Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
Cns drugs 2004; 18 (5): 269-284
CNS Drugs 2004; 18 (5): 269-284
2004 Adis Data Information BV. All rights reserved.
σ
-1 Receptor Ligands
Potential in the Treatment of Neuropsychiatric Disorders
Teruo Hayashi and
Tsung-Ping Su
Cellular Pathobiology Unit, Cellular Neurobiology Research Branch, Intramural ResearchProgram, Department of Health and Human Sciences, National Institute on Drug Abuse,National Institutes of Health, Baltimore, Maryland, USA
The σ receptor was originally proposed to be a subtype of the opioid receptor.
However, it is now clear that σ receptors are unique non-opioid,non-phencyclidine brain proteins. Two types of σ receptor exist, the σ-1 receptorand the σ-2 receptor. σ-1 receptors have been cloned and their distribution,physiological functions and roles in signal transduction were recently character-ised. Certain sex hormones in the brain (neurosteroids) are known to interact with
σ-1 receptors. σ-1 receptors regulate glutamate NMDA receptor function and therelease of neurotransmitters such as dopamine. They are thus proposed to beinvolved in learning and memory as well as in certain neuropsychiatric disorders.
Selective σ-1 receptor ligands have been suggested to represent a new class of
therapeutic agents for neuropsychiatric disorders, although none have yet beenintroduced into therapeutic use. Early studies showed that psychotomimeticbenzomorphans, as well as several antipsychotics, can bind to σ-1 receptors. As aresult of these findings, σ-1 receptor ligands have been proposed as being ofpotential use in the treatment of schizophrenia. Nevertheless, the relationship of
σ-1 receptors to the underlying pathogenesis of schizophrenia is still unclear. σ-1receptor ligands have failed to improve acute psychotic symptoms of schizo-phrenia in clinical trials, but, interestingly, a few studies have shown an improve-ment in negative symptoms in schizophrenic patients.
A number of preclinical studies have shown that selective agonists of σ-1
receptors affect higher-ordered brain functions such as learning and memory,cognition and mood. These studies indicate that σ-1 receptor agonists may exerttherapeutic effects in depression and senile dementia. Indeed, the σ-1 receptoragonist igmesine, has been shown to improve depression in a clinical trial. Themost distinctive feature of the action of σ-1 receptor ligands is their ‘modulatory'role. In behavioural studies of depression and memory, they exert beneficialeffects only when brain functions are perturbed.
Given the recently accumulated preclinical and clinical data, it is time to
reconstruct the concept of σ-1 receptors and the associated pathophysiologicalconditions that ligands of these receptors target. This would allow clinical trials tobe performed more efficiently, and the results may confirm a long-speculatedpossibility that σ-1 receptor ligands represent a new class of therapeutic agents forneuropsychiatric disorders.
σ receptors were originally proposed by Martin
benzomorphans such as SKF-10047 (N-allyl-
et al.[1] to be a subtype of opioid receptor. Since
normetazocine) and pentazocine are known to bind
Hayashi & Su
selectively to σ receptors and cause delusions and
introduction into clinical trials. However, recently
hallucinations in animals and humans,[1-6] Martin et
accumulated evidence on σ-1 receptors from basic
al.[1] speculated that these drugs have a
and preclinical research has resulted in a significant
psychotomimetic action through an interaction with
advance in our understanding of this orphan recep-
σ receptors. However, a series of later experiments
tor. In particular, behavioural studies using animals
demonstrated that the σ receptor is insensitive to
have contributed greatly to understanding the physi-
naloxone, a universal antagonist of opioid recep-
ological function of σ-1 receptors.
tors,[7,8] and confirmed that the σ receptor is a non-
In this article, findings on σ-1 receptors from
opioid receptor. However, because SKF-10047 can
preclinical and clinical research are summarised,
bind to the phencyclidine (PCP) site on glutamate
and the physiological function of σ-1 receptors, as
NMDA receptors and, conversely, PCP can bind to
well as the potential therapeutic targets of σ-1 recep-
the σ receptor, confusion arose between the σ recep-
tor ligands in clinical pharmacotherapy, discussed.
tor and the PCP site on NMDA receptors.[9] Furtherexperiments using more selective ligands for the σ
1. σ
-1 Receptors
receptor, such as (+)-3-(3-hydroxyphenyl)-N-propylpiperidine [(+)-3-PPP] and for the PCP site,
such as thienylcyclohexylpiperidine (TCP), showedthat the binding sites and brain distribution of σ and
The σ-1 receptor was originally identified using a
NMDA receptors are different,[10-13] leading to the
dextrorotatory isomer of the benzomorphan
conclusion that the σ receptor is a non-opioid, non-
SKF-10047 [(+)SKF-10047],[7,9-11,13] and further
PCP unique brain-enriched receptor.
characterised by more selective compounds such as
Data from receptor binding studies indicate that
(+)pentazocine.[19] (–)-Isomers [e.g. (–)SKF-10047]
at least two subtypes of σ receptor exist: σ-1 (high
have significantly lower affinities for the σ-1 recep-
affinity site) and σ-2 (low affinity site) receptors.[14]
The σ-1 receptor was cloned in 1996.[15] The cloned
It is now known that many structurally diverse
protein contains an endoplasmic reticulum (ER) re-
compounds can bind to the σ-1 receptor (table I).
tention signal (therefore residing at the ER) and at
Interestingly, several clinically used psychotropic
least one putative transmembrane domain.[15-18] The
drugs such as haloperidol, imipramine and SSRIs
σ-2 receptor has not been cloned.
can bind to the receptor with low nanomolar affini-
In this article, we focus on the σ-1 receptor. As
ty.[7,20-23] Some σ-1 receptor ligands such as halo-
mentioned above, some benzomorphans that can
peridol, NE-100 and MS-377 have been shown to
bind to the σ-1 receptor are known to cause psycho-
block, especially in
in vivo studies, the action of
sis in humans.[2,4-6] Furthermore, it was discovered
(+)pentazocine and other selective σ-1 ligands and,
in the 1980s that some antipsychotics, especially
therefore, have been categorised as σ-1 receptor
haloperidol, have relatively high affinities for σ-1
antagonists.[24,25] However, it is still unclear exactly
receptors.[7] Thus, σ-1 ligands were originally pro-
which pharmacological actions exerted by these
posed to be a new class of antipsychotics for the
therapeutic compounds are mediated by the σ-1
treatment for schizophrenia. However, until the ear-
receptor.[7,20,22,23] As such, in drug development, in
ly 1990s, methods for studying the σ-1 receptor
addition to receptor binding assays, a thorough eval-
were confined to mainly receptor binding assays and
uation of σ-1 ligands by recently established
in vitro
there were no established biochemical and behav-
and
in vivo assays (see sections 1.3 and 2.1) is
ioural tools to characterise the agonist-antagonist
necessary, as evidence from binding assays alone
action of σ-1 ligands and their implications for
may not be sufficient to fully characterise the ligand.
certain neurophysiological conditions. In addition,
In other words, more criteria should be applied when
endogenous ligands, intracellular signal transduc-
interpreting a pharmacological action of ‘so-called'
tion and receptor localisation had not been clarified.
σ receptor ligands, as the compounds that have
These impeded the identification of the specific
affinity for the σ binding site may not always be
therapeutic targets of σ-1 receptor ligands and their
selective and functional.
2004 Adis Data Information BV. All rights reserved.
CNS Drugs 2004; 18 (5)
σ-1 Receptors in Neuropsychiatric Disorders
Table I. σ-1 receptor ligands
Subtype selectivity Function
Also binds to the PCP site
Highly selective agonist
Dopamine receptor agonist
Dopamine D2 receptor antagonist
Monoamine reuptake inhibitor
Partial agonist in some assays
Pregnenolone sulphate
PB-008 (dihydroepiandrosterone sulphate)
Dopamine transporter inhibitor
Other synthetic compounds
Igmesine (JO-1784)
High affinities for both σ-1receptor and C8-C7 sterolisomerase
Serotonin 5-HT1A receptoragonist
Rimcazole (BW-234U)
Ineffective against the positivesymptoms of schizophrenia[27]b
BMS-181100 (BMY-14802)
Ineffective against the positivesymptoms of schizophrenia[28]c
Panamesin (EMD-57445)
Effective against the positive/negative symptoms ofschizophrenia[29,30]d
Metabolite is a dopamineantagonist
Eliprodil (SL-82.0715)
Effective against negativesymptoms of schizophrenia[31]d
Glutamate NMDA polyamine siteantagonist
Inhibition constant (Ki) values, where +++ indicates <50 nmol/L, ++ indicates <500 nmo/L and + indicates <10 µmol/L.
Double-blind study.
Single-blind study.
Open-label study.
3-PPP = 3-(3-hydroxyphenyl)-N-propylpiperidine;
DTG = 1,3-di-o-tolylguanidine;
PCP = phencyclidine.
