Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Gamalift.com.br
Arm et al. Lipids in Health and Disease 2013, 12:141http://www.lipidworld.com/content/12/1/141
Impact of botanical oils on polyunsaturated fattyacid metabolism and leukotriene generation inmild asthmatics
Jonathan P Arm1,4,5,9, Joshua A Boyce4,5,7, Lin Wang1, Heng Chhay1, Muhammad Zahid2, Vaishali Patil2,Usha Govindarajulu1, Priscilla Ivester6,7, Kelly L Weaver6,10, Susan Sergeant7,8, Elliot Israel2,3,5 and Floyd H Chilton6,7*
Background: Dietary supplementation with botanical oils that contain n-6 and n-3 eighteen carbon chain (18C)-PUFAsuch as γ linolenic acid (GLA, 18:3n-6), stearidonic acid (SDA, 18:4n-3) and α linolenic acid (ALA, 18:3n-3) have beenshown to impact PUFA metabolism, alter inflammatory processes including arachidonic acid (AA) metabolism andimprove inflammatory disorders.
Methods: The diet of mild asthmatics patients was supplemented for three weeks with varying doses of two botanicalseed oils (borage oil [Borago officinalis, BO] and echium seed oil [Echium plantagineum; EO]) that contain SDA, ALA andGLA. A three week wash out period followed. The impact of these dietary manipulations was evaluated for severalbiochemical endpoints, including in vivo PUFA metabolism and ex vivo leukotriene generation from stimulatedleukocytes.
Results: Supplementation with several EO/BO combinations increased circulating 20–22 carbon (20–22C) PUFAs,including eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and dihommo-gammalinolenic acid (DGLA),which have been shown to inhibit AA metabolism and inflammation without impacting circulating AA levels. BO/EOcombinations also inhibited ex vivo leukotriene generation with some combinations attenuating cysteinyl leukotrienegeneration in stimulated basophils by >50% and in stimulated neutrophils by >35%.
Conclusions: This study shows that dietary supplementation with BO/EO alters 20–22C PUFA levels and attenuatesleukotriene production in a manner consistent with a reduction in inflammation.
Keywords: Asthma, Gammalinolenic acid, Stearidonic acid, Inflammation, Leukotrienes, Borage oil, Echium oil
effects, which include contraction of bronchial smooth
Asthma is a complex disease which involves smooth
muscle, vasodilatation, and mucus secretion within the
muscle contraction and inflammation that result in
airways [Additionally, LTB4 is a potent chemo-
narrowing and obstruction of the airway. Arachidonic
attractant for granulocytes, effector T cells, and mono-
acid (AA) metabolism via the 5-lipoxygenease pathway
cytes, acting at a specific GPCR, BLT1 Collectively,
to form leukotrienes has been demonstrated to be par-
leukotrienes have numerous proinflammatory properties,
ticularly important to the pathology of asthma. Specific-
and leukotriene modifying drugs have proven effective in
ally, the cysteinyl leukotrienes, LTC4, LTD4, and LTE4,
the management of asthma and allergic rhinitis
act at GPCRs, CysLT1R and CysLT2R, to elicit their
In addition to pharmacologic modifiers, supplementa-
tion of diets with fatty acid-based marine and botanical oil
* Correspondence:
supplements have been demonstrated to reduce the sever-
6Department of Physiology/Pharmacology, Wake Forest School of Medicine,
ity of several inflammatory disorders including asthma.
Medical Center Blvd, 27157, Winston-Salem, NC, USA7
One of the primary mechanisms thought to be responsible
Center for Botanical Lipids and Inflammatory Disease Prevention, Wake
Forest School of Medicine, Medical Center Blvd, 27157, Winston-Salem, NC,
for their efficacy has been the capacity of n-6 and n-3
polyunsaturated fatty acids to alter AA metabolism to
Full list of author information is available at the end of the article
2013 Arm et al.; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the CreativeCommons Attribution License which permits unrestricted use, distribution, andreproduction in any medium, provided the original work is properly cited.

Arm et al. Lipids in Health and Disease 2013, 12:141
form eicosanoids. For example, dietary supplementation
It has been long appreciated that natural products may
with fish oils that contain eicosapentaenoic acid (EPA;
contain a complex mixture of several active ingredients
20:5, n-3) inhibits leukotriene generation, likely through
with synergistic biological effects. As discussed above, bo-
substrate competition of EPA with AA for the action of
tanical seed oils contain several 18C-PUFAs that have the
cytosolic phospholipase A2 and 5-lipoxygenase .
potential to impact diseases that are driven by eicosanoid
In regard to botanical oils, borage seed oils contain the
generation. Several studies have examined the impact of
18 carbon PUFA, gammalinolenic acid (GLA; 18:3, n-6)
providing individual botanical oils that contain a high pro-
that is elongated by most tissues and inflammatory cells to
portion of their total fatty acids as a putative PUFA active
dihommo-gammalinolenic acid (DGLA) (Figure Newly-
ingredient; however, little is known regarding the bio-
formed DGLA is then incorporated into membrane phos-
chemical interactions of adding more than one botanical
pholipids. Like EPA, DGLA has the capacity to compete
oil containing several potential PUFA active ingredients.
with AA for eicosanoid production. DGLA is also
The primary objective of this current study was to exam-
converted to prostaglandin (PG)H1 via cyclooxygenase en-
ine the biochemical impact of adding PUFAs found in two
zyme(s), which is then converted to PGE1. PGE1 has been
botanical seed oils on PUFA metabolism and leukotriene
illustrated to have anti-inflammatory effect in both animals
generation in mild asthmatic patients.
