Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Print layout
Clinical and Laboratory StudiesFreeStyle™ Blood GlucoseTest Strip Performance
Executive Summary
2.0 FreeStyle Overview . . . . . . . . . 2
Objective:
To evaluate the performance of the Abbott FreeStyle blood glucose test strip for accuracy, precision,
2.1 Coulometry Technology . . . . . . . . 2
linearity, interference, hematocrit effect, and ease of use.
2.2 Glucose Dehydrogenase (GDH) . . . . . 3
2.3 Reliable Sample Detection . . . . . . . 3
To assess accuracy, clinical studies were conducted with the FreeStyle test strip:
2.4 Low Potential (Voltage) Measurement. . . 4
(1) Accuracy in testing arterial blood specimens was assessed at Duke University Medical Center and
at Hartford Hospital. Duplicate results obtained with the FreeStyle system were compared against
3.0 Clinical Accuracy Studies . . . . . . . 4
plasma glucose results from the YSI 2300 Glucose Analyzer and another laboratory analyzer fromthe study site laboratory. A total of 301 arterial blood samples were included in this study.
3.1 Comparative Methods . . . . . . . . 4
(2) Accuracy in testing neonatal blood specimens was assessed at St. Louis Children's Hospital.
3.2 Statistical Methods . . . . . . . . . 4
Results were compared against the VITROS System. A total of 206 neonatal capillary (heelstick)
3.3 Arterial Studies: Sites and Patients. . . . 4
blood specimens were included in this study.
To assess precision, linearity, interference and hematocrit effect, laboratory studies were conducted
3.4 Arterial Studies: Results. . . . . . . . 5
by Abbott Diabetes Care.
3.5 Neonatal Study: Site and Patients . . . . 6
To assess ease of use, a lay user study was conducted with 185 participants at 3 clinical sites.
3.6 Neonatal Study: Results. . . . . . . . 6
4.0 Laboratory Studies . . . . . . . . . 7
(1) In the arterial studies, 100% of the FreeStyle results were in Zone A (clinically accurate) of the Clarke
and Consensus Error Grids compared to the YSI. Compared to the analyzers from the study site labo-
ratories, 99.8% of the results were in Zone A; 1 result (0.2%) was in Zone B (clinically acceptable).
(2) In the neonatal study, 100% of the FreeStyle results were in Zone A (clinically accurate) or Zone B
4.3 Interference Study . . . . . . . . . 8
(clinically acceptable) of the Leroux Error Grid compared to the YSI. 93% of the results were in Zone A.
4.4 Hematocrit Effect Study . . . . . . . 9
In the laboratory studies:
Precision: Variability in blood tests from strip to strip was 5.6% or less.
Linearity: Linearity of the FreeStyle test strip was demonstrated across the measurement range of
20–600 mg/dL.
Interference: None of 16 substances tested at high concentrations showed clinically significant effect
on FreeStyle results.
Hematocrit: In tests of blood samples with a hematocrit range of 15% to 65%, 98.8% of 497 results
were in Zone A (clinically accurate) of the Clarke Error Grid; 6 results (1.2%) were in Zone B (clinicallyacceptable).
In the user tests evaluating FreeStyle ease of use, lay participants gave an overall mean score of 5.5 ona scale of 1–6 (with 6 reflecting greatest ease), confirming that the FreeStyle blood glucose monitoringsystem is easy to use.
Conclusions:
These studies verified the accuracy of the FreeStyle test strip for arterial and neonatal blood samples
when compared to laboratory results. Additional laboratory studies demonstrated that the FreeStyle test
strip performed well in precision, linearity and interference. The user study confirmed that the FreeStyle
test strip and system are easy to use. Taken together, these clinical, laboratory and user studies show
that the FreeStyle system is well suited for meeting the challenges of today's point-of-care blood glu-
cose monitoring.
FreeStyle Blood Glucose Test Strip Performance
The FreeStyle blood glucose test strip (Abbott Laboratories, Abbott Diabetes Care) utilizes coulometry technology toquantify glucose, requiring only 0.3µL of whole blood for analysis.
Multicenter clinical studies were conducted to evaluate accuracy performance with various specimen types. Additionallaboratory studies were performed to validate performance in precision, linearity, hematocrit effect and interference. Auser study was conducted to evaluate ease-of-use of the FreeStyle test strip.