2004 Adis Data Information BV. All rights reserved.
CNS Drugs 2004; 18 (5)


Hayashi & Su
Sex steroid hormones such as progesterone and
tritiated benzomorphans may contain multiple sites
pregnenolone sulphate are known to bind to the σ-1
including the σ-1 receptor.[50-52]
receptor.[32] These hormones are synthesised denovo in the brain (and are termed neurosteroids) and
1.3 Effect on Cellular Function
are known to regulate NMDA and GABAA recep-tor-coupled channels.[33] Specifically, progesterone
As mentioned in section 1.2, recent studies using
possesses a significant affinity for the σ-1 receptor
a specific antibody against the σ-1 receptor suggest
at physiologically relevant concentrations; the affin-
that the receptor exists predominantly on the ER,
ity of progesterone at the σ-1 receptor has been
especially the smooth ER.[47,53] In the rat primary
shown to be about 250 nmol/L in binding stud-
neuron, we found that the majority of σ-1 receptors
ies.[32,34] Brain levels of neurosteroids are known tobe affected by aging, emotion and certain patho-physiological states such as dementia and depres-sion.[33,35-37] Several behavioural and physiological
experiments suggest the involvement of σ-1 recep-
Lung HeartAortaVein
tors in certain behaviours caused by neuroster-
oids.[38-41] The physiological relevance of neuroster-
oid binding to σ-1 receptors has been under intense
Pancreas renal glandpose tissue
-1 receptors are present in high density in the
CNS. The early experiments using binding assaysand autoradiography suggested that σ-1 receptorsare enriched in several brain regions including thecerebellum, and exist also at a high density in theliver and adrenal gland.[10-12,42-44]
σ-1 receptors are enriched in microsomal mem-
branes from the brain,[45] suggesting that they maylocalise on the endoplasmic reticulum (ER).
After the cloning of the σ-1 receptor, it became
possible to measure the mRNA and protein levels of
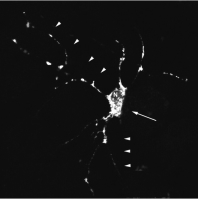
σ-1 receptors using nucleotide probes or anti-bodies.[46,47] mRNA of σ-1 receptors is expressed atmoderate levels in the brain and several peripheralorgans including the stomach, liver, adrenal glandand testis.[15-17] However, protein levels measuredby a specific antibody against σ-1 receptors exhibitquite a different pattern (figure 1),[48,49] with thelevel being highest in the brain, spinal cord andperipheral nerves, but is lower in peripheral organs
Fig. 1. Cellular and whole body distribution of σ-1 receptors. (a)
including the liver and adrenal grand. Furthermore,
Body distribution of σ-1 receptors in the mouse. Protein extractsfrom each organ or region were separated by sodium dodecyl
in the brain, the σ-1 receptor protein level is high in
sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and blot-
the frontal cortex, hippocampus, and striatum, but
ted by a σ-1 receptor antibody. (b) A rat brain primary neuron
lower in the cerebellum.[48,49] Therefore, the distri-
expressing yellow fluorescent protein (YFP)-tagged σ-1 receptors(shown in white). EYFP-N vectors (clontech) containing σ-1 re-
bution of the σ-1 receptor protein is much more
ceptor cDNA were transfected to neurons from the postnatal rat
restricted than previously reported. The distribution
brain. The cell body is indicated by the arrow and the dendrites by
of σ-1 binding sites as originally characterised by
the arrowheads. N = nucleus.
2004 Adis Data Information BV. All rights reserved.
CNS Drugs 2004; 18 (5)
σ-1 Receptors in Neuropsychiatric Disorders
are localised on the ER of the cell body and the
search is needed to understand the mechanisms by
dendrites, and are highly clustered (figure 1).[48]
which the σ-1 receptor modulates diverse types of
These results are consistent with the enrichment of
ion channels on the plasma membrane.
σ-1 binding sites in brain microsomes[45] and with
As mentioned in section 1.1, tricyclic antidepres-
the existence of an ER retention motif on the N-
sants and SSRIs possess high affinity for the σ-1
terminus of the cloned σ-1 receptor.[15]
receptor.[7,20,22,23] We recently found that these anti-
Recent studies using green fluorescence protein-
depressants potentiate nerve growth factor-induced
fused σ-1 receptors demonstrated that the receptors
neurite growth in PC-12 cells via σ-1 receptors.[64]
localise on lipid droplets associated with the ER in
Therefore, the affinity of these antidepressants for
addition to being on the ER reticular network.[1,54,55]
the σ-1 receptor may contribute to certain pharma-
Functional dominant negative σ-1 receptors failed to
cological actions, specifically their neurotropic ac-
target ER-lipid droplets, which led to retention of
neutral lipids and cholesterol in the ER, a decrease
Several lines of research demonstrated that the
of cholesterol in plasma membranes and a bulbous
σ-1 receptor modulates neuronal firing and neuro-
aggregation of ER.[54] The ER synthesises most
transmitter release.[41,65-67,93-96] Monnet et al.[66]
lipids inside cells and exports them to the periphery
found that σ-1 receptor ligands, although exhibiting
of cells for the constitution of the plasma membrane.
no effect by themselves, selectively enhance
Therefore, these findings suggest that σ-1 receptors
NMDA-induced neuronal firing in the CA3 region
on the ER regulate the compartmentalisation and
of hippocampus in rats. σ-1 receptor ligands also
export of ER lipids, and the resultant are lipid com-
modulate NMDA-mediated neuronal firing in the
positions in the plasma membranes. Interestingly,
CA1 region in a biphasic dose-response manner.[65]
upon stimulation with σ-1 receptor agonists, σ-1
A 2-day treatment with σ-1 receptor agonists mark-
receptors can translocate at the ER (i.e. from ER
edly increases neuronal firing of serotonergic neu-
lipid droplets to periphery ER networks that are
rons in the dorsal raphe nucleus.[67] Interestingly, a
close to the plasma membrane or to the tips of
selective σ-1 receptor agonist SA-4503 potentiates
neurites[55]). The physiological role of σ-1 receptor
dopamine neuronal activity in ventral regimental
translocation is unknown at present; however, be-
area, but supresses it in substantia nigra pars com-
cause σ-1 receptors specifically target ER lipid
pacta in the rat brain.[68] On the other hand, σ-1
droplets and compartmentalise neutral lipids, the
receptor ligands were shown to modulate depo-
dynamic translocation of the receptors might affect
larisation-induced dopamine release from brain
lipid transport and distribution in neuronal cells.
slices.[94] Nuwayhid and Werling[95] recently dem-
Localisation of σ-1 receptors on the ER is in
onstrated that σ-1 receptor agonists regulate
perfect agreement with σ-1 receptors regulating
NMDA-induced dopamine release from rat striatal
Ca2+ signaling via inositol 1,4,5-trisphosphate (IP3)
slices via the protein kinase C pathway. σ-1 receptor
receptors on the ER.[47] σ-1 receptor agonists were
agonists and neurosteroids, on the other hand, poten-
found to potentiate IP3-induced Ca2+ mobilisation
tiate NMDA-induced noradrenaline release from rat
in a biphasic dose-dependent manner in a neuronal
hippocampus slices.[41] It has also been shown, using
cell line.[47] The regulation is mediated by the asso-
in vivo microdialysis, that σ-1 receptor agonists
ciation of the σ-1 receptor with cytoskeletal proteins
increase spontaneous acetylcholine release in the
frontal cortex.[96]
In addition to its action at the ER, the σ-1 recep-
Although the exact molecular action of the σ-1
tor can also modulate several physiological and cel-
receptor remain unclear, the receptor appears to
lular events on the plasma membrane (table II). The
regulate neuronal excitability by modulating plasma
receptor regulates ion channels such as K+ chan-
membrane potentials and/or intracellular Ca2+ sig-
nels,[56] NMDA receptors[57-62] and voltage-depen-
naling. It should be emphasised that most of the
dent Ca2+ channels.[47,63] It is suggested that the σ-1
pharmacological effects of σ-1 receptor ligands are
receptor inhibits the K+ channel current by a direct
‘modulatory'. σ-1 receptor agonists did not cause
coupling as a modulatory subunit.[56] Further re-
Ca2+ mobilisation, neuronal firing and dopamine
2004 Adis Data Information BV. All rights reserved.
CNS Drugs 2004; 18 (5)
Hayashi & Su
release by themselves in the above-mentioned stud-
pose that σ-1 receptors might be involved in regulat-ing the remodelling of neuronal membranes.[54,55,99]
ies;[47,66,95] they exerted their modulatory actionwhen the IP3 receptor, K+ channel or NMDA chan-
2. Effects of σ-1 Receptor Ligands in
nel was activated by transmitters or depolarisa-
Animal Models of Behaviour
tion[47,56,66,95] (also see review by Su and Hayashi[97]for details). Taken together, σ-1 receptors appear to
σ-1 receptor ligands have been tested in several
play an important role in the CNS as a modulator or
animal models of behaviour. Actions of σ-1 receptor
an amplifier of signal transduction in systems such
ligands include: (i) improvement of memory and
as NMDA channel activity, Ca2+ signalling and
cognitive function; (ii) antidepressant-like actions;
NGF-induced cell differentation.[97] These systems
and (iii) modulation of psychostimulant-induced be-
have recently been shown to be essential factors in
the formation of neural plasticity.[98] Thus, we pro-
2.1 Drug-Induced Amnesia
Table II. Modulatory pharmacological action of σ-1 receptor ligands
System/model effected
It is well established that cholinergic and gluta-
matergic (especially via the NMDA receptor sub-
In vitro studies
type) neurotransmitter systems play a crucial role in
Ion channel activity[47,56-64] Glutamate NMDA
the neurophysiological processes underlying learn-
ing and memory.[100,101]
Selective σ-1 agonists have been shown to re-
verse amnesia induced by acetylcholine muscarinic
and nicotinic receptor antagonists in animals.[69,102]
It is interesting that σ-1 receptor agonists including
(+)pentazocine, (+)SKF-10047 and 1,3-di-o-tolyl-
guanidine (DTG) exert an anti-amnesic action when
Neuron firing[39,66-68]
Glutamatergic (NMDA)
administered either at pre-training, immediately
neuron in hippocampus
after training or before retention test time points of
Serotonergic neuron in
dorsal raphe nucleus
mnemonic examinations.[69,102,103] These results sug-
Dopaminergic neuron in
gest that σ-1 receptor agonists can improve acetyl-
choline-related cognitive processes encompassing
all phases of memory formation: acquisition, con-
Y-maze test and passive Improvedavoidance[39,40,49,67,69-72]
solidation and retention.