In addition, seed oil from another member of the
Materials and methods
Boraginaceae family, Echium plantagineum contains both
n-6 and n-3 18C-PUFAs, including a GLA, α-linolenic acid
Thirty-seven asthmatic subjects (ages 18–64) were
(ALA; 18:3, n-3) and stearidonic acid (SDA; 18:4, n-3). The
recruited. Written informed consent was obtained from
conversion of ALA to EPA and DHA is poor in humans
all subjects prior to enrollment. Asthma was diagnosed by
which is believed to be a result of the inefficiency of
the presence of variable airflow obstruction or by a history
the initial rate-limiting step (Δ-6 desaturase, FADS2 gene)
of treatment for asthma with documented airways hyper-
involved in 20–22 carbon PUFA biosynthesis. However
responsiveness to methacholine []. Histories (medical
SDA is downstream of Δ-6 desaturase and is 4 to 5-fold
and respiratory), a brief physical examination and routine
more efficiently converted to EPA than ALA
clinical test were used to exclude the presence of signifi-
Additionally, SDA has been demonstrated to block in vitro
cant co-morbid diseases. The inclusion criteria were: 1)
leukotriene generation and in vivo inflammatory processes
male or female 18 years to 65 years of age; 2) asthma with
FEV1 50 to 90% of predicted, or personal best; and 3)
ω-3 series
ω-6 series
α-linolenic acid 18:3
Stearidonic acid 18:4
γ-linolenic acid
ω -3 Arachidonic acid 20:4
Dihomo- -
γ linolenic acid
Eicosapentaenoic acid 20:5
Arachidonic acid
Docosapentaenoic acid 22:5
Docosahexaenoic acid 22:6
Figure 1 Pathways for metabolism of n-6 (left) and n-3 (right) PUFAs in humans. The pathway depicts the synthesis of 20 and 22 carbonPUFAs from the essential, dietary PUFAs, α-linolenic acid (n-3) and linoleic acid (n-6). The PUFAs derived from borage oil (linoleic and gamma-linolenic acids, both n-6) and echium (α-linolenic, n-3; stearidonic, n-3; linoleic and gamma-linolenic acids, both n-6) would be expected to enterthe pathways at the indicated points.
Arm et al. Lipids in Health and Disease 2013, 12:141
improvement in FEV1 > 12% after administration of a
for three months prior to entry into the study no subject
beta-2 agonist. The exclusion criteria were: 1) pregnant or
used omalizumab, a monoclonal antibody against IgE.
nursing; 2) smoking history of > 10 pack years or active
The study was approved by the Partners Human Sub-
smoking within the previous year; 3) use of asthma treat-
jects Research Committee. An investigator-initiated IND
ments that potentially alter leukotriene biosynthesis includ-
was obtained from the Food and Drug Administration
ing theophylline and oral steroids; 4) dietary supplements
(IND number 74,110). Because the outcomes in this
with fatty acids or other products that may interfere with
study were entirely biochemical with no assessment
leukotriene generation; 5) treatment within the previous
of clinical efficacy, the study was not registered at
three months with omalizumab (monoclonal antibody di-
rected against IgE); 6) use of non-steroidal anti-inflammatory drugs in the week prior to any measurements
Dietary fatty acids
of ex vivo leukotriene generation; 7) a history of aspirin-
Capsules containing echium seed oil (1000 mg) or borage
sensitive asthma; 8) significant abnormalities in CBC, differ-
seed oil (1300 mg) were supplied by Bioriginal (Saskatoon,
ential white cell count, renal function, and liver function, or
SA, Canada). The fatty acid contents of the oils were vali-
urinalysis; and 9) any serious co-morbid medical condition.
dated by gas chromatography (Table Since the leaves of
Subjects meeting the entry criteria were randomized
Echium plantagineum contain pyrrolizidine alkaloid, an in-
to one of the four study groups based on botanical oil
dependent assay of this toxin was obtained, assessed by
dosing (Table Subjects used rescue albuterol less than
high performance TLC, and found it to be less than 4 ng/g
twice a week, had nocturnal asthma less than twice a
of oil (Chemisches Laboratorium Dr. Hermann Ulex Nachf,
month, and had a forced expiratory volume in one sec-
ond ≥75% predicted. During the month prior to entryinto the study, no subject used leukotriene modifiers,
oral or high dose inhaled steroids, or theophylline, and
The study goal was to test combinations of Boraginaceaefamily seed oils derived from Borago officinalis (BO) and
Table 1 Study group compositions and daily dosing
Echium plantagineum L. (EO) for their capacity to impact
scheme for borage and echium seed oils in the four
circulating PUFA levels and inhibit ex vivo leukotriene
groups of subjects
generation. After baseline determinations of plasma fatty
acid levels and ex vivo leukotriene generation, subjects
were randomized to dosing groups (Table ingesting a
Table 2 Fatty acid profile of botanical oils consumed by
asthmatic subjects
No. of subjects withdrawn
% of Total Fatty Acids
Echium Oil Dose (g)
No. of daily capsules
Borage Oil Dose (g)
No. of daily capsules
No. of daily capsules
The number of daily capsules, containing 1 g (EO) or 1.3 g (BO) oil per capsule,
and the amount of ALA, SDA and GLA provided by the encapsulated echiumand borage oils is shown for each dosing group. Recruitment into Group 1
was stopped after the interim analyses showed that the highest dose of EO
was not required to prevent elevations of AA levels in circulating plasma.
Arm et al. Lipids in Health and Disease 2013, 12:141
constant dose of GLA ( 1.5 g daily), 0.25 g to 1.75 g SDA
constipation, loose stools, and/or abdominal pain or dis-
and 0.57 g to 4.02 g ALA in divided doses (three times a
comfort. With the exception of the 3 subjects who with-
day) for 3 weeks.
drew due to gastrointestinal discomfort (Groups 2, 3 and
Aside from the addition of supplements, the subject's
4), these symptoms were mild and transient, occurred in
diets were not altered before or during the study. The
the first few days of the study and resolved within 2 to
‘typical' western diet provides very small quantities of
3 days while still taking BO/EO combinations. Other ad-
GLA and SDA, and it is difficult to measure these PUFA
verse events included cold-like symptoms (n = 7: Group 1
in circulating or cellular lipids. GLA and SDA are typic-
ally only observed when individuals are consuming a
ryngitis (n = 2; one each in Groups 2 and 4), mild symp-
GLA- or SDA- containing supplement such as borage,
toms of asthma associated with seasonal allergies (n = 2,
evening primrose, black currant, or echium, and this was an
one each in Groups 3 and 4), transient wrist pain (n = 1;
exclusion criterion of the study. ALA (n-3) makes up 1%
group 3), and transient itchy rash (n = 1; Group 4). Two
of the typical western diet and so the supplement may have
other subjects withdrew from the study due to the develop-
some impact on dietary levels. Approximately 7% of energy
ment of itch and mild asthma flare (Group 4) and unfore-
in the western diet is LA, and thus the quantities of
seen extension of an out-of-town business trip (Group 1).
LA in the supplements would have little total impact
There were no significant changes in vital signs in any
on overall linoleic acid (n-6) consumption.
study subjects.
