.,
Harsh, J.R. (Eds.), Sleep Onset. APA Press, Washington, pp.
219 – 236.
This research was supported by the Deutsche
Hirayasu, Y., Shenton, M.F., Salisbury, D.F., Frumin, M., Fischer,
L.A., Farrell, D., Yurgelu-Todd, D.A., Zarate, C., McCarley,
Forschungsgemeinschaft and the Volkswagen-Stif-
R.W., 1999. Progressive change in posterior superior temporal
tung. We thank Drs. K. Pro¨pster, B. Schuller and P.
gyrus in schizophrenia. Biol. Psychiatry 45S, 117S, abstract.
Ro¨ssner for accomplishing the diagnostics and clinical
Iramina, K., Ueno, S., Matsuoka, S., 1996. MEG and EEG topog-
status of the patients.
raphy of the frontal midline theta rhythm and source localiza-tion. Brain Topogr. 8, 329 – 331.
Jahn, T., Mussgay, L., 1989. Die statistische Kontrolle mo¨glicher
Medikamenteneinflu¨sse in experimentalpsychologischen Schiz-
ophreniestudien. Z. Klin. Psychol. 18, 257 – 267.
Johnstone, E.C., Owens, D.G., Crow, T.J., Frith, C.D., Alexandrop-
Badia, P., Wright, K., Wauquier, A., 1994. Fluctuation in single-
olis, K., Bydder, G., Colter, N., 1989. Temporal lobe structure as
Hertz EEG activity during the transition to sleep. In: Ogilvie,
determined by nuclear magnetic resonance in schizophrenia and
T. Fehr et al. / Schizophrenia Research 63 (2003) 63–71
bipolar affective disorder. J. Neurol., Neurosurg. Psychiatry 52,
on appearance of frontal midline theta activity in EEG. J. Hu-
736 – 741.
man Ergol. 21, 201 – 206.
Kay, S.R., Fiszbein, A., Opler, L.A., 1987. The positive and neg-
Niedermeyer, E., Lopes da Silva, F., 1987. Electroencephalography:
ative syndrome scale (PANSS) for schizophrenia. Schizophr.
Basic Principles, Clinical Applications and Related Fields. Ur-
Bull. 13, 261 – 276.
ban & Schwarzenberg, Baltimore.
Klimesch, W., Doppelmayr, M., Russegger, H., Pachinger, T., 1996.
Oldfield, R., 1971. The assessment and analysis of handedness: the
Theta band power in the human scalp EEG and the encoding of
Edinburgh Questionnaire. Neuropsychologia 9, 97 – 113.
new information. NeuroReport 7, 1235 – 1240.
Rockstroh, B., Elbert, T., Berg, P., 1997. Die Untersuchung von
Klimesch, W., Doppelmayr, M., Schimke, H., Ripper, B., 1997.
Strukturen und Funktionen des Gehirns in der experimentellen
Theta synchronization and alpha desynchronization in a memo-
Psychopathologie am Beispiel der Schizophrenien. In: Rock-
ry task. Psychophysiology 34, 169 – 176.
stroh, B., Elbert, T., Watzl, H. (Eds.), Impulse fu¨r die Klinische
Klimesch, W., Doppelmayr, M., Stadler, W., Pollhuber, D., Suu-
Psychologie. Hogrefe, Go¨ttingen, pp. 1 – 29.
seng, P., Rohm, D., 2001. Episodic retrieval is reflected by a
Rockstroh, B., Wienbruch, C., Moratti, S., Kissler, J., Fehr, T.,
process specific increase in human electroencephalographic the-
Elbert, T., 2000. Magnetic source imaging of slow wave activity
ta activity. Neurosci. Lett. 13, 49 – 52.
in psychiatric samples. Proceedings of the 12th Int. Conference
Koshino, Y., Murata, I., Morata, T., Omori, M., Hamada, T., Miya-
on Biomagnetism, Helsinki, pp. 395 – 397.
goshi, M., Isaki, K., 1993. Frontal intermittent delta activity in
Saletu, B., Kuefferle, B., Grunberger, J., Foldes, P., Topitz, A.,
schizophrenic patients receiving antipsychotic drugs. Clin. En-
Anderer, P., 1994. Clinical EEG mapping and psychometric
cephalogr. 24, 13 – 18.
studies in negative schizophrenia: comparative trials with ami-
Kubicki, S., Herrmann, W.M., 1996. The future of computer-assis-
sulpiride and fluphenazine. Neuropsychobiology 29, 125 – 135.
ted investigation of the polysomnogram: sleep microstructure.