Depression and stress
Selective σ-1 agonists also improve amnesia in-
duced by a NMDA receptor antagonist or by a Ca2+
channel antagonist.[70,71] Using different behavioural
tests, Maurice et al.[70] demonstrated that σ-1 recep-
Stress-induced colon
tor agonists produce significant attenuation of short-
PCP-induced cognitive
and long-term memory disturbances induced by
dizocilpine (MK-801). This action was blocked by
Psychostimulant-induced Inhibited by
NE-100, a σ-1 receptor antagonist.
Notably, in all behavioural tests for learning and
memory, σ-1 receptor agonists again show a modu-
conditioned place
latory pharmacological action. They show anti-am-
nesic action only when memory and cognition are
Cocaine toxicity[85-88]
impaired by chemicals that inhibit neural transmis-
sion. By themselves, they do not further augment
memory in normal animals. It is reported that σ-1
IP3 = inositol 1,4,5-triphosphate; PCP = phencyclidine.
receptor ligands also show beneficial effect against
2004 Adis Data Information BV. All rights reserved.
CNS Drugs 2004; 18 (5)
σ-1 Receptors in Neuropsychiatric Disorders
memory impairment induced by physiological pro-
and dementia is unclear at present, σ-1 receptor
cesses such as aging.[72]
agonists may improve memory and cognition in
σ-1 ligands may not cause the adverse effect,
certain types of dementia.
such as peripheral cholinomimetic responses, thatare often associated with acetylcholinesterase inhib-
2.2 Depression and Anxiety
itors (the current gold standard treatment for demen-tia).
Because σ-1 ligands improve amnesia induced
The effects of σ-1 receptor ligands in animal
by Ca2+, acetylcholine or NMDA channel blockers,
models of depression have recently been investigat-
intracellular Ca2+ appears to be a candidate as a
ed. The immobility time in the mouse forced swim-
common factor regulated by σ-1 receptors. This
ming test was dose-dependently reduced by σ-1
possibility has been further investigated[104] and it
receptor agonists.[73] Structurally diverse σ receptor
has been found that σ-1 receptors regulate Ca2+
agonists such as (+)pentazocine, SA-4503 and
release from intracellular Ca2+ storage sites.[47,53]
igmesine (JO-1784) all decreased the immobility
Furthermore, it has been shown that injections of
time in the test.[73,74,111] This action was blocked by
inhibitors of intracellular Ca2+ release into the brain
the σ-1 receptor antagonist NE-100.
abolish the effects of σ-1 receptor agonists in beha-
Neurosteroids such as pregnenolone sulphate and
viour tests.[104]
PB-008 act as σ-1 receptor agonists, although pro-
Cognitive function depends partly on neuroster-
gesterone acts apparently as a σ-1 receptor ant-
oid levels, which are decreased by aging. It has been
agonist.[3,40,47] Since sulphated neurosteroids act as
shown that levels of pregnenolone sulphate are sig-
reservoirs for the unsulphated neurosteroids in the
nificantly lower in the hippocampus of aged (24-
brain, their levels are much higher than those of
month) than in young ( 3-month) male animals.[105]
unsulphated forms.[33] Pregnenolone sulphate and
Low levels of pregnenolone sulphate in the hippo-
PB-008 have higher affinities for the σ-1 receptor
campus are correlated with poor performance in
than their unsulphated forms.[32] Therefore, their
spatial memory tasks.[105] Central or systematic ad-
concentrations in the brain may reach a sufficient
ministration of PB-008 (dihydroepiandrosterone
level to interact with σ-1 receptors under certain
[DHEA] sulphate) and pregnenolone sulphate im-
psychological states such as stress, anxiety and ag-
proves/enhances mnemonic performances.[105]
gression.[33] The interaction between the σ-1 recep-
These actions of neurosteroids involve NMDA-de-
tor and neurosteroids has been shown in the forced
pendent processes, but have also been shown to be
swimming test.[73,74,111] The above neurosteroids sig-
blocked by selective σ-1 receptor antagonists.[40]
nificantly decreased the immobility time in these
Therefore, σ-1 receptors regulating cognitive func-
studies, an effect that was blocked by NE-100 and
tion may involve an interaction between neuroster-
BD-1047, suggesting that certain neurosteroids ex-
oids and NMDA receptors. A recent study demon-
ert antidepressant actions by interacting with σ-1receptors.[73,74]
strated that there is a general trend toward lowerlevels of neurosteroids in different brain regions of
Extract of the flowering plant St John's wort
individuals with Alzheimer's disease or aged nor-
(Hypericum perforatum) is prescribed as an antide-
mal subjects. The metabolism of PB-008 is also
pressant in Germany. However, the mechanism of
shown to decrease in these subjects.[106] A study
the antidepressant action of St John's wort remains
using post-mortem brains showed a 26% reduction
unknown. Raffa[112] tested the binding of hypericin
of [3H]DTG binding in the CA1 region of the hippo-
(a major component of St John's wort) to 30 recep-
campi in patients affected by Alzheimer's dis-
tors or uptake sites that are suspected to be involved
ease.[107] Some clinical studies suggest that hormone
in the action of clinically used antidepressants. At
replacement therapy using estrogen and PB-008 im-
1.0 µmol/L, hypericin inhibited less than 40% of
proves cognitive function and may protect against
specific binding at all receptors or uptake sites ex-
age-associated memory decline.[106,108-110] Thus, al-
cept acetylcholine muscarinic receptors and σ recep-
though the relationship between the σ-1 receptor
tors.[112] Hypericine (1.0 µmol/L) inhibits 48% of
2004 Adis Data Information BV. All rights reserved.
CNS Drugs 2004; 18 (5)
Hayashi & Su
[3H]DTG binding. Because the author used subtype-
2.3 Psychosis, Drug-Dependence
nonselective ligands in his assay, it is unclear at
present which subtype of σ receptor (1 or 2) binds tohypericine. The antidepressant action of hypericin in
the forced swimming test was inhibited by a σ-1
Since σ-1 receptor ligands were originally expec-
receptor antagonist.[75] Bennett et al.[113] systemical-
ted to represent a new class of antipsychotics, a
ly evaluated all articles on hypericin identified from
substantial number of experiments have been per-
a database and suggested that the σ receptor is a
formed to examine the potential involvement of σ-1
potential site of action for hypericin.
receptors in schizophrenia using animal models.
Because the pharmacological effect of σ-1 recep-
Amphetamine and PCP are frequently employed as
tor ligands is typically modulatory, it is reasonable
the animal model of schizophrenia. Amphetamine
to propose developing compounds that possess a
mimicks acute positive symptoms of schizophrenia,such as delusion and hallucination. Methamphetam-
high affinity for both σ-1 receptors and other recep-
ine and apomorphine are also known to induce simi-
tors that are known to be related to certain pathologi-
lar effects. Because PCP causes not only acute psy-
cal states such as depression. A newly synthesised
chosis mimicking positive symptoms but also long-
antidepressant OPC-14523 has a high affinity for
lasting psychosocial dysfunction similar to the nega-
both σ-1 receptors (47–56 nmol/L) and serotonin
tive symptoms of schizophrenia, PCP-induced psy-
5-HT1A receptors (2.3 nmol/L).[76] A single oral
chosis is suggested to be a suitable model for schizo-
administration of OPC-14523 produced a marked
antidepressant-like effect in the forced swimmingtest, whereas fluoxetine and imipramine required at
Some putative σ-1 receptor antagonists (e.g.
least four days of repeated administration to show
BMS-181100 [BMY-14802]) inhibit methamphet-
the same activity.[76] This potent antidepressant-like
amine-induced locomotor activity and apomor-
action of OPC-14523 can be mimicked by combined
phine-induced stereotypy.[115] However, highly se-
administration of σ-1 and 5-HT
σ-1 antagonists such as NE-100 and MS-377
do not affect locomotor activity induced by an injec-tion of amphetamine.[24,25,81] Some of these experi-
ments used σ receptor ligands at high doses (>20mg/kg);[24] therefore, the inhibitory effect seen on
σ-1 ligands also exert anxiolytic action in animal
psychostimulant-induced locomotor activity may
behavioural tests.[78] Animals exhibit a marked sup-
not be solely due to interaction with the σ-1 recep-
pression of motility when they are placed in the
tor. In fact, the action of σ-1 ligands on amnesia (see
same environment in which they had previously
section 2.1) is usually seen at doses below 1 mg/kg,
received an electric footshock (conditioned fear
and the effect usually disappears at higher doses (> 3
stress). (+)SKF-10047 reversed the conditioned fear
mg/kg).[70] Selective antagonism of dopamine D2
stress and this effect was blocked by NE-100.[78]
receptors seems to be more effective in attenuating
However, this anxiolytic action was not seen with
locomotion and stereotypy induced by the acute
(+)pentazocine and DTG.