Concentrations of GLA provided to human subjects as
There were no consistent or significant changes in bio-
borage oil alone have been shown to induce increases in
chemical laboratory parameters or urinalysis during the
circulating AA levels []. It was our hypothesis that the
study (data not shown). However, in four of the individ-
presence of the n-3 18C-PUFA SDA in echium oil would
uals who entered the study (2 male, 2 female) there was a
prevent such an increase. However, to assure that there
decrease in circulating hemoglobin of >1.0 g/dl. Across all
were no elevations of circulating AA out of the normal
study groups there was on average a decrease in circulat-
range, an interim analysis was carried out during the early
ing hemoglobin of 0.45 g/dl (13.8 to 13.3 g/dl; p < 0.001),
phase of the study. The interim analyses revealed that AA
which did not vary significantly between study groups
levels remained constant in all groups and consequently,
(p = 0.09 for differences among groups across time). In
the study was then completed. Blood and urine were col-
three individuals, the value dipped just below the range of
lected weekly for assessment of hematological indices,
normal. There was an accompanying decrease in red cell
liver and renal function, plasma fatty acids, ex vivo leuko-
count from 4.58 to 4.39x1012 /liter with no change in
triene generation. After three weeks of supplementation,
mean corpuscular volume. A decrease in hemoglobin
subjects entered a 3 week wash-out phase, in which
concentration was not seen in all subjects. There were
plasma fatty acids and ex vivo leukotriene generation were
no accompanying decreases in white cell count or
measured weekly. After dropouts, 6 to 11 individuals com-
platelet count.
pleted each arm of the study.
Subjects reported any change in their medical condi-
Plasma and botanical oil fatty acids analysis
tion at each study visit and used diary cards to record all
Fatty acid methyl esters were prepared in duplicate
medical symptoms and to log intake of study oils. Com-
plasma samples (100 μl) following a modification of
pliance was monitored by medication diaries and counts
Metcalfe et al. and analyzed by gas chromatography
of returned capsules. These assessments indicated at
as previously described [Encapsulated oils were suit-
least 90% compliance by all subjects and plasma fatty
ably diluted in hexane and submitted to fatty acid ana-
acids measurements, in which no outliers were seen, also
lysis in a manner comparable to that for plasma. Fatty
reflected in a comparable adherence to the supplementa-
acids in sample were identified based on retention times
tion. As the end points of the study were entirely bio-
of authentic fatty acid methyl esters (Supleco, Bellefonte,
chemical, the subjects were not blinded (i.e. subjects
PA, USA; Cayman Chemicals, Ann Arbor MI, USA;
took different numbers of capsules depending on the
Matreya, Pleasant Gap PA, USA; NuChek Prep, Elysian,
group to which they were assigned). The objective of this
biochemical study was to determine the impact of po-tentially bioactive PUFAs in combinations of BO and EO
Ex vivo leukotriene generation
on plasma fatty acid levels and ex vivo leukotriene pro-
FcεRI-dependent generation of cysteinyl leukotrienes from
duction. All individuals performing the biochemical as-
basophils. Peripheral blood mononuclear cells (PBMC),
says were blinded to the subjects' study group.
typically containing 3 to 4% basophils, were isolated
The most common adverse events associated with taking
from 20 mL of blood by density gradient centrifugation
BO/EO combinations were gastrointestinal symptoms, oc-
over Percoll (GE Healthcare, Piscataway, NJ) as de-
curring in thirteen subjects, variously reported as gas,
scribed ]. As previously described basophils
Arm et al. Lipids in Health and Disease 2013, 12:141
were stimulated in PBMC by cross-linking of the high
(p < 0.05, Figure white bar) than baseline in Group 1
affinity Fc receptor for IgE (FcεRI). Under these circum-
(wk 3) and Group 3 (wk 2). SDA (18:4n-3) was not detect-
stances, the basophil is the predominant, if not the only,
able in the plasma at baseline but rose significantly during
source of cysteinyl leukotrienes. Briefly, cells were
supplementation with BO and EO (p < 0.0001, Figure
primed with 10 μg/L IL-3 on ice and stimulated with
black bar). As expected, the increase in plasma SDA varied
0.01 to 1.0 μg/mL of 15A5, an activating monoclonal
between groups and was most marked in Group 1 in
antibody to FcεRI, for 30 min at 37°C. Cysteinyl leukotri-
which subjects consumed the largest dose of echium oil
enes in the supernatant fluids were stored at −80°C until
(p = 0.004 for differences among groups across time). In
assayed by immunoassay (Cayman Chemical Company,
each group, the plasma levels of EPA (20:5n-3; Figure
Ann Arbor, MI or GE Healthcare) for LTC4 with 100%
gray bar) and its elongation product DPA (22:5n-3;
cross-reactivity with LTC5, 48% and 46% cross-reactivity
Figure cross-hatched bar) rose significantly (p < 0.0001
with LTD4 and LTD5, respectively, and <10% cross-
for each fatty acid) in a manner that was dependent on
reactivity with LTE4 and LTE5.
the dose of echium oil ingested (p = 0.004 for group by
A23187-stimulated generation of leukotrienes from neu-
time interactions for each fatty acid). However, there was
trophils. Neutrophils were isolated from heparinized blood
no rise in circulating plasma DHA (22:6n-3; Figure
by dextran sedimentation, density gradient centrifugation
white striped bar) in any group (p = 0.14). During the
through Ficoll Paque (GE Healthcare, Uppsala, Sweden),
washout phase, plasma SDA, EPA and DPA rapidly
and hypotonic lysis of contaminating red cells []. Neu-
returned to baseline levels.
trophils (2 × 106) were stimulated (5 min, 37°C) with 0.1
With respect to n-6 fatty acids, plasma concentrations
to 10 μmol/L A23187 (Sigma-Aldrich, St Louis, MO) in
of GLA (18:3n-6; Figure black bar) and DGLA (20:3n-6;
Hanks buffered salt solution containing 1.25 mmol/L cal-
Figure white bar) rose in each group (p < 0.0001 for
cium and magnesium salts, 25 mmol/L Hepes, and 1 g/L
each fatty acid) with no significant difference between
fatty acid-free bovine serum albumin. Leukotrienes in the
groups (p = 0.06 and p = 0.63, respectively, for differences
supernatants were resolved by reverse-phase HPLC (RP-
among groups across time). Importantly, concentrations
HPLC) as described and quantified by absorbance
of plasma AA (20:4n-6; Figure , gray bar) remained con-
at 280 nm (LTB4 and all-trans LTB4) and 235 nm (5-
stant throughout with no significant change during sup-
hydroxyeicosatetraenoic acid, 5-HETE).
plementation with BO and EO in any group (p = 0.39 foreffect of time, and p = 0.87 for group by time interactions).
Statistical analysis
During the washout phase, plasma GLA and DGLA rap-
Statistical analyses were performed using SAS Version
idly returned to baseline levels.