2.0 FreeStyle Overview
2.1 Coulometric Technology
The FreeStyle test strip utilizes coulometric biosensor technology to quantify glucose in a whole blood sample, requiring
just 0.3µL of blood for analysis.
Coulometry is an electrochemical method for blood glucose measurement. Glucose in the sample is oxidized to glucono-lactone via its reaction with glucose dehydrogenase (GDH) in the presence of a proprietary electron-transfer mediator.
The reaction proceeds as follows:
2 mediator (oxidized) + glucose
→ 2 mediator (reduced) + gluconolactone
Other electrochemical glucose meters use amperometry, which measures the amplitude of a current during a narrowwindow of time in the reaction. Consequently, only a portion of the glucose in the sample is reacted and measured, andthe amplitude of the electrical current is sensitive to hematocrit.
Suitable for larger
sample volume
Ideal for small
sample volume
Small (variable)
percentage of
Virtually all of
glucose reacts
glucose reacts
Reacted glucose =
Unreacted glucose =
In contrast to amperometric meters, the FreeStyle measures the total charge generated by the reaction and virtually all the glucose in the sample is reacted. This "area-under-the-curve" signal is larger than the signal in amperometry.
Hematocrit fluctuations can change the shape of the curve in electrochemical assays, but it has little influence on thearea-under-the-curve. Thus, it impacts coulometry much less than amperometry.
Amperometry Response Curves
Coulometry Response Curves
With amperometry, current
Amperometry — 40 mg/dL
Coulometry — 40 mg/dL
is measured at a singletime, or averaged over abrief time interval.
With coulometry, chargeresulting from the exhaus-
Amperometry — 180 mg/dL
Coulometry — 180 mg/dL
tive electrolysis of the
analyte is integrated.
Coulometry measures the
area under the current/
Amperometry — 400 mg/dL
Coulometry — 400 mg/dL
time curve, which is lessvariable than the height of
the curve at a particular
time (amperometry).
2.2 Glucose Dehydrogenase (GDH)
Glucose Dehydrogenase (GDH) is the enzyme used for oxidation of glucose in the FreeStyle test strip. The electrons
released in this reaction are shuttled by electron mediator to the working electrode, and the electrical charge is meas-
ured. Test time depends on glucose concentration, and averages 15 seconds.
Clinical and Laboratory Studies
GDH can be used to perform electrochemical glucose measurements without interference by oxygen in the blood sam-ple. The commonly used enzyme in most other electrochemical strips, Glucose Oxidase (GOX), is capable of reacting with oxygen to produce interference in the measurement.
Glucose reaction in FreeStyle—
Glucose reaction in most other
All electrons flow from glucose (G) to emzyme (GDH) to
In a side reaction, some electrons flow from glucose (G) to
mediator molecules (M), where they canbe measured.
enzyme (GOX) to oxygen (O2). These electrons cannot be
No side reactions.
measured which can lead to inaccurate results.
Use of GDH in the FreeStyle test strip helps ensure accuracy of results at extreme levels of blood oxygen. This helpsensure accuracy with patients undergoing oxygen therapy, patients with pulmonary problems, or for tests at high altitudes.
The chart below shows the effect of oxygen on FreeStyle measurements: Both the high and low oxygen samples givesimilar results. The chart on the right shows the oxygen effect on an electrochemical strip using GOX: The high and lowoxygen samples give distinctly different results.
FreeStyle – GDH
Low Oxygen
Low Oxygen
High Oxygen
High Oxygen
Average YSI Glucose (mg/dL)
Average YSI Glucose (mg/dL)
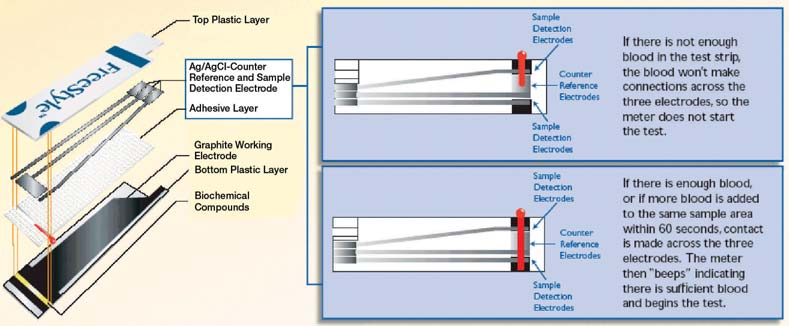
2.3 Reliable Sample Detection
The FreeStyle test strip is designed to ensure reliable sampling. Facing the working electrode on the test strip are three
other electrodes: two sample detection electrodes and a counter/reference electrode in between the sample detection
electrodes. The circuit connecting all three electrodes must be detected before the test will begin. This design minimizes
the possibility of inaccurate results due to low sample volume.