administration of psychostimulants. Taken together,
Gue et al.[79,80] reported that the σ-1 receptor
the data on the direct involvement of σ-1 receptors
agonist igmesine attenuates an increase of colonic
in schizophrenia, especially acute psychotic symp-
spike bursts induced by conditioned fear stress or
toms, is equivocal.
corticotropin releasing hormone. Their findings are
NE-100 is highly selective for σ-1 receptors and
relevant with regard to gastrointestinal tract disor-
is the most well-defined functional σ-1 receptor
ders that are frequently seen in anxiety and mood
antagonist.[24] NE-100 does not affect behaviours
induced by amphetamine, but inhibits several types
More studies using structurally different σ-1
of behaviour induced by PCP.[24] The order of inhib-
ligands may confirm the possible involvement of the
iting potencies on PCP-induced head weaving by
σ-1 receptor in anxiety.
σ-1 receptor antagonists (NE-100 > haloperidol >
2004 Adis Data Information BV. All rights reserved.
CNS Drugs 2004; 18 (5)
σ-1 Receptors in Neuropsychiatric Disorders
BMS-181100 > Dup-734) correlates well with their
agonists, indicating the potential involvement of σ-1receptors in cocaine-induced toxicity.[88]
affinities for σ-1 receptors.[24] PCP-induced beha-viour is insensitive to selective D2 receptor antagon-
Recently, the σ receptor antagonists BD-1047
ists, but can be attenuated by very low doses of
and LR-172 were shown to inhibit cocaine-induced
NE-100 (<0.1 mg/kg).[24] In addition, NE-100 alone
locomotor activity.[86] Both compounds possess sim-
did not cause any extrapyramidal symptoms. Selec-
ilar high affinities for σ-1 receptors. However,
tive σ-1 receptor agonists such as (+)pentazocine, at
LR-172, which possesses much greater affinity at
low doses that caused no behavioural activity, en-
σ-2 receptors, was a more potent inhibitor than
hanced the behavioural effect of dizocilpine, and
BD-1047 against cocaine-induced locomotor ac-
this enhancement was blocked by NE-100 (0.01–0.1
tivity.[86] These data may suggest the involvement of
mg/kg).[24] Thus, it is very likely that one potential
σ-2 receptors in this action.
site of action of the σ-1 receptor is the regulation of
The affinity of cocaine for σ-1 receptors is about
ion channels coupled to NMDA receptors. A dys-
2 µmol/L.[118] The intracellular concentration of co-
function of glutamatergic transmission, specifically,
caine in the brain of cocaine abusers might not reach
in the frontal cortex is implicated in schizo-
a sufficient level to occupy σ-1 receptors on the ER.
phrenia.[114,116] σ-1 receptor ligands may thus affect
Romieu et al.[84] reported that conditioned place
certain schizophrenic symptoms that are related to a
preference induced either by cocaine or a selective
dysfunction of glutamatergic transmission.
dopamine transporter inhibitor without any σ-1 re-ceptor affinity is inhibited by a selective σ-1 recep-
It has been shown that the microinjection of σ
tor antagonist, suggesting that σ-1 receptors may
receptor ligands, including DTG and
play a role downstream of dopaminergic transduc-
(+)SKF-10047, into the red nucleus causes dystonia
tion. Further studies are required to clarify the mech-
in animals.[117] Antipsychotics lacking affinity for σ
anism by which σ-1 receptors may be involved in
receptors such as clozapine fail to induce this move-
cocaine abuse.
ment disorder. Interestingly, the microinjection of
Ujike et al.[82,83] reported that putative σ receptor
these σ ligands into the substantia nigra produces
antagonists, at doses per se not affecting spontan-
vigourous contralateral circling behaviour at ex-
eous locomotor activity, block the development of
tremely low doses. These data suggest the possibili-
behavioural sensitisation induced by cocaine and
ty that σ receptors are involved in the motor adverse
methamphetamine. This action of σ receptor ant-
effects of antipsychotics. The σ ligand-induced
agonists was confirmed by other researchers using
movement disorders seem to involve both σ-1 and
the selective σ-1 antagonist MS-377.[81]
σ-2 subtypes.[85] This information will be importantwhen the safety of σ-1 receptor ligands is evaluated
in clinical trials.
Several reports indicate that σ-1 receptor ago-
nists can inhibit opioid-induced analgesia.[89-91] As
2.3.2 Drug Dependence
shown in figure 1, σ-1 receptors are enriched in thespinal cord and peripheral nerves where opioid re-
Recently, the involvement of the σ-1 receptor in
ceptors are enriched. σ-1 receptors and ligands may
the pharmacological action of cocaine has been in-
thus regulate opiate receptor activities at these loci.
tensively investigated. Because cocaine has a mod-erate affinity for σ-1 receptors,[118] it is suggested
3. Potential Therapeutic Targets of
that certain actions of cocaine, inducing its toxic
Selective σ-1 Receptor Ligands
effects, may be attributed to its direct interactionwith σ-1 receptors.[99]
3.1 Schizophrenia
Matsumoto and colleagues[86-88] clearly demon-
strated that novel σ receptor antagonists at low
The demonstration that benzomorphans, such as
doses (1 mg/kg) significantly inhibit convulsions
SKF-10047, produce ‘canine delirium' and psycho-
and lethality induced by a toxic dose of cocaine. The
sis in dogs[1] led to the proposal that σ-1 receptors
toxicity of cocaine was potentiated by σ-1 receptor
mediate these effects. Binding studies using
2004 Adis Data Information BV. All rights reserved.
CNS Drugs 2004; 18 (5)
Hayashi & Su
[3H]SKF-10047 and (+)[3H]-3-PPP identified an
BPRS scores were ≥35. In a single-blind trial, treat-
abundance of σ receptors in the brain.[7,10-13] An
ment with BMS-181100 (500–1500mg twice daily
important role of σ receptors in schizophrenia is also
up to a maximum of 3000 mg/day) for 4 weeks in 28
suggested by the high affinity of many established
patients was not associated with any improvements
antipsychotic drugs for σ receptors.[7] However, in
in BPRS and CGI score.[28] The most frequent ad-
the early reports on benzomorphan-induced psycho-
verse effect was mild to moderate sinus tachycardia.
sis in humans, very high dose of pentazocine were
Elevations in creatine phosphokinase (CPK) levels,
usually administered.[5,6] In most case, a mixture of
agitation and insomnia were seen in some patients.
both (+) and (–) isomers were given.[5,6] Considering
There are a number of issues associated with
that these benzomorphans have moderate to high
these two trials[27,28] that need to be considered: (i)
affinities for the PCP site,[9] the psychotomimetic
the functional activity of the ligands and their selec-
action of benzomorphans might be mainly attributed
tivity for the σ-1 receptor were not fully character-
to their interaction with the PCP site. In fact, recent-
ised via in vivo and in vitro assays; (ii) only schizo-
ly synthesised highly selective σ-1 receptor agonists
phrenic patients in the acute psychotic phase were
do not have any psychotomimetic activity in animal
studied; (iii) the subclass of symptoms was assessed
studies,[76,102] negating a direct relationship between
only using the BPRS, which mainly reflects the
activation of σ-1 receptors and acute psychosis.
degree of positive symptoms; (iv) the duration of
Binding assays using post-mortem brains of schizo-
evaluation might not have been long enough (evalu-
phrenic patients[119,120] and association studies of
ation longer than 4 weeks is usually required for
polymorphism of σ-1 receptor genes in schizo-
examining changes in negative symptoms); and (v)
phrenic patients[121,122] have been performed. How-
an effective dose might not have been used in the
ever, results vary and are not conclusive.
BMS-181100 study[28] (too high in this trial).
Some σ receptor ligands have been tested in
Modell et al.[31] tested the effect of eliprodil
clinical trials (see table I). Rimcazole (BW-234U)
(SL-82.0715), a potent σ-1 receptor ligand, on
was the first σ receptor ligand to be tested in a
schizophrenia in an open-label, dose-ranging study.
clinical trial. After a 1-week placebo washout
This compound exacerbated acute psychotic symp-
period, rimcazole was administered for 4 weeks to
toms in some patients in an earlier study,[125] as was
11 patients with schizophrenia who were experienc-
seen in the rimcazole and BMS-181100 stud-
ing an acute exacerbation of symptoms.[27] Subjects
ies.[27,28,123,124] Therefore, subjects with the diagnosis
were assigned on a double-blind, randomised basis
of residual-type schizophrenia with a predominant
to one of four flexible dosing treatment regimens
negative symptomatology (score of >28 on the nega-
(rimcazole 20–80 mg/day, rimcazole 100–400 mg/
tive subscale of the Positive and Negative Syndrome
day, chlorpromazine 400–1600 mg/day or placebo).