9.2. We employed a repeated measures mixed model(rmm) analysis for each fatty acid to predict the response
Ex vivo leukotriene generation
in which repeated measures were taken on each subject
Figure illustrates the effect of dietary supplementation
at each visit (time) and the group and time interactions
with a constant dose of BO and variable doses of EO on
were modeled along with the main effects. The model
ex vivo cysteinyl leukotriene generation from basophils
utilized a compound symmetry correlation structure
stimulated through FcεRI. Basophils are the only cell
since the correlation between pairs of times would be
among peripheral blood mononuclear cells that express
similar and not expected to change across time. For ana-
the fully functional heterotetrameric αβγ2 form of FcεRI
lyses of ex vivo leukotriene generation by neutrophils
and are the principal, if not the only, source of cysteinyl
and basophils, we also considered the effect of a range
leukotrienes when this population of cells is stimulated
of doses of each stimulus at each visit within the same
through this receptor []. We therefore stimulated baso-
modeling. We then used adjusted least square mean dif-
phils at 3 to 4% purity in PBMC with an activating anti-
ferences obtained from each rmm model to compare the
body to FcεRI, before (baseline) and at weekly intervals for
differences between groups and between times within a
three weeks while subjects supplemented their diet with
single group. All analyses were conducted at a 0.05 level
borage and echium seed oils. Because of the marked dif-
of significance.
ference among individuals in absolute quantities ofcysteinyl leukotrienes generated by basophils, varying
from 1 to 90 ng/106 basophils, data are expressed as a per-
Plasma fatty acids
centage of maximal leukotriene generation per million ba-
The effects of dietary supplementation with BO and EO
sophils prior to treatment with BO and EO. Compared to
on n-3 and n-6 PUFA levels are provided in Figure and
baseline leukotriene generation (Figure closed circles),
Figure respectively. ALA (18:3n-3), present in echium
supplementation with BO and EO resulted in decreased
oil, tended to increase in all groups during the supplemen-
FcεRI-mediated leukotriene generation. This inhibitory
tation period (weeks 1–3) and was significantly higher
effect was statistically significant in all groups during
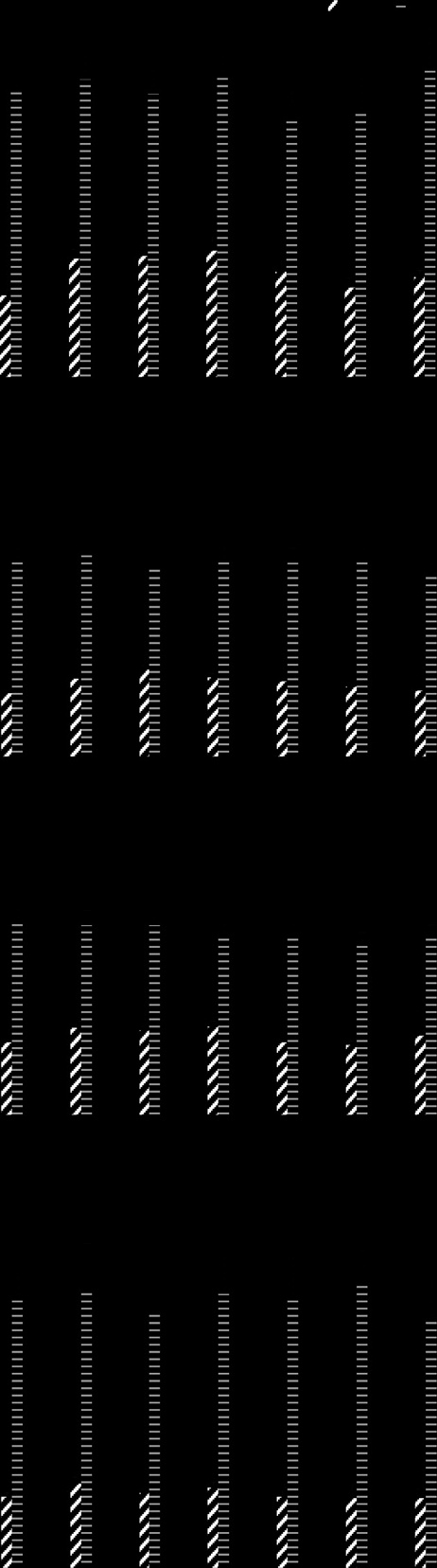
Arm et al. Lipids in Health and Disease 2013, 12:141
Figure 2 Effects of dietary supplementation with borage andechium seed oils on concentrations of plasma n-3 fatty acids.
Data are expressed as a percentage of total plasma fatty acids andare shown for ALA (open bar), SDA (black bars), EPA (gray bars), DPA(cross-hatched bars), and DHA (white striped bars). Fatty acid profiles
were monitored during the time course of the study beginning atbaseline (Pre), during supplementation (weeks 1–3) and during thewashout phase (w/o1-3). Data are mean ± SEM for each group.
Statistically significant rises in SDA, EPA and DPA were noted overtime (p < 0.0001) with significant differences amonggroups (p = 0.004).
treatment with BO and EO (p < 0.0001) with a significantdifference between study groups (p < 0.0001 for differ-
ences among groups across time). The most stable and ro-
w/o 1 w/o 2 w/o 3
bust inhibition of leukotriene generation was seen inGroup 2 (Figure , in which individuals ingested EO/BO
combination that provided 1.7 g of GLA, 2.01 g ALA and
0.9 g SDA daily. In this group there was a time-dependentinhibition of cysteinyl leukotriene generation from baso-
phils, stimulated with all concentrations of the antibody to
εRI that reached 57% and 50% inhibition of the maximal
response by weeks 2 and 3, respectively. A similar inhib-
ition of FcεRI-dependent cysteinyl leukotriene generation
was observed in Group 3 (Figure in which individuals
ingested a BO/EO combination that provided 1.6 g of
w/o 1 w/o 2 w/o 3
GLA, 1.15 g ALA and 0.5 g SDA daily (n = 8). In Group 3,inhibition was maximal (60%) at week one and cysteinyl
leukotriene generation remained suppressed at weeks 2
and 3. Inhibition of FcεRI-dependent cysteinyl leukotrienegeneration from peripheral blood basophils was less ro-
bust in Groups 1 and 4.
The inhibitory effect of dietary BO and EO on baso-
philic leukotriene generation was transient. Figure shows the reversal of ex vivo cysteinyl leukotriene gener-
ation from basophils (stimulated with a maximum con-
w/o 1 w/o 2 w/o 3
centration of stimulating antibody) during the washoutperiod after individuals stopped taking capsules of bor-
age and echium oil supplements. Data are expressed aspercentage of the maximum cysteinyl leukotriene gener-
ation prior to starting supplements. In all groups, therewas a return to baseline values within 3 weeks.