In the left chart below, the FreeStyle system does not report a result when an insufficient sample is applied to the strip.
When a sufficient sample is applied, a correct result is obtained. In the chart on the right, showing results from a systemthat does not detect the presence of an adequate sample, an inaccurately low or inaccurately high result can beobtained when an insufficient sample is applied.
FreeStyle Blood Glucose Test Strip Performance
FreeStyle – Sample Detection
A Strip Without Sample Detection
ometric System (mg/dL)
eeStyle Glucose (mg/dL)
Strip window not full
0 denotes no result
Strip window full
0 denotes no result
2.4 Low Potential (Voltage) Measurement
Low Potential Measurements offer a method for minimizing the interference by substances present in blood. In order for
an electrochemical reaction to occur, a potential (voltage) is usually applied between a working and counter electrode.
The larger the applied potential, the greater the number of interfering substances that can react and produce a signal.
The proprietary osmium-based mediator used in the FreeStyle test strip allows the glucose reaction to occur at a poten-tial very near zero. For this reason, very few substances can interfere with FreeStyle readings.
The effect of various common substances on FreeStyle test strip results was evaluated in a laboratory study, which issummarized in section 4.3 of this paper.
3.0 Clinical Accuracy Studies
To evaluate the accuracy of the FreeStyle test test strip, three clinical studies were conducted: two replicate studiesusing arterial blood samples and one study using neonatal blood samples. Each study was approved by the IRB at eachparticipating medical center.
3.1 Comparative Methods
Duplicate test results of the FreeStyle were compared to plasma glucose results of:
(1) YSI 2300™ Glucose Analyzer (YSI Inc.)—tested in duplicate at Duke University Medical Center and Hartford Hospital
(2) VITROS® System (Ortho-Clinical Diagnostics)—tested singly at Duke University Medical Center and St. Louis
Children's Hospital
(3) COBAS® Integra System (Roche Diagnostics)—tested singly at Hartford Hospital
3.2 Statistical Methods
Passing and Bablok regression1 was used to calculate the slope and intercept, comparing the FreeStyle Response (Y or
dependent variable) to the reference method (X or independent variable). The correlation coefficient (r) was calculated
using the standard regression method.
In the arterial study, the FreeStyle results were plotted against the reference results on the Clarke Error Grid2 and theConsensus Error Grid3. In the neonatal study, FreeStyle results were plotted against the reference results on the LerouxNeonatal Error Grid4. The number and percent of readings in each zone of the Error Grid were calculated.
3.3 Arterial Studies: Sites and Patients
The arterial studies were conducted at Hartford Hospital, Hartford, CT, and at Duke University Medical Center, Durham, NC.
A total of 301 arterial blood samples were tested. Three test strip lots were included in the evaluation at HartfordHospital (lots 0423138 and 0423152) and Duke University Medical Center (lots 0423138 and 0423201). Two replicatetests on the FreeStyle test strip were performed on each arterial blood sample.
A summary of the YSI Plasma Glucose results, other analyzer Plasma Glucose results and hematocrit values are given inthe table below.
Summary of YSI Plasma Glucose, Other Analyzer Plasma Glucose and Hematocrit Values
Other Analyzers (mg/dL)
1 Passing H, Bablok W: A new biochemical procedure for testing the equality of measurements from two different analytical methods. Applications of linear regression procedures for
method comparison studies in clinical chemistry, Part I, Journal of Clinical Chemistry and Clinical Biochemistry, November 1983, volume 21, no. 11 p709-720
2 Cox DJ, Gonder-Frederick L, Kovachev BP, Julian DM, Clarke WL: Understanding error grid analysis. Diabetes Care 20:911-912, 1997.
3 Parkes JL, Slatin SL, Pardo S, Ginsberg B: A New Consensus Error Grid to Evaluate the Clinical Significance of Inaccuracies in the Measurement of Blood Glucose. Diabetes Care.
23:1143-1148, 2000.