Scale [PANSS]) were included in this study. After a
Rimcazole was not associated with any significant
1- to 2-week washout period, patients received
improvement in the Brief Psychiatric Rating Scale
eliprodil for 4 weeks (2.5 mg/day for the first week,
(BPRS) and Clinical Global Impressions (CGI)
then up to 75 mg/day). Although the number of
scores. However, it did not show any neurological
subjects was limited (n = 10), a significant reduction
adverse effects. Although rimcazole selectively
of scores in negative symptoms, but not in positive
binds to σ-1 receptors, it is a weak antagonist (inhi-
symptoms, according to the PANSS was found.
bition constant [Ki] = 2.4 µmol/L).[24] In contrast to
Panamesine (EMD-57445), a potent σ receptor
these results, in earlier open studies, rimcazole was
ligand with antidopaminergic activity, was shown to
found to cause improvement of negative symptoms
improve both positive and negative symptoms in an
such as depression and anergia, but a worsening of
open clinical trial.[29,30] In this study, panamesine
acute positive symptoms.[123,124]
(15mg on day one, 30mg on day two, 45mg on day
BMS-181100, a relatively selective and potent
three and 60mg from day four onwards) was admin-
σ-1 receptor antagonist, was also tested in patients
istered to 12 schizophrenic patients for 4 weeks, and
with acute exacerbation of schizophrenic symptom-
effects were assessed by both the BPRS and the
atology. Subjects were included in this study if their
PANSS. Statistical analyses showed a significant
2004 Adis Data Information BV. All rights reserved.
CNS Drugs 2004; 18 (5)
σ-1 Receptors in Neuropsychiatric Disorders
reduction of PANSS scores related to both positive
significant importance in depression.[36,37] Several
and negative symptoms after 3 weeks of panamesine
studies have also suggested the involvement of
use. However, panamesine induced mild dyskinetic
neurosteroids in premenstrual dysphoric disorder
movements in 5 of the 12 patients. It needs to be
(PMDD)[128] and attention-deficit hyperactivity dis-
further characterised whether these effects, or a part
of them, are mediated by a direct interaction with
In patients with major depression, neurosteroid
σ-1 receptors, because a metabolite of the drug has
levels in the CSF are lower than in control subjects
potent antidopaminergic activity.[126]
and these levels normalise after successful treatment
Taken together, these results suggest that selec-
with antidepressants.[36,37] SSRIs have been shown
tive σ-1 receptor antagonists appear to have thera-
to normalise levels of allopregnenolone in a seroto-
peutic effects on some symptoms of schizophrenia
nin-independent manner.[37] It is well known that
but not acute positive symptoms. As mentioned in
plasma cortisol levels are increased in depressed
section 2.3, selective σ-1 receptor antagonists may
patients,[36,130] and that PB-008 regulates cortisol
not affect acute psychosis mediated by the activation
levels.[131] Some studies found significantly higher
of dopaminergic transduction, but can inhibit PCP-
cortisol/PB-008 ratios in depressed patients com-
induced behaviours in animals. So far, no anti-
pared with controls.[36,132] Administration of PB-008
psychotic which manipulates glutamatergic neural
has been shown to reduce plasma cortisol levels.[132]
transmission is available. Therefore, the beneficial
The important neurosteroid-targeted receptors in
effect of σ-1 receptor ligands might be seen when
the brain include the GABAA, NMDA and σ-1
they are used to target symptoms of schizophrenia
receptors.[33,36] PB-008 interacts with σ-1 receptors
that are mediated predominantly via glutamatergic
as an agonist and exerts antidepressant-like action in
systems rather than dopaminergic systems. Thus,
an animal model of depression.[73,74] The molecular
σ-1 receptor ligands may affect negative symptoms
mechanism underlying this action of σ-1 receptors is
of schizophrenia, which are hypothesised to be me-
unclear at present. However, it is very likely that the
diated, at least partly, by glutamatergic transmis-
σ-1 receptor plays an important role in the biologi-
cal action of neurosteroids (or neuroactive steroids)
At low doses, psychostimulants exacerbate psy-
in depression. Orally administered neuroactive ster-
chosis in patients with remitting schizophrenia.
oids may not result in an increase in levels in the
Long-term drug abusers sometimes show psychotic
brain due to a rapid metabolism. Selective σ-1 re-
symptoms similar to those seen in schizophrenia
ceptor agonists may act in a complementarily fash-
when taking a low dose of psychostimulants or even
ion to neurosteroids in the brain.
when stressed. Therefore, the behavioural sensitisa-
So far, the only reports of the clinical use of a σ-1
tion to psychostimulants has been used as a model of
receptor ligand as an antidepressant are of igmesine.
schizophrenia, especially for studies of deterioration
In an open trial,[26] igmesine showed a significant
and exacerbation of manifested symptoms.[127] Judg-
improvement in 31 severely depressed inpatients. A
ing from the behavioural sensitisation data in ani-
double-blind placebo-controlled study comparing
mals,[81-83] σ-1 receptor antagonists might have a
igmesine 25 and 100 mg/day with fluoxetine 20 mg/
prophylactic effect in preventing the recurrence of
day in 348 patients with major depressive disorder
schizophrenia. However, so far, no clinical study
was performed in three different countries (UK,
regarding this issue has been performed.
Poland and the Czech Republic). Although no sig-nificant effect was found comparing total samples, a
3.2 Depression and Senile Dementia
subset of patients receiving igmesine 25 mg/day
It has been established that abnormalities of sero-
from UK sites (n = 263) showed a 3 point greater
tonergic and noradrenergic neural transmission are
decrease in total Hamilton Depression Rating Scalescore than the placebo group (p < 0.05).[26]
involved in the pathophysiology of depression.
However, recent studies have also demonstrated the
To date, there have been no reports of the use of a
involvement of neurosteroids in depression.[36] Al-
σ-1 receptor ligand to treat dementia. According to
lopregnenolone and PB-008, in particular, are of
aforementioned recent preclinical studies, however,
2004 Adis Data Information BV. All rights reserved.
CNS Drugs 2004; 18 (5)
Hayashi & Su
it is highly possible that σ-1 receptor agonists may
Pathological state(e.g. depression, cognition impairment)
augment cognitive functions and improve mood inpatients with psychiatric disorders. Because the ac-tion of σ-1 receptor ligands is mainly modulatory,
σ-1 receptor ligands are expected to restore the
existing (but functionally declining) neural trans-mission.
Because the σ-1 receptor is involved in higher-
ordered brain functions such as memory, cognition
and mood, it may be involved in the development of
neuronal plasticity. The pathophysiology of certain
neuropsychiatric disorders, such as drug dependence
Fig. 2. A hypothetical scheme of the ‘modulatory' action of σ-1
and depression, has been shown to involve neural
receptor ligands. σ-1 receptors may possess innate biological ac-
plasticity that may be related to dentrite outgrowth
tivity. In the normal brain, the activity of σ-1 receptors would bemaintained within a sufficient range (shaded area) for regulating
and synapse formation.[92,133-135] Thus, σ-1 receptor
neurophysiological functions. The factor that maintains the activity
ligands might show therapeutic actions by affecting
of σ-1 receptors is unknown at present. If neuronal activity de-
the remodelling of neural membranes, as we have
creases due to certain pathological states or influences from exoge-
nous compounds, σ-1 receptors would become hypoactive (line 2).
σ-1 receptor agonists at appropriate doses may act as modulators
A unique pharmacological feature of selective
to compensate for the reduced activity of the receptor (line 1). σ-1
σ-1 receptor ligands is their ‘modulatory' action.
antagonists would eliminate the augmentation of the σ-1 receptor
For example, in in vitro studies, σ-1 receptor ligands
activity induced by σ-1 agonists (line 3). At excessive doses of anagonist, the effects of σ-1 receptors could disappear (shifting from
did not affect intracellular Ca2+ levels by them-
line 1 to 2) as has been shown in in vivo and in vitro studies.[39,47,65]
selves, but potentiated IP3-induced Ca2+ mobilisa-tion.[47] In behavioural studies examining mnemonic
tors become hypoactive (or hyperactive) under cer-
processes, σ-1 receptor agonists showed effects only
tain pathological states, σ-1 ligands might compen-
when memory was impaired.[70]
sate for (or normalise) the biological activity of the
We do not know exactly how σ-1 receptor
protein. This hypothesis should be further addressed
ligands exert this modulatory action, but we propose
in the future.
here a hypothetical scheme of this action (figure 2).