A23187 is a calcium ionophore that stimulates leukotri-
ene generation by eliciting calcium flux in a receptor-
independent manner. In neutrophils, it provides a robuststimulus for leukotriene generation and allows assessment
of the integrity of the whole leukotriene biosynthetic path-way including the terminal product, LTB4, and the stable
non-enzymatic degradation products of its intermediates,
w/o 1 w/o 2 w/o 3
5-HETE (from 5-hydroperoxyeicosatetraenoic acid) and
all-trans-LTB4 (from LTA4). We therefore examined theeffects of dietary supplementation with BO and EO onA23187-stimulated leukotriene generation from neutro-phils. The shape and position of the dose response curvesfor release of LTB4 and 5-HETE were similar at baseline,
Arm et al. Lipids in Health and Disease 2013, 12:141
Figure 3 Effects of dietary supplementation with borage and
echium seed oils on concentrations of plasma n-6 fatty acids.
Data are expressed as a percentage of total plasma fatty acids and
are shown for GLA (black bars), DGLA (white bars), and AA (graybars). Fatty acid profiles were monitored during the time course of
the study beginning at baseline (Pre), during supplementation
(weeks 1–3) and during the washout phase (w/o1-3). Data are mean± SEM for each group. Statistically significant rises in GLA and DGLA
were noted over time (p < 0.0001) with no statistically significantdifferences among groups.
and changes in generation of LTB4 and 5-HETE during
w/o 1 w/o 2 w/o 3
dietary supplementation with BO and EO were similar(data not shown). Therefore, data are presented as total
Group 2
leukotriene generation; i.e. the sum of 5-HETE, LTB4, and
all-trans-LTB4. A23187 elicited the dose-dependent re-
lease of leukotrienes that was apparent at a concentration
of 0.3 μM and reached a maximum response at a concen-tration of 3 to 10 μM A23187 (Figure . The quantities of
all-trans LTB4 diastereoisomers that were generated were
small and were not always readily measured by UV ab-
sorbance; they are included in the data where possible.
Treatment with BO/EO combinations led to a significant
w/o 1 w/o 2 w/o 3
inhibition of A23187-stimulated leukotriene generation
from neutrophils (p < 0.0001) with a significant difference
Group 3
in effect between groups (p = 0.02 for differences among
groups across time). The group-dependent effects of diet-
ary supplementation with BO and EO on A23187-
stimulated leukotriene generation from neutrophils were
similar to those observed for FcεRI-dependent cysteinyl
leukotriene generation from basophils (Figure ). The
greatest effect was seen in Group 2 (Figure ), in which
maximal total leukotriene generation was inhibited 43%after three weeks of dietary supplementation with BO/EO
combinations. The inhibitory effect of the dietary supple-
w/o 1 w/o 2 w/o 3
mentation on neutrophil leukotriene generation declined
Group 4
over time in Group 3 and was less robust in Group 4
(Figure ). As in the case of basophils, A23187-stimulated
leukotriene generation from neutrophils returned to base-
line during the 3-week wash-out period (data not shown).
Botanical seed oils from plants such as borage andechium have shown modest efficacy in a number of ani-
mal and human inflammation models and disease. These
botanicals contain 18C-PUFAs (ALA, SDA and GLA)
that can be metabolized into 20–22 carbon PUFAs suchas EPA, DHA, DGLA and AA. All of these have beenshown to impact eicosanoid generation. However, a bet-ter understanding of the in vivo biochemistry of poten-tially bioactive PUFAs found in these botanical seed oilsand oil combinations and their capacity to block inflam-matory processes including eicosanoid production isneeded to enhance the effectiveness of botanical seed
Arm et al. Lipids in Health and Disease 2013, 12:141
Figure 4 Dietary supplementation with borage and echium
seed oils decreases FcεRI-dependent cysteinyl leukotriene
generation by peripheral blood basophils. Cysteinyl leukotriene
generation is shown prior to (, closed circles) and one week
(Δ, open triangles), two weeks (□, open squares), and three weeks
(○, open circles) after dietary supplementation with one of four
borage and echium seed oil combinations (Groups 1–4, respectively)
in response to buffer alone, control IgG1 (1.0 μg/ml, IgG), and 15A5,
an activating antibody against FcεRI (0.01 to 1.0 μg/ml). Due totechnical errors, data are not available for one subject in each of
groups 1, 3, and 4. In addition, basophils from one subject in eachof groups 3 and 4 failed to release leukotrienes upon stimulation, a
well described phenomenon due to impaired signaling through Syk.
Data are expressed as a percentage of maximal cysteinyl leukotriene
generation in each subject and are expressed as means ± SEM.
Statistically significant suppression of ex vivo leukotriene generation
was noted (p < 0.0001) with a significant difference between groups(p < 0.0001).
oils. The current study utilized various BO/EO combina-
tions to understand these processes.
Supplementation with BO/EO combinations increases
plasma levels of n-3 and n-6, 18 carbon and 20–22 carbon
PUFAs during the supplementation periods (Figure and
Figure . Of note, circulating levels of three PUFAs,DGLA, EPA and DPA increased after supplementation. It
is likely that DGLA increased as a result of GLA found in
both BO and EO. As discussed above, GLA is readily elon-
gated to DGLA in cells and tissues utilizing an enzyme
encoded for by a gene known as elongase 5 (ELOVL5).
Once formed, DGLA is incorporated into inflammatory
cells and tissues and competes with AA for the actionof cytosolic phospholipase A2 and cyclooxygenase to
3wks supplementation
Wk 1 washout
Wk 2 washout
Wk 3 washout
% of Pre-Diet Maxim
Figure 5 Cessation of dietary supplementation reversesinhibition of FcεRI-dependent cysteinyl leukotriene generationby peripheral blood basophils. After 3 weeks of dietarysupplementation (open bars), subjects stopped ingesting borageand echium seed oils. FcεRI-stimulated basophilic cysteinylleukotriene generation was measured at one (gray bar), two(cross-hatched bar) and three weeks (solid bar) of washout. Data areshown for maximal cysteinyl leukotriene generation in response to1.0 μg/ml 15A5. Data are expressed as a percentage of maximalcysteinyl leukotriene generation in each subject and are expressedas means ± SEM.