4 Leroux M: Glucose Meter Use In Nurseries, Laboratory Medicine, 9 September 1994, volume 25
Clinical and Laboratory Studies
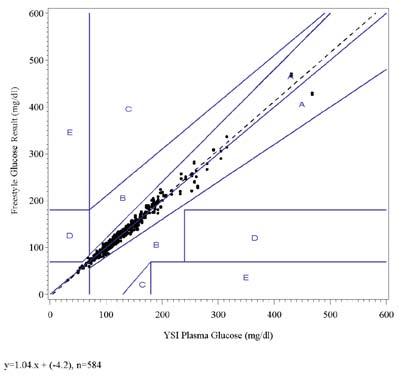
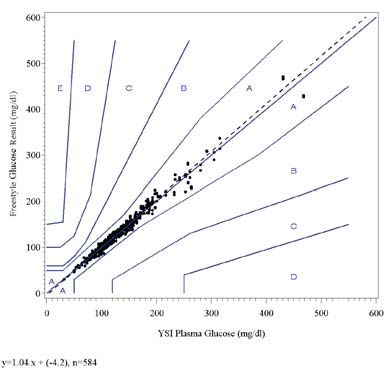
3.4 Arterial Studies: Results
The result of the linear regression analysis indicates good correlation of the FreeStyle test strips with the YSI Plasma
Glucose Reference and Laboratory Plasma Glucose Reference methods.
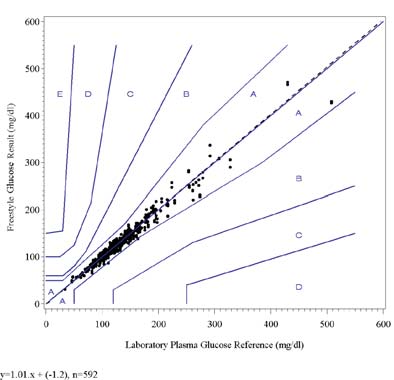
The results of the Clarke Error Grid and the Consensus Error Grid analyses show that all data points fall within zones Aand B indicating that they are clinically accurate or clinically acceptable.
The tables below show the results of the regression analysis against the YSI Plasma Glucose reference and theLaboratory Plasma Glucose Reference respectively.
Regression Statistics, FreeStyle vs. YSI Plasma Glucose
confidence interval
confidence interval
Regression Statistics, FreeStyle vs. Other Laboratory Analyzers' Plasma Glucose
confidence interval
confidence interval
The following graphs show the FreeStyle test strip results for all lots combined plotted against the YSI Plasma Glucoseresults and the plasma glucose results from other analyzers in the laboratory on the Clarke Error Grid and the ConsensusError Grid. The fitted regression line is shown on each graph.
FreeStyle Glucose vs. YSI Plasma Glucose, Clark Error Grid
FreeStyle Glucose vs. YSI Plasma Glucose, Consensus Error Grid
FreeStyle Glucose vs. Other Laboratory Analyzer Plasma
FreeStyle Glucose vs. Other Laboratory Analyzer Plasma
Glucose, Clark Error Grid
Glucose, Consensus Error Grid
FreeStyle Blood Glucose Test Strip Performance
The tables below show the number and percentage of results within each zone of the Clarke Error Grid and theConsensus Error Grid respectively.
Number and percentage of readings within each zone
of the Clarke Error Grid
FreeStyle vs. YSI Plasma Glucose
FreeStyle vs. Laboratory Plasma Glucose
Number and percentage of readings within each zone
of the Consensus Error Grid
FreeStyle vs. YSI Plasma Glucose
FreeStyle vs. Laboratory Plasma Glucose
3.5 Neonatal Study: Site and Patients
In the neonatal study, a total of 206 neonatal blood samples from neonatal capillary (heelstick) specimens, destined for
discard. Three test strip lots were included in the evaluation at St. Louis Children's Hospital (lots 0423138, 0423152 and
0423201). Two replicate tests on the FreeStyle test strip were performed on each neonatal blood sample.
A summary of the Laboratory Plasma Glucose results, hematocrit values and age of subject after exclusions are given inthe table below.
Summary of Laboratory Plasma Glucose, Hematocrit and Age of Subject
Laboratory Plasma Glucose (mg/dL)
Age of Subject (days)
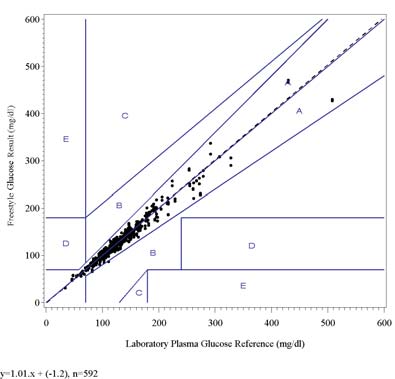
3.6 Neonatal Study: Results
The result of the linear regression analysis indicates good correlation of the FreeStyle with the Laboratory PlasmaGlucose method.