When dopamine receptor ligands are administered
Potential therapeutic targets of σ-1 ligands may
to normal animals, agonists per se induce physiolog-
include depression and senile dementia. The action
ical or behavioural alterations by activating the re-
of σ-l ligands in these diseases might be, at least in
ceptors. Conversely, antagonists induce certain ac-
part, related to an effect on neurosteroids via an
tions by displacing endogenous dopamine. These
unknown mechanism. The effects of σ-1 receptor
actions are not seen with σ-1 receptor ligands. Both
ligands in schizophrenia seem to be equivocal, al-
σ-1 receptor agonists and antagonists apparently
though their effects on certain symptoms (e.g. nega-
show no effects when administered to normal ani-
tive symptoms, depressive symptoms and prophy-
mals (see section 2.1). σ-1 receptor proteins per se
laxis) warrant further investigation.
may possess certain unknown biological activity,
Because of their modulatory action, σ-1 ligands
even in the absence of ligands. However, the exis-
may be active only in patients who have neurop-
tence of endogenous σ-1 receptor ligands does not
sychiatric diseases. Therefore, they might be expec-
explain the existing biological activity of σ-1 recep-
ted to show only minimal adverse effects. Also
tors, because σ-1 receptor antagonists lack pharma-cological effects in normal subjects. σ-1 receptor
because of their unique action, it is reasonable to
ligands may merely work as a modulator of the
propose developing compounds that possess a high
existing activity of σ-1 receptors. When σ-1 recep-
affinity for both σ-1 receptors and other receptors
2004 Adis Data Information BV. All rights reserved.
CNS Drugs 2004; 18 (5)
σ-1 Receptors in Neuropsychiatric Disorders
sigma receptor. Biochem Biophys Res Commun 1997; 241:
which are known to be involved in certain patholog-
ical states such as depression.
18. Kekuda R, Prasad PD, Fei YJ, et al. Cloning and functional
expression of the human type 1 sigma receptor (hSigmaR1).
Biochem Biophys Res Commun 1996; 229: 553-8
19. de Costa BR, Bowen WD, Hellewell SB, et al. Synthesis and
evaluation of optically pure [3H]-(+)-pentazocine, a highly
The authors are supported by the Intramural Research
potent and selective radioligand for sigma receptors. FEBS
Program of the National Institute on Drug Abuse, National
Lett 1989; 251: 53-8
20. Narita N, Hashimoto K, Tomitaka S, et al. Interactions of
Institutes of Health, Department of Health and Human Ser-
selective serotonin reuptake inhibitors with subtypes of sigma
vices, USA. The authors have no financial interest that may
receptors in rat brain. Eur J Pharmacol 1996; 307: 117-9
confound the publication of this article.
21. Shirayama Y, Takahashi K, Nishikawa T. Uncompetitive inhibi-
tion of [3H]1,3-di-o-tolyl-guanidine-defined sigma bindingsites by desipramine, propranolol and alprenolol in rat brain.
Eur J Pharmacol 1997; 331: 319-23
1. Martin WR, Eades CG, Thompson JA, et al. The effects of
22. O'Dell LE, George FR, Ritz MC. Antidepressant drugs appear
morphine and nalorphine-like drugs in the non-dependent and
to enhance cocaine-induced toxicity. Exp Clin Psychopharma-
morphine-dependent chronic spinal dog. J Pharmacol Exp
col 2000; 8: 133-41
Ther 1976; 197: 517-32
23. Sanchez C, Meier E. Behavioral profiles of SSRIs in animal
2. Haertzen CA. Subjective effects of narcotic antagonists
models of depression, anxiety and aggression: are they all
cyclazocine and nalorphine on the Addiction Research Center
alike? Psychopharmacology (Berl) 1997; 129: 197-205
Inventory (ARCI). Psychopharmacologia 1970; 18: 366-77
24. Okuyama S, Nakazato A. NE-100: a novel sigma receptor
3. Brady KT, Balster RL, May EL. Stereoisomers of N-al-
antagonist. CNS Drug Rev 1996; 2: 226-37
lylnormetazocine: phencyclidine-like behavioral effects in
25. Takahashi S, Sonehara K, Takagi K, et al. Pharmacological
squirrel monkeys and rats. Science 1982; 215: 178-80
profile of MS-377, a novel antipsychotic agent with selective
4. Lahmeyer HW, Steingold RG. Pentazocine and tripelennamine:
affinity for sigma receptors. Psychopharmacology (Berl) 1999;
a drug abuse epidemic? Int J Addict 1980; 15: 1219-32
5. Blazer DG, Haller L. Pentazocine psychosis: a case of persistent
26. Pande AC, Geneve J, Scherrer B. Igmesine, a novel sigma
delusions. Dis Nerv Syst 1975; 36: 404-5
ligand, has antidepressant properties. Congress of Collegium
6. Yost Jr MA, McKegney FP. Acute organic psychosis due to
Internationale Neuro-Psychopharmacologicum; 1998 Jul 5-10;
Talwin (pentazocine). Conn Med 1970; 34: 259-60
Glasgow, UK. 30S, SM 0505
7. Su TP. Evidence for sigma opioid receptor: binding of
27. Borison RL, Diamond BI, Dren AT. Does sigma receptor ant-
[3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig
agonism predict clinical antipsychotic efficacy? Psycho-
brain. J Pharmacol Exp Ther 1982; 223: 284-90
pharmacol Bull 1991; 27: 103-6
8. Vaupel DB. Naltrexone fails to antagonize the sigma effects of
28. Gewirtz GR, Gorman JM, Volavka J, et al. BMY 14802, a
PCP and SKF 10,047 in the dog. Eur J Pharmacol 1983; 92:
sigma receptor ligand for the treatment of schizophrenia.
Neuropsychopharmacology 1994; 10: 37-40
9. Quirion R, Bowen WD, Itzhak Y, et al. A proposal for the
29. Frieboes RM, Murck H, Wiedemann K, et al. Open clinical trial
classification of sigma binding sites. Trends Pharmacol Sci
on the sigma ligand panamesine in patients with schizophrenia.
Psychopharmacology (Berl) 1997; 132: 82-8
10. Largent BL, Gundlach AL, Snyder SH. Pharmacological and
30. Muller MJ, Grunder G, Wetzel H, et al. Antipsychotic effects
autoradiographic discrimination of sigma and phencyclidine
and tolerability of the sigma ligand EMD 57445 (panamesine)
receptor binding sites in brain with (+)-[3H]SKF 10,047, (+)-
and its metabolites in acute schizophrenia: an open clinical
trial. Psychiatry Res 1999; 89: 275-80
[-(2-thienyl)cyclohexyl]iperidine. J Pharmacol Exp Ther 1986;
31. Modell S, Naber D, Holzbach R. Efficacy and safety of an
opiate sigma-receptor antagonist (SL 82.0715) in schizo-
11. Tam SW. (+)-[3H]SKF 10,047, (+)-[3H]ethylketocyclazocine,
phrenic patients with negative symptoms: an open dose-range
mu, kappa, delta and phencyclidine binding sites in guinea pig
study. Pharmacopsychiatry 1996; 29: 63-6
brain membranes. Eur J Pharmacol 1985; 109: 33-41
32. Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors
12. Gundlach AL, Largent BL, Snyder SH. Autoradiographic local-
suggests a link between endocrine, nervous, and immune sys-
ization of sigma receptor binding sites in guinea pig and rat
tems. Science 1988; 240: 219-21
central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-
33. Baulieu EE. Neurosteroids: a novel function of the brain.
propyl)piperidine. J Neurosci 1986; 6: 1757-70
Psychoneuroendocrinology 1998; 23: 963-87
13. Largent BL, Gundlach AL, Snyder SH. Sigma receptors on
NCB-20 hybrid neurotumor cells labeled with (+)[3H]SKF
34. McCann DJ, Weissman AD, Su TP. Sigma-1 and sigma-2 sites
10,047 and (+)[3H]3-PPP. Eur J Pharmacol 1986; 124: 183-7
in rat brain: comparison of regional, ontogenetic, and subcellu-lar patterns. Synapse 1994; 17: 182-9
14. Bowen WD, Hellewell SB, McGarry KA. Evidence for a multi-
site model of the rat brain sigma receptor. Eur J Pharmacol
35. Stoffel-Wagner B. Neurosteroid metabolism in the human brain.
1989; 163: 309-18
Eur J Endocrinol 2001; 145: 669-79
15. Hanner M, Moebius FF, Flandorfer A, et al. Purification,
36. Van Broekhoven F, Verkes RJ. Neurosteroids in depression: a
molecular cloning, and expression of the mammalian sigma1-
review. Psychopharmacology (Berl) 2003; 165 (2): 97-110
binding site. Proc Natl Acad Sci U S A 1996; 93: 8072-7
37. Uzunova V, Sheline Y, Davis JM, et al. Increase in the
16. Seth P, Fei YJ, Li HW, et al. Cloning and functional characteri-
cerebrospinal fluid content of neurosteroids in patients with
zation of a sigma receptor from rat brain. J Neurochem 1998;