Arm et al. Lipids in Health and Disease 2013, 12:141
Figure 6 Dietary supplementation with borage and echium oilsdecreases A23187-stimulated total leukotriene generation by
Group 1
peripheral blood neutrophil. Leukotriene generation is shownprior to (, closed circles) and one week (Δ, open triangles), two
weeks (□, open squares), and three weeks (○, open circles) after
dietary supplementation with one of four borage and echium seed
oil combinations (Groups 1–4, respectively) in response to buffer
alone and increasing concentrations of the calcium ionophore,
A23187 (0.1 to 10 μM). Total leukotriene generation is the sum ofLTB4, 5-HETE, and, where measurable, all-trans-LTB4 isomers. Data are
expressed as means ± SEM. Statistically significant suppression of
ex vivo leukotriene generation was noted (p < 0.0001) with asignificant difference in effect between groups (p = 0.02).
form PGE1. Additionally DGLA is converted to a 15-
Group 2
lipoxygenase product, 15-hydroxyeicosatrienoic acid
(15 HeTrE) by human mononuclear leukocytes 15-HETrE has been demonstrated to be a potent
blocker of LTB4 formation.
Additionally this botanical oil combination increased
circulating levels of EPA and DPA This contribution waslike due to EO addition since it contains the precursor
PUFAs, ALA and SDA. Providing 0.25 g/d to 1.75 g/d of
otrienes
Buffer 0.1
SDA and 0.57 g/d to 4.02 g/d of ALA from EO led tosignificant and dose-dependent increases in circulating
EPA and DPA; plasma EPA concentrations rose morethan 2-fold in the group receiving the highest concentra-
tion of SDA (Figure This increase in EPA and DPA is
Group 3
likely a result of SDA and not ALA as in vivo SDA con-
version to EPA is 4–5 fold more efficient than ALA.
However, some epidemiological studies suggest that
ALA-containing oils (from seed oils such as flax; Linumusitatissimum L.) ] may provide independent protec-
tion from cardiovascular disease.
Numerous studies show the biological impact of EPA
and DPA. EPA reduces AA metabolism through several
Buffer 0.1
mechanisms including decreasing AA mobilization from
membrane phospholipids, competition for cylooxygenaseand 5-lipoxygenease and reducing the expression of AA
metabolizing enzymes and proinflammatory cytokines.
Group 4
Additionally, EPA can serve as a substrate for prostaglan-
din formation generating "3-series" prostaglandin products
including PGD3, PGE3, PGF3α, PGI3, and TxA3 and "5series" leukotriene products including LTB5 and LTC5
[. Reduced asthma symptoms with n-3 PUFA ingestionhave been shown to be related to 5-series leukotriene
production []. With regard to inflammation, DPA is
A21387 (µM)
beginning to receive attention. DPA is converted to 11-hydroxy-7,9,13,16,19-DPA and 14-hydroxy7,10,12,16,19-DPA, which inhibit aggregation of platelets and contain10-fold greater capacity to elicit endothelial cell migrationthan EPA, a biological process critical to wound healing[]. There were no changes in plasma levels of DHA,likely reflecting the poor bioconversion of EPA to DHA.
Arm et al. Lipids in Health and Disease 2013, 12:141
Previous studies have shown that GLA-containing oils
combinations led to a significant inhibition of A23187-
such as BO have the potential to increase circulating AA
stimulated leukotriene generation (Figure that was
which could enhance inflammation and platelet aggrega-
almost as great as the inhibition of basophil cysteinyl leu-
tion through increased thromboxane formation
kotriene generation (Figure We recently reported that
However, there were no changes in AA levels as a result
dietary supplementation with BO and fish oil led to re-
of BO/EO supplementation. It is possible that the ob-
duced expression of phosphatidylinositol 3-kinase, a key
served increase in EPA resulting from of the botanical
signaling molecule, in circulating mononuclear cells
combination is a feedback inhibitor of AA production
It is therefore possible that the inhibition of cysteinyl leu-
via the Δ5 desaturation step. EPA has been demon-
kotriene generation that we observed in basophils was
strated to inhibit the in vivo and in vitro desaturation of
due, at least in part, to inhibition of signaling through
DGLA to form AA In any event, the BO/EO
FcεRI. However, the inhibition of leukotriene biosyn-
combination led to an increase in three 20–22 carbon
thesis in neutrophils stimulated through A23187, a re-
PUFAs, DGLA, EPA and DPA that have been demon-
ceptor independent stimulus, argues for a more direct
strated to inhibit AA metabolism and attenuate inflam-
effect of BO and EO on leukotriene biosynthesis.
mation without increasing circulating levels of AA.
The final objective of this paper was to determine
whether these botanical oil combinations had the capacity
Our data demonstrate that ingestion of certain combina-
to inhibit leukotriene generation from two inflammatory
tions of BO and EO increases circulating levels of both
cells, basophils and neutrophils, isolated from subjects
n-6 and n-3, 20–22 carbon PUFAs. Supplementation of
with mild asthma who had supplemented their diet with
human and animal diets with these fatty acids has been
BO/EO. Prior studies of dietary supplementation with
shown to inhibit metabolism of AA to pro-inflammatory
GLA have demonstrated a reduction in ex vivo leukotriene
lipid mediators and ameliorate inflammatory disease
generation in whole blood or neutrophils stimulated with
models including asthma, arthritis and coronary artery
calcium ionophore A23187 or with zymosan ,].
disease These same combinations do not increase
Basophils [], IgE ], and cysteinyl leukotrienes []
circulating AA levels, as has been observed with BO
have been strongly implicated in the pathobiology of
alone. Importantly, the observed biochemical changes
asthma. We therefore assessed the effects of BO/EO com-
occur concomitantly with a reduced capacity of inflam-
binations on the generation of cysteinyl leukotrienes from
matory cells from mild asthmatics supplemented with
basophils stimulated through the high affinity IgE re-
BO/EO combinations to produce leukotrienes that have
ceptor, a physiologically relevant stimulus for asthma.