The results of the Leroux Error Grid analysis shows that all data points (within the concentration range of the grid) fallwithin zones A and B indicating that they are clinically accurate or clinically acceptable.
The results of the Consensus Error Grid analysis shows that all data points fall within zone A indicating that they are clinically accurate.
Regression Statistics, FreeStyle vs. Laboratory Plasma Glucose
confidence interval
confidence interval
The following graph shows the FreeStyle results for all lots combined plotted against the Laboratory Plasma Glucoseresults on the Leroux Error Grid. The fitted regression line is also shown on the graph.
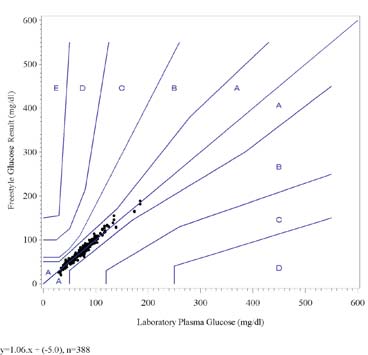
Clinical and Laboratory Studies
FreeStyle Glucose vs. Laboratory Plasma Glucose, Leroux Error Grid
The table below shows the number and percentage of results within each zone of the Leroux Error Grid.
Number and percentage of results within each zone of the Leroux Error Grid
Note: 4 observations fall outside the concentration range of the Leroux Error Grid.
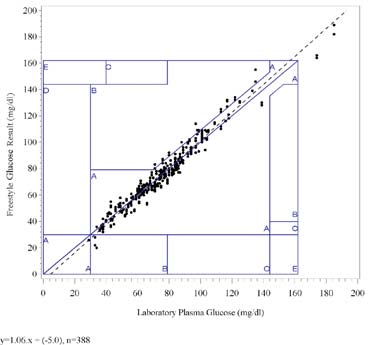
The following graph shows the FreeStyle results for all lots combined plotted against the Laboratory Plasma Glucoseresults on the Consensus Error Grid.
FreeStyle Glucose vs. Laboratory Plasma Glucose, Consensus Error Grid
All observations fall within zone A of the Consensus Error Grid.
4.0 Laboratory Studies
Studies were performed at Abbott Laboratories, Abbott Diabetes Care, to evaluate FreeStyle for precision, linearity,interfering substances and effect of hematocrit levels on results.
4.1 Precision Study
Within-lot and within-vial precision of FreeStyle test strips were measured with venous blood samples in the laboratory.
A total of 54 test strip lots were tested and the results pooled.
Results:
The pooled precision data for 54 test strip lots (n=5184) is shown in the following tables. Variability in blood tests from
strip to strip was 5.6% or less.
FreeStyle Blood Glucose Test Strip Performance
Within-Lot Precision
Average glucose concentration mg/dL (mmol/L)
SD mg/dL (mmol/L)
Within-Vial Precision
Average glucose concentration mg/dL (mmol/L)
SD mg/dL (mmol/L)
4.2 Linearity Study
Testing was performed to evaluate the linearity of the FreeStyle test strip across the dynamic range of 20–600 mg/dL.
The study was conducted over two days; on each day, capillary whole blood samples were collected from 24 donors. Thecapillary samples were spiked to varying glucose concentrations, distributed throughout the blood glucose testing range,and four donors were included in each of the six glucose ranges. Three lots of FreeStyle test strips were tested.
Results:
The results of the capillary linearity study are shown in the tables below. The Mean Bias is given in mg/dL for levels 1
and 2 and as Mean % Bias for levels 3–6. At glucose levels below 120 mg/dL, the Mean Bias values are –1.05 mg/dL
and –1.56 mg/dL respectively, and at glucose levels above 120 mg/dL, the Mean % biases are within ±2%.
Mean Bias in mg/dL at Levels 1 and 2
Average Bias
over Batches
Mean % Bias in mg/dL at Levels 3–6
Average Bias
over Batches
4.3 Interference Study
The Food and Drug Administration (FDA) recommends that a series of drugs and endogenous substances be tested for
possible interference with blood glucose assays. The drugs are tested at several times of their therapeutic level and
endogenous substances are screened at their maximum expected level. Sixteen substances commonly found in blood
were tested. The experiments consisted of comparing the results for samples to which the interferents were added with
results of samples to which no interferent was added. The effect of each interferent on the results was determined at 50,
200, and 400mg/dL glucose concentration.