unipolar major depression who are receiving fluoxetine or
fluvoxamine. Proc Natl Acad Sci U S A 1998; 95: 3239-44
17. Seth P, Leibach FH, Ganapathy V. Cloning and structural ana-
38. Bergeron R, de Montigny C, Debonnel G. Potentiation of neu-
lysis of the cDNA and the gene encoding the murine type 1
ronal NMDA response induced by dehydroepiandrosterone
2004 Adis Data Information BV. All rights reserved.
CNS Drugs 2004; 18 (5)
Hayashi & Su
and its suppression by progesterone: effects mediated via
plex in primary cultured rat neuronal cells. Life Sci 2002; 70:
sigma receptors. J Neurosci 1996; 16: 1193-202
39. Maurice T, Su TP, Privat A. Sigma1 (σ1) receptor agonists and
58. Hayashi T, Su TP, Kagaya A, et al. Neuroleptics with differ-
neurosteroids attenuate β25-35-amyloid peptide-induced am-
ential affinities at dopamine D2 receptors and sigma receptors
nesia in mice through a common mechanism. Neuroscience
affect differently the N-methyl-D-aspartate-induced increase
in intracellular calcium concentration: involvement of protein
40. Maurice T, Junien JL, Privat A. Dehydroepiandrosterone sulfate
kinase. Synapse 1999; 31: 20-8
attenuates dizocilpine-induced learning impairment in mice
59. Hayashi T, Kagaya A, Takebayashi M, et al. Modulation by
via sigma 1-receptors. Behav Brain Res 1997; 83: 159-64
sigma ligands of intracellular free Ca++ mobilization by N-methyl-D-aspartate in primary culture of rat frontal cortical
41. Monnet FP, Mahe V, Robel P, et al. Neurosteroids, via sigma
neurons. J Pharmacol Exp Ther 1995; 275: 207-14
receptors, modulate the [3H]norepinephrine release evoked byN-methyl-D-aspartate in the rat hippocampus. Proc Natl Acad
60. Yamamoto H, Yamamoto T, Sagi N, et al. Sigma ligands
Sci U S A 1995; 92: 3774-8
indirectly modulate the NMDA receptor-ion channel complexon intact neuronal cells via sigma 1 site. J Neurosci 1995; 15:
42. Bouchard P, Quirion R. [3H]1,3-di(2-tolyl)guanidine and
[3H](+)pentazocine binding sites in the rat brain: autoradio-
61. Church J, Lodge D, Berry SC. Differential effects of dex-
graphic visualization of the putative sigma1 and sigma2 recep-
trorphan and levorphanol on the excitation of rat spinal neu-
tor subtypes. Neuroscience 1997; 76: 467-77
rons by amino acids. Eur J Pharmacol 1985; 111: 185-90
43. Wolfe Jr SA, Culp SG, De Souza EB. Sigma-receptors in
62. Klette KL, Lin Y, Clapp LE, et al. Neuroprotective sigma
endocrine organs: identification, characterization, and autora-
ligands attenuate NMDA and trans-ACPD-induced calcium
diographic localization in rat pituitary, adrenal, testis, and
signaling in rat primary neurons. Brain Res 1997; 756: 231-40
ovary. Endocrinology 1989; 124: 1160-72
63. Zhang H, Cuevas J. Sigma receptors inhibit high-voltage-acti-
44. Graybiel AM, Besson MJ, Weber E. Neuroleptic-sensitive bind-
vated calcium channels in rat sympathetic and parasympathetic
ing sites in the nigrostriatal system: evidence for differential
neurons. J Neurophysiol 2002; 87: 2867-79
distribution of sigma sites in the substantia nigra, pars com-
64. Takebayashi M, Hayashi T, Su TP. Nerve growth factor-in-
pacta of the cat. J Neurosci 1989; 9: 326-38
duced neurite sprouting in PC12 cells involves sigma-1 recep-
45. McCann DJ, Su TP. Haloperidol-sensitive (+)[3H]SKF-10,047
tors: implications for antidepressants. J Pharmacol Exp Ther
binding sites (sigma sites) exhibit a unique distribution in rat
2002; 303: 1227-37
brain subcellular fractions. Eur J Pharmacol 1990; 188: 211-8
65. Ishihara K, Sasa M. Modulation of neuronal activities in the
46. Inoue A, Sugita S, Shoji H, et al. Repeated haloperidol treat-
central nervous system via sigma receptors. Nihon Shinkei
ment decreases σ1 receptor binding but does not affect its
Seishin Yakurigaku Zasshi 2002; 22: 23-30
mRNA levels in the guinea pig or rat brain. Eur J Pharmacol
66. Monnet FP, Debonnel G, Junien JL, et al. N-methyl-D-as-
2000; 401: 307-16
partate-induced neuronal activation is selectively modulated
47. Hayashi T, Maurice T, Su TP. Ca2+ signaling via σ1-receptors:
by sigma receptors. Eur J Pharmacol 1990; 179: 441-5
novel regulatory mechanism affecting intracellular Ca2+ con-
67. Bermack JE, Debonnel G. Modulation of serotonergic neuro-
centration. J Pharmacol Exp Ther 2000; 293: 788-98
transmission by short- and long-term treatments with sigma
48. Hayashi T, Su TP. Intracellular and body distributions of sigma-
ligands. Br J Pharmacol 2001; 134: 691-9
1 receptors. Intramural Research Program, the National Insti-
68. Minabe Y, Matsuno K, Ashby Jr CR. Acute and chronic admin-
tute on Drug Abuse, NIH. Bethesda, MD, 2002. (Data on file)
istration of the selective sigma1 receptor agonist SA4503
49. Phan VL, Urani A, Romieu P, et al. Strain differences in σ1
significantly alters the activity of midbrain dopamine neurons
receptor-mediated behaviours are related to neurosteroid
in rats: an in vivo electrophysiological study. Synapse 1999;
levels. Eur J Neurosci 2002; 15: 1523-34
50. Tsao LI, Su TP. Naloxone-sensitive, haloperidol-sensitive,
69. Earley B, Burke M, Leonard BE, et al. Evidence for an anti-
[3H](+)SKF-10047-binding protein partially purified from rat
amnesic effect of JO 1784 in the rat: a potent and selective
liver and rat brain membranes: an opioid/sigma receptor?
ligand for the sigma receptor. Brain Res 1991; 546: 282-6
Synapse 1997; 25: 117-24
70. Maurice T, Su TP, Parish DW, et al. PRE-084, a sigma selective
51. Ueda H, Inoue M, Yoshida A, et al. Metabotropic neurosteroid/
PCP derivative, attenuates MK-801-induced impairment of
sigma-receptor involved in stimulation of nociceptor endings
learning in mice. Pharmacol Biochem Behav 1994; 49: 859-69
of mice. J Pharmacol Exp Ther 2001; 298: 703-10
71. Maurice T, Su TP, Parish DW, et al. Prevention of nimodipine-
52. Couture S, Debonnel G. Some of the effects of the selective
induced impairment of learning by the selective sigma ligand
sigma ligand (+)pentazocine are mediated via a naloxone-
PRE-084. J Neural Transm Gen Sect 1995; 102: 1-18
sensitive receptor. Synapse 2001; 39: 323-31
72. Maurice T, Roman FJ, Su TP, et al. Beneficial effects of sigma
53. Hayashi T, Su TP. Regulating ankyrin dynamics: roles of sigma-
agonists on the age-related learning impairment in the senes-
1 receptors. Proc Natl Acad Sci U S A 2001; 98: 491-6
cence-accelerated mouse (SAM). Brain Res 1996; 733: 219-30
54. Hayashi T, Su TP. Sigma-1 receptors (sigma(1) binding site)
73. Reddy DS, Kaur G, Kulkarni SK. Sigma (σ1) receptor mediated
form raft-like microdomains and target lipid droplets on the
anti-depressant-like effects of neurosteroids in the Porsolt
endoplasmic reticulum: roles in endoplasmic reticulum lipid
forced swim test. Neuroreport 1998; 9: 3069-73
compartmentalization and export. J Pharmacol Exp Ther 2003;
74. Urani A, Roman FJ, Phan VL, et al. The antidepressant-like
effect induced by σ1-receptor agonists and neuroactive ster-
55. Hayashi T, Su TP. Intracellular dynamics of sigma-1 receptors
oids in mice submitted to the forced swimming test. J
(sigma(1) binding sites) in NG108-15 cells. J Pharmaco Exp
Pharmacol Exp Ther 2001; 298: 1269-79
Ther 2003; 206: 726-33
75. Panocka I, Perfumi M, Angeletti S, et al. Effects of Hypericum
56. Aydar E, Palmer CP, Klyachko VA, et al. The sigma receptor as
perforatum extract on ethanol intake, and on behavioral de-
a ligand-regulated auxiliary potassium channel subunit.
spair: a search for the neurochemical systems involved.