Schober, F., Schellenberg, R., Dimpfel, W., 1996. Reflection of
Clin. Neurophysiol. 13, 285 – 294.
mental exercise in the dynamic quantitative topographical
Lewine, J.D., Orrison, W.W., 1995. Magnetoencephalography and
EEG. Neuropsychobiology 31, 98 – 112.
magnetic source imaging. In: Orrison, W.W., Lewine, J.D.,
Shagass, C., 1991. EEG studies of schizophrenia. In: Steinhauer,
Sanders, J.A., Hartshorne, M.F. (Eds.), Functional Brain Imag-
S.R., Gruzelier, J.H., et al., (Eds.), Neuropsychology, Psycho-
ing. Mosby-Year Book, St. Louis, pp. 404 – 413.
physiology, and Information Processing. Handbook of Schizo-
Malow, B.A., Reese, K.B., Sato, S., Bogard, P.J., Malhotra, A.K.,
phrenia. Elsevier, Amsterdam, pp. 39 – 69.
Su, T.P., Pickar, D., 1994. Spectrum of EEG abnormalities dur-
Shenton, M.F., Dickey, C.C., Frumin, M., McCarley, R.W., 2001. A
ing clozapine treatment. Electroencephalogr. Clin. Neurophy-
review of MRI findings in schizophrenia. Schizophr. Res. 15,
siol. 91, 205 – 211.
Matsuoka, S., 1990. Theta rhythms: state of consciousness. Brain
Vieth, J.B., Kober, H., Ganslandt, O., Mo¨ller, M., Kamada, K.,
Topogr. 3, 203 – 208.
2000. The clinical use of MEG activity associated with brain
McCarley, R.W., Shenton, M.E., O'Donnell, B.F., Faux, F.S., Kiki-
lesions. Proceedings of the 12th Int. Conference on Biomagnet-
nis, R., Nestor, P.G., Jolesz, F.A., 1993. Auditory P300 abnor-
ism, Helsinki, pp. 387 – 394.
malities and left posterior superior temporal gyris volume
Wienbruch, C., Moratti, S, Vogel, U., Fehr, T., Kissler, J., Elbert, T.,
reduction in schizophrenia. Arch. Gen. Psychiatry 50, 190 – 197.
Schiller, A., Rockstroh, B. Source distribution of neuromagnetic
McCarley, R.W., Wible, C.G., Frumin, M., Hirayasu, Y., Levitt, J.J.,
slow wave activity in schizophrenic and depressive patients,
Fischer, I.A., Shenton, M.E., 1999. MRI anatomy of schizophre-
submitted for publication.
nia. Biol. Psychiatry 45, 1099 – 1119.
Winterer, G., Herrmann, W.M., 1995. U
¨ ber das Elektroenzephalo-
Morikawa, T., Hayashi, M., Hori, T., 1997. Auto power and coher-
gramm in der Psychiatrie: Eine kritische Bewertung. Z. Elek-
ence analysis of delta – theta band EEG during waking – sleeping
troenzephalogr., Elektromyogr. Verw. Geb. 26, 19 – 37.
transition. Electroencephalogr. Clin. Neurophysiol. 103, 633 –
Yamamoto, S., Matsuoka, S., 1990. Topographic EEG study of
visual display terminal (VDT) performance with special refer-
Nakashima, K., Sato, H., 1992. The effects of various mental tasks
ence to frontal midline theta waves. Brain Topogr. 2, 257 – 267.
Source: http://www.exzellentforschen.de/psychologie/personen/ae02/KisslerPDFs/FehrKissler_et_al_SZres2003.pdf
Reishi or Ling Zhi (Ganoderma lucidum) Solomon P. WasserInstitute of Evolution, University of Haifa, Mount Carmel, Haifa, Israel and locust (Quercus, Acer, Alnus, Betula, Castanea,Coryolus, Fagus, Fraxinus, Populus, Pyrus, Magnolia, Ganoderma lucidum (reishi mushroom, Ling Zhi) has Tilia). G. lucidum is less frequently found on conifer- been an economically important species, particularly
Benefits You Can Count On Prism EPO/Prism PPO/Blue View Vision Country Life LLC February 25, 2010 Dear Country Life employee, This is an important decision and this booklet is designed to help. Basically, it's a snapshot of the benef ts come with your Empire coverage. It summarizes what's available to you, what you get with each benef t your plans work. Of course, you should always refer to your evidence of coverage or medical policy for full plan details.