Results:
The table below shows the list of the tested electro-oxidizable constituents of blood, which are potential interferents.
The list includes acetaminophen (Tylenol™), ascorbic acid (vitamin C) and others, all tested at very high levels. None was
shown to alter the FreeStyle readings with clinical significance. Effects on FreeStyle results were less than ±10 mg/dL at
glucose concentrations below 100 mg/dL and less than ±10% at glucose concentrations above 100 mg/dL.
Clinical and Laboratory Studies
Upper Limit of Therapeutic
or Normal Concentration
Acetaminophen mg/dL
Bilirubin (unconjugated)
(1) Intravenous therapy solutions such as some immunoglobulin and peritoneal dialysis solutions containing icodextrin may cause overestimation of
blood glucose results. Compounds of galactose ≥13 mg/dL or maltose ≥20 mg/dL may cause overestimation of blood glucose results.
(2) Do not use during xylose absorption testing
4.4 Hematocrit Effect Study
The FreeStyle test strips were tested with blood samples with a hematocrit range of 15%–65% to evaluate the effect of
varying hematocrit levels on test results. Three glucose concentrations were tested using three lots of FreeStyle test
strips.
Results:
The acceptance criteria are that 95% of results must be in the A Zone of the Clarke Error Grid and 99% must be within
the A and B Zones. The FreeStyle results passed the hematocrit acceptance criteria.
Hematocrit Data for FreeStyle Distribution in Error Grid
Total Points
A total of 185 lay users participated in the FreeStyle user study. Age of the users ranged from 13–84, 54% male and46% female, with 46% college educated.
After reading the testing instructions and performing a glucose test on their own, the lay users were asked to complete aquestionnaire rating 10 topics regarding FreeStyle ease of use. Participants were asked to evaluate the FreeStyle bloodglucose monitoring system by rating a number of statements according to their level of agreement using a six-pointscale (1= strongly disagree, 6 = strongly agree).
Results:
The results of the lay user responses in the six-point scale are summarized in the chart below. An overall ease-of-use rat-
ing of 5.5 was obtained when all responses were averaged, indicating that the lay users found the FreeStyle easy to use.
FreeStyle Blood Glucose Test Strip Performance
Mean Result
The meter fits comfortably in my hand
Easy to identify the test port on the meter
Easy to calibrate (scan barcode)
Easy to insert test strip
Easy to apply blood to the test strip
Had enough time to apply blood to the glucose strip
The display is easy to read
The test instructions are easy to follow
The buttons are easy to identify
Mean over all statements
1= strongly disagree, 6 = strongly agree
In clinical, laboratory and user studies, the FreeStyle glucose monitoring strip showed excellent performance in accuracy,precision, ease of use and capability in reducing use error.
6.1 Accuracy
The FreeStyle test strip demonstrated reliable accuracy under real-world clinical conditions:
Accuracy: In the studies cited, the FreeStyle test strip consistently delivered accurate results; 99.7–100% of
FreeStyle results were in the "clinically accurate" A Zone of the Clarke and Consensus Error Grids. Precision wasgood, with variability from strip to strip of 5.6% or less.
Hematocrit effect: Due to the exceptionally low potential (voltage) in the FreeStyle glucose reaction, the FreeStyle teststrip maintains accuracy across broad ranges of hematocrit. In testing with hematocrit levels of 15%–65%, 98.8% ofthe results were in the "clinically accurate" A Zone.
Interference: The FreeStyle test strip's low voltage also minimizes the potential for interference from substances com-monly found in blood. Of the 16 substances tested (including Acetaminophen, Ibuprofen, Uric acid, Tetracycline,Ephedrine, Salicyclic Acid) at high concentrations, none was found to have a clinically significant effect on testresults.
6.2 Ease of Use
In User Testing, lay participants found the FreeStyle test strip to be easy and convenient for point-of-care personnel to use:
Requires less blood: The FreeStyle test strip requires 0.3µL of blood—significantly less than other strips requiringfrom 2.5µL to 5µL of blood.
Easy fill strip: Capillary action "pulls" in the correct amount of blood.
Autostarts when adequate sample is detected.