been illustrated to be important to promoting the asth-
Significant inhibition of basophil cysteinyl leukotriene
matic response. Consequently, an important next step
generation was noted within one week of dietary supple-
would be to determine whether such BO/EO combina-
mentation (Figure . Interestingly, the time dependence
tions have the capacity to improve clinical symptoms of
of this inhibition varied between groups. The least robust
inhibition was observed in Group 4, in which subjects re-ceived the lowest dose of SDA. Although there is consid-
erable variation in the extent of inhibition of ex vivo
AA: Arachidonic acid; ALA: α linolenic acid; BLT1: B leukotriene receptor 1;
leukotriene generation among individuals and the groups
BO: Borage oil; CysLT1R: Cysteinyl leukotriene receptor 1; CysLT2R: Cysteinylleukotriene receptor 2; DGLA: Dihommo-gammalinolenic acid;
were relatively small, the between group variation was sta-
DHA: Docosahexaenoic acid; DPA: Docosapentaenoic acid; EO: Echium oil;
tistically significant. Furthermore, a comparable variation
EPA: Eicosapentaenoic acid; GLA: γ linolenic acid; GPCR: G protein coupled
in supplementation-induced inhibition of leukotriene gen-
receptor; IND: Investigational new drug; LTB4: Leukotriene B4;LTC4: Leukotriene C4; LTD4: Leukotriene D4; LTE4: Leukotriene E4;
eration was observed in response to A23187- stimulation
PBMC: Peripheral blood mononuclear cells; SDA: Stearidonic acid.
of neutrophils (Figure . The data therefore suggest thatproviding SDA in the diet contributed to the extent of in-
Competing interests
hibition of leukotriene generation, consistent with data
FHC is an unpaid consultant for Gene Smart Health and receives nocompensation or equity in this role. This information has been disclosed to
showing that dietary supplementation with EPA leads to
WFUHS and outside sponsors, as appropriate, and is institutionally managed.
inhibition of ex vivo leukotriene generation .
All other authors declare no competing interest.
In a receptor-independent manner, A23187 robustly
stimulates human neutrophils to elicit maximal gener-
Authors' contributionsJPA and FHC designed the study and protocols, oversaw the conduct of the
ation of LTB4, the product of leukotriene biosynthesis
study and wrote the manuscript; EI participated in the conduct of the study;
in neutrophils. Utilization of this stimulus allows an as-
LW and HC performed ex vivo experiments and sample analyses; PI and KLW
sessment by RP-HPLC of the non-enzymatic degradation
analyzed fatty acids and biomarkers; MZ and VP were study coordinators; UGperformed statistical analyses; JAB and SS helped with data analysis and
products of the proximal intermediates of leukotriene
interpretation and manuscript preparation. All authors read and approved
biosynthesis. Dietary supplementation with BO/EO
the final manuscript.
Arm et al. Lipids in Health and Disease 2013, 12:141
Metcalfe LD, Schmitz AA, Pelka JR: Rapid preparation of fatty acid esters from
Supported by a grant from the National Institutes of Health, P50 AT002782, a
lipids for gas chromatographic analysis. Anal Chem 1966, 38:514–515.
bridge grant from the American Academy of Allergy Asthma and
Weaver KL, Ivester P, Seeds MC, Case LD, Arm J, Chilton FH: Effect of
Immunology and the Fund to Sustain Research Excellence, Brigham and
dietary fatty acids on inflammatory gene expression in healthy humans.
Women's Hospital Biomedical Research Institute.
J Biol Chem 2009, 284:15400–15407.
Sloane DE, Tedla N, Awoniyi M, MacGlashan DW Jr, Borges L, Austen KF,
Arm JP: Leukocyte immunoglobulin-like receptors: novel innate receptors
1Division of Rheumatology, Immunology and Allergy, Brigham and Women's
for human basophil activation and inhibition. Blood 2004, 104:2832–2839.
Hospital, 75 Francis St 02115, Boston, MA, USA. 2Pulmonary Division, Brigham
Nauseef WM: Isolation of human neutrophils from venous blood. Meth
and Women's Hospital, 75 Francis St 02115, Boston, MA, USA. 3Center for
Mol Biol 2007, 412:15–20.
Clinical Investigation, Brigham and Women's Hospital, 75 Francis St 02115,
Iversen L, Fogh K, Kragballe K: Effect of dihomogammalinolenic acid and
Boston, MA, USA. 4Partners Asthma Center, Brigham and Women's Hospital,
its 15-lipoxygenase metabolite on eicosanoid metabolism by human
75 Francis St 02115, Boston, MA, USA. 5Department of Medicine, Harvard
mononuclear leukocytes in vitro: selective inhibition of the 5-
Medical School, 25 Shattuck Street, 02115, Boston, MA, USA. 6Department of
lipoxygenase pathway. Archives of Dermatol Res 1992, 284:222–226.
Physiology/Pharmacology, Wake Forest School of Medicine, Medical Center
de Lorgeril M, Salen P: Alpha-linolenic acid and coronary heart disease.
Blvd, 27157, Winston-Salem, NC, USA. 7Center for Botanical Lipids and
Nutr Metab Cardiovas Dis 2004, 14:162–169.
Inflammatory Disease Prevention, Wake Forest School of Medicine, Medical
Wada M, DeLong CJ, Hong YH, Rieke CJ, Song I, Sidhu RS, Yuan C, Warnock
Center Blvd, 27157, Winston-Salem, NC, USA. 8Department of Biochemistry,
M, Schmaier AH, Yokoyama C, et al: Enzymes and receptors of
Wake Forest School of Medicine, Medical Center Blvd, 27157, Winston-Salem,
prostaglandin pathways with arachidonic acid-derived versus
NC, USA. 9Current address: Novartis International AG, CH-4002, Basel,
eicosapentaenoic acid-derived substrates and products. J Biol Chem 2007,
Switzerland. 10Current address: Department of Surgery, University of Miami
School of Medicine, 1600 NW 10th Ave, 33136, Miami, FL, USA.
Broughton KS, Johnson CS, Pace BK, Liebman M, Kleppinger KM: Reducedasthma symptoms with n-3 fatty acid ingestion are related to 5-series
Received: 23 May 2013 Accepted: 26 September 2013
leukotriene production. Am J Clin Nutr 1997, 65:1011–1017.
Published: 2 October 2013
Kaur G, Cameron-Smith D, Garg M, Sinclair AJ: Docosapentaenoic acid(22:5n-3): a review of its biological effects. Prog Lipid Res 2011, 50:28–34.
Calder P: n − 3 Polyunsaturated fatty acids and inflammation: from
molecular biology to the clinic. Lipids 2003, 38:343–352.
Heise CE, O'Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, Stocco R,
Seyberth HW, Oelz O, Kennedy T, Sweetman BJ, Danon A, Frolich JC,
Bellefeuille JN, Abramovitz M, Cheng R, et al: Characterization of the human
Heimberg M, Oates JA: Increased arachidonate in lipids after
cysteinyl leukotriene 2 receptor. J Biol Chem 2000, 275:30531–30536.
administration to man: effects on prostaglandin biosynthesis. Clin
Lynch KR, O'Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, Coulombe N,
Pharmacol Ther 1975, 18:521–529.
Abramovitz M, Figueroa DJ, Zeng Z, et al: Characterization of the human
Barham JB, Edens MB, Fonteh AN, Johnson MM, Easter L, Chilton FH:
cysteinyl leukotriene CysLT1 receptor. Nature 1999, 399:789–793.
Addition of eicosapentaenoic acid to g-linolenic acid-supplemented
Henz BM, Jabolonska S, van de Kerhof PC, Stingl G, Blazczyk M, Vandervalk
diets prevents serum arachidonic acid accumulation in humans. J Nutr
PG, Veenhuizen R, Muggli R, Raederstorff D: Double-blind, multicentre
analysis of the efficacy of borage oil in patients with atopic eczema.
Surette ME, Koumenis IL, Edens MB, Tramposch KM, Chilton FH: Inhibition
Br J Dermatol 1999, 140:685–688.
of leukotriene synthesis, pharmacokinetics, and tolerability of a novel
Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Pineiro A, Wei LX,
dietary fatty acid formulation in healthy adult subjects. Clin Therapeut
Seidenberg BC, Reiss TF: Oral montelukast, inhaled beclomethasone, and
2003, 25:948–971.
placebo for chronic asthma. Ann Intern Med 1999, 130:487–495.
Surette ME, Koumenis IL, Edens MB, Tramposch KM, Clayton B, Bowton D,
Philip G, Malmstrom K, Hampel FC, Weinstein SF, LaForce CF, Ratner PH,
Chilton FH: Inhibition of leukotriene biosynthesis by a novel dietary fatty
Malice MP, Reiss TF: Montelukast for treating seasonal allergic rhinitis: a
acid formulation in patients with atopic asthma: a randomized, placebo-
randomized, double-blind, placebo-controlled trial performed in the
controlled, parallel-group, prospective trial. Clin Therapeut 2003, 25:972–979.
spring. Clin Exp Allergy 2002, 32:1020–1028.
Nouri-Aria KT, Irani AM, Jacobson MR, O'Brien F, Varga EM, Till SJ, Durham
Lee TH, Hoover RL, Williams JD, Sperling RI, Ravalese Jr J, Spur BW, Robinson
SR, Schwartz LB: Basophil recruitment and IL-4 production during human
DR, Corey EJ, Lewis RA, Austen KF: Effect of dietary enrichment with
allergen-induced late asthma. J Allergy Clin Immunol 2001, 108:205–211.
eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and
Milgrom H, Fick RB, Su JQ, Reimann JD, Bush RK, Watrous ML, Metzger WJ:
monocyte leukotriene generation and neutrophil function. N Eng J Med
Treatment of allergic asthma with monoclonal anti-IgE antibody. N Eng J
Med 1999, 341:1966–1973.
Arisaka M, Arisaka O, Yamashiro Y: Fatty acid and prostaglandin metabolism
Arm JP, Lee TH: Sulphidopeptide leukotrienes in asthma. Clin Sci 1993,
in children with diabetes mellitus. II. The effect of evening primrose oil
supplementation on serum fatty acid and plasma prostaglandin levels.
Arm JP, Horton CE, Mencia-Huerta JM, House F, Eiser NM, Clark TJ, Spur BW,
Prostaglandin Leukot Essential Fatty Acids 1991, 43:197–201.
Lee TH: Effect of dietary supplementation with fish oil lipids on mild
Kapoor R, Huang YS: Gamma linolenic acid: an antiinflammatory omega-6
asthma. Thorax 1988, 43:84–92.
fatty acid. Current Pharmaceut Biotech 2006, 7:531–534.
Arm JP, Horton CE, Spur BW, Mencia-Huerta JM, Lee TH: The effects of dietary
Takai S, Jin D, Kawashima H, Kimura M, Shiraishi-Tateishi A, Tanaka T,
supplementation with fish oil lipids on the airways response to inhaled
kakutani S, Tanaka K, Kisa Y, Miyazaki M: Anti-atherosclerotic effects of
allergen in bronchial asthma. Am Rev Respir Dis 1989, 139:1395–1400.
dihomo-gamma-linolenic acid in ApoE-deficient mice. J Atherosclerosis
Lee TH, Mencia-Huerta JM, Shih C, Corey EJ, Lewis RA, Austen KF: Effects of
Thromb 2009, 16:480–489.
exogenous arachidonic, eicosapentaenoic, and docosahexaenoic acids
Mantzioris E, James MJ, Gibson RA, Cleland LG: Dietary substitution with
on the generation of 5-lipoxygenase pathway products by ionophore-
an alpha-linolenic acid-rich vegetable oil increases eicosapentaenoic
activated human neutrophils. J Clin Invest 1984, 74:1922–1933.
acid concentrations in tissues. Am J Clin Nutr 1994, 59:1304–1309.
Calder PC: n − 3 Polyunsaturated fatty acids, inflammation, and
Whelan J: Dietary stearidonic acid is a long chain (n-3) polyunsaturated
inflammatory diseases. Am J Clin Nutr 2006, 83:S1505–S1519.
fatty acid with potential health benefits. J Nutr 2009, 139:5–10.
Department Health and Human Services N, NHLBI: Expert panel report 2:
guidelines for the diagnosis and management of asthma. NIH Publication
Cite this article as: Arm et al.: Impact of botanical oils on
No 07-4051 2007.
polyunsaturated fatty acid metabolism and leukotriene generation in
Johnson MM, Swan DD, Surette ME, Stegner J, Chilton T, Fonteh AN, Chilton
mild asthmatics. Lipids in Health and Disease 2013 12:141.
FH: Dietary supplementation with gamma -linolenic acid alters fatty acidcontent and eicosanoid production in healthy humans. J Nutr 1997,127:1435–1444.
Source: http://gamalift.com.br/site/artigos/26.pdf
Allam Appa Rao Page 1 of 24 Professor Allam Appa Rao Allam Appa Rao Page 2 of 24 Jawaharlal Nehru Technological University: Kakinada, AP, India +91-884 2300 888 (Office), +91-884 2300 800 (Fax), +91-98481 85922 (Personal Cell) E Mails: [email protected],Web SPermanent Home Address: 4-51-19/1/2, Lawson's Bay Colony, Visakhapatnam - 530 017
"Las prácticas de intervención corporal de los movimientos sociosexuales frente al sistema experto de salud (legitimado para decir y hacer sobre los sujetos-cuerpos)". Mgter. Cecilia Rugna (UNL-UNSAM) Correo electrónico: [email protected] Resumen: En el trabajo de campo de mi investigación de tesis de maestría (Rugna, 2014) surgía