Speed: Glucose results are available within 15 seconds on average.
Allows re-application of sample: If there is insufficient blood on the test strip to start a test, the user may add a sec-ond blood drop to the same test strip within 60 seconds of the first blood drop. This minimizes the need to repeat thetest with a new test strip, thus reducing strip waste. It may also reduce the need to perform additional finger-sticks.
With many glucose meters, adding a second blood drop can produce erroneous results5.
No Cleaning: No test strip holder or meter optics to clean.
"Universal" sampling convenience: The FreeStyle test strip can be used for capillary, arterial, venous and neonatal
blood samples.
5SureStep® Pro‚ Test Strip Insert, LifeScan, Inc., AW 052-595-148, 01/2004.
Clinical and Laboratory Studies
6.3 Reduced Use Error
The FreeStyle test strip is designed to reduce use error in real point-of-care testing conditions:
Most glucose meters, including some newer models, produce erroneous results when a small drop of blood is used[6,7,8,9]. Erroneous results ranging from 85% lower to 39% higher have been reported when a small drop of blood wasused. This is a common and significant error that users may not be aware of. The FreeStyle strip's sample detectionelectrode minimizes error from insufficient sample. The small sample size requirement also helps reduce the potentialfor short-sampling errors.
Maintains accuracy when double-dosing (re-application with a second drop of blood within 60 seconds).
Maintains accuracy across broad ranges of hematocrit (15%–65% Hct) and at extreme levels of blood oxygen, offeringgreater clinical confidence.
No timing error (no "off-meter dosing," thus no user timing error) Biosensor technology is not affected by errors dueto dirty optics and there are no optical components to clean10.
Minimizes interference from medications and endogenous substances. Because of the low voltage in the FreeStyleelectrochemical reaction, substances such as acetaminophen, uric acid or gentisic acid have no clinically significanteffect on test results.
6.4 Conclusion
In conclusion, the FreeStyle glucose test strip is uniquely designed to assure accuracy, safety, ease of use, and cost
savings when used by point-of-care testing personnel.
6Haag BL, Leed LA. Susceptibility of two new glucose test strips to testing errors. Diabetes1999;48: A415.
7 Velazquez, FR, Wright L, Ko K. Influence of glucose test strip design on the accuracy of test results. Diabetes1999;48: A349.
8 Lewandrowski KB, Dan L. Effects of small sample volumes and interfering substances on two glucose meters. Diabetes 1999;48: A387.
9 Velazquez FR, Wright L, Herbert M, Wilson L. Effect of small sample volume, acetaminophen and uric acid on two glucose meters, Diabetes1998;47: A102.
10Banks R, Howard S, Velazquez FR. Letter to the Editor. Clinica Chimica Acta 2005.
To learn how FreeStyle Blood Glucose Test Strips can revolutionize point-of-care glucose testing in your facility, call your Abbott Sales Representative at 1-800-366-8020 or visit us at www.abbottpointofcare.com.
Abbott Point of Care
104 Windsor Center Drive
East Windsor, NJ 08520
www.abbottpointofcare.com
2005 Abbott PRT06911 Rev A
Source: https://freestylediabetes.co.uk/images/uploads/documents/CP013_freeStyle_white_paper.pdf
zur Prüfung der Richtlinienverfahren gemäß §§ 13 – 15 der Psychotherapie-Richtlinie für die psychoanalytisch begründeten Verfahren Diese Stellungnahme wurde als fachliche Einschätzung zum Fragenkatalog des Gemeinsamen Bundessausschusses zur Prüfung der Richtlinienverfahren gem. §§ 13 – 15 der Psychotherapierichtlinie – Psychoanalytisch begründete Verfahren – von folgenden Verbänden und wissenschaftlichen Fachgesellschaften gemeinsam mit der DGPT erarbeitet: Deutsche Gesellschaft für Psychosomatische Medizin und
from World on the Edge: How to Prevent Environmentaland Economic Collapse, by Lester R. Brown© 2011 Earth Policy Institute We need an economy for the twenty-first century, onethat is in sync with the earth and its natural support sys-tems, not one that is destroying them. The fossil fuel-based, automobile-centered, throwaway economy thatevolved in western industrial societies is no longer aviable model—not for the countries that shaped it or forthose that are emulating them. In short, we need to builda new economy, one powered with carbon-free sources ofenergy—wind, solar, and geothermal—one that has adiversified transport system and that reuses and recycleseverything.