Neuron 2002; 34: 399-410
Pharmacol Biochem Behav 2000; 66: 105-11
57. Karasawa J, Yamamoto H, Yamamoto T, et al. MS-377, a
76. Tottori K, Miwa T, Uwahodo Y, et al. Antidepressant-like
selective sigma receptor ligand, indirectly blocks the action of
responses to the combined sigma and 5-HT1A receptor agonist
PCP in the N-methyl-D-aspartate receptor ion-channel com-
OPC-14523. Neuropharmacology 2001; 41: 976-88
2004 Adis Data Information BV. All rights reserved.
CNS Drugs 2004; 18 (5)
σ-1 Receptors in Neuropsychiatric Disorders
77. Yamada S, Uwahodo Y, Tottori K, et al. Role for sigma and 5-
96. Kobayashi T, Matsuno K, Nakata K, et al. Enhancement of
HT1A receptors in the forced swimming test supports the
acetylcholine release by SA4503, a novel sigma 1 receptor
mechanism of action of OPC-14523 [abstract]. Abs Soc
agonist, in the rat brain. J Pharmacol Exp Ther 1996; 279: 106-
Neurosci 2000; 26: 2326
78. Kamei H, Noda Y, Kameyama T, et al. Role of (+)-SKF-
97. Su TP, Hayashi T. Understanding the molecular mechanism of
10,047-sensitive sub-population of sigma 1 receptors in ame-
sigma-1 receptors: toward a hypothesis that sigma-1 receptors
lioration of conditioned fear stress in rats: association with
are intracellular amplifiers for signal transduction. Curr Opin
mesolimbic dopaminergic systems. Eur J Pharmacol 1997;
Med Chem 2003; 10: 2073-80
98. Wheal HV, Chen Y, Mitchell J, et al. Molecular mechanisms
79. Gue M, Yoneda M, Monnikes H, et al. Central neuropeptide Y
that underlie structural and functional changes at the post-
and the sigma ligand, JO 1784, reverse corticotropin-releasing
synapic membrane during synaptic plasticity. Prog Neurobiol
factor-induced inhibition of gastric acid secretion in rats. Br J
Pharmacol 1992; 107: 642-7
99. Su TP, Hayashi T. Cocaine affects the dynamics of cytoskeletal
80. Gue M, Junien JL, Del Rio C, et al. Neuropeptide Y and sigma
proteins via σ1 receptors. Trends Pharmacol Sci 2001; 22:
ligand (JO 1784) suppress stress-induced colonic motor distur-
bances in rats through sigma and cholecystokinin receptors. J
100. Brown TH, Chapman PF, Kairiss EW, et al. Long-term synaptic
Pharmacol Exp Ther 1992; 261: 850-5
potentiation. Science 1988; 242: 724-8
81. Takahashi S, Miwa T, Horikomi K. Involvement of sigma 1
101. Deutsch JA. The cholinergic synapse and the site of memory.
receptors in methamphetamine-induced behavioral sensitiza-
Science 1971; 174: 788-94
tion in rats. Neurosci Lett 2000; 289: 21-4
102. Matsuno K, Senda T, Matsunaga K, et al. Ameliorating effects
82. Ujike H, Kuroda S, Otsuki S. Sigma receptor antagonists block
of sigma receptor ligands on the impairment of passive avoid-
the development of sensitization to cocaine. Eur J Pharmacol
ance tasks in mice: involvement in the central
acetylcholinergic system. Eur J Pharmacol 1994; 261: 43-51
83. Ujike H, Okumura K, Zushi Y, et al. Persistent supersensitivity
103. Maurice T, Lockhart BP. Neuroprotective and anti-amnesic
of sigma receptors develops during repeated methamphetam-
potentials of sigma (σ) receptor ligands. Prog Neuropsycho-
ine treatment. Eur J Pharmacol 1992; 211: 323-8
pharmacol Biol Psychiatry 1997; 21: 69-102
84. Romieu P, Phan VL, Martin-Fardon R, et al. Involvement of the
104. Urani A, Romieu P, Portales-Casamar E, et al. The antidepres-
σ1 receptor in cocaine-induced conditioned place preference:
sant-like effect induced by the sigma(1) (sigma(1)) receptor
possible dependence on dopamine uptake blockade. Neuropsy-
agonist igmesine involves modulation of intracellular calcium
chopharmacology 2002; 26: 444-55
mobilization. Psychopharmacology (Berl) 2002; 163: 26-35
85. Matsumoto RR, Pouw B. Correlation between neuroleptic bind-
105. Baulieu EE, Robel P, Schumacher M. Neurosteroids: beginning
ing to sigma-1 and sigma-2 receptors and acute dystonic
of the story. Int Rev Neurobiol 2001; 46: 1-32
reactions. Eur J Pharmacol 2000; 401: 155-60
106. Schumacher M, Weill-Engerer S, Liere P, et al. Steroid hor-
86. McCracken KA, Bowen WD, de Costa BR, et al. Two novel
mones and neurosteroids in normal and pathological aging of
sigma receptor ligands, BD1047 and LR172, attenuate co-
the nervous system. Prog Neurobiol 2003; 71: 3-29
caine-induced toxicity and locomotor activity. Eur J
107. Jansen KL, Faull RL, Storey P, et al. Loss of sigma binding
Pharmacol 1999; 370: 225-32
sites in the CA1 area of the anterior hippocampus in
87. Matsumoto RR, McCracken KA, Pouw B, et al. Involvement of
Alzheimer's disease correlates with CA1 pyramidal cell loss.
sigma receptors in the behavioral effects of cocaine: evidence
Brain Res 1993; 623: 299-302
from novel ligands and antisense oligodeoxynucleotides.
108. Jacobs DM, Tang MX, Stern Y, et al. Cognitive function in
Neuropharmacology 2002; 42: 1043-55
nondemeted older women who took estrogen after menopause.
88. Matsumoto RR, McCracken KA, Pouw B, et al. N-alkyl substi-
Neurology 1998; 50: 368-73
tuted analogs of the sigma receptor ligand BD1008 and tradi-
109. Phillips SM, Sherwin BB. Effects of estrogen on memory func-
tional sigma receptor ligands affect cocaine-induced convul-
tion in surgically menopausal women. Psychoneuroendocrin-
sions and lethality in mice. Eur J Pharmacol 2001; 411: 261-73
ology 1992; 17: 485-95
89. Chien CC, Pasternak GW. Selective antagonism of opioid anal-
110. Morales AJ, Nolan JJ, Nelson JC, et al. Effects of replacement
gesia by a sigma system. J Pharmacol Exp Ther 1994; 271:
dose of dehydroepiandrosterone. J Clin Endocrinol Metab
90. King M, Pan YX, Mei J, et al. Enhanced kappa-opioid receptor-
111. Maurice T, Urani A, Phan VL, et al. The interaction between
mediated analgesia by antisense targeting the sigma1 receptor.
neuroactive steroids and the sigma1 receptor function: behav-
Eur J Pharmacol 1997; 331: R5-6
ioral consequences and therapeutic opportunities. Brain Res
91. Mei J, Pasternak GW. Sigma1 receptor modulation of opioid
Brain Res Rev 2001; 37: 116-32
analgesia in the mouse. J Pharmacol Exp Ther 2002; 300:
112. Raffa RB. Screen of receptor and uptake-site activity of hyper-
icin component of St John's wort reveals sigma receptor
92. Nestler EJ, Barrot M, DiLeone R, et al. Neurobiology of
binding. Life Sci 1998; 62: PL265-70
depression. Neuron 2002; 34: 13-25
113. Bennett Jr DA, Phun L, Polk JF, et al. Neuropharmacology of St
93. Takahashi S, Horikomi K, Kato T. MS-377, a novel selective σ1
John's Wort (hypericum). Ann Pharmacother 1998; 32: 1201-8
receptor ligand, reverses phencyclidine-induced release ofdopamine and serotonin in rat brain. Eur J Pharmacol 2001;
114. Tamminga CA. Schizophrenia and glutamatergic transmission.
Crit Rev Neurobiol 1998; 12: 21-36
94. Ault DT, Werling LL. Phencyclidine and dizocilpine modulate
115. Matthews RT, McMillen BA, Sallis R, et al. Effects of BMY
dopamine release from rat nucleus accumbens via sigma recep-
14802, a potential antipsychotic drug, on rat brain dopaminer-
tors. Eur J Pharmacol 1999; 386: 145-53
gic function. J Pharmacol Exp Ther 1986; 239: 124-31
95. Nuwayhid SJ, Werling LL. Sigma1 receptor agonist-mediated
116. Jentsch JD, Roth RH. The neuropsychopharmacology of
regulation of N-methyl-D-aspartate-stimulated [3H]dopamine
phencyclidine: from NMDA receptor hypofunction to the dop-
release is dependent upon protein kinase C. J Pharmacol Exp
amine hypothesis of schizophrenia. Neuropsychopharmacol-
Ther 2003; 304: 364-9
ogy 1999; 20: 201-25
2004 Adis Data Information BV. All rights reserved.
CNS Drugs 2004; 18 (5)
Hayashi & Su
117. Walker JM, Matsumoto RR, Bowen WD, et al. Evidence for a
129. Strous RD, Spivak B, Yoran-Hegesh R, et al. Analysis of
role of haloperidol-sensitive sigma-‘opiate' receptors in the
neurosteroid levels in attention deficit hyperactivity disorder.
motor effects of antipsychotic drugs. Neurology 1988; 38:
Int J Neuropsychopharmacol 2001; 4: 259-64
130. Osran H, Reist C, Chen CC, et al. Adrenal androgens and
118. Sharkey J, Glen KA, Wolfe S, et al. Cocaine binding at sigma
receptors. Eur J Pharmacol 1988; 149: 171-4
cortisol in major depression. Am J Psychiatry 1993; 150: 806-
119. Shibuya H, Mori H, Toru M. Sigma receptors in schizophrenic
cerebral cortices. Neurochem Res 1992; 17: 983-90
131. Browne ES, Wright BE, Porter JR, et al. Dehydroepiandroster-
one: antiglucocorticoid action in mice. Am J Med Sci 1992;
120. Helmeste DM, Tang SW, Bunney Jr WE, et al. Decrease in
sigma but no increase in striatal dopamine D4 sites in schizo-
phrenic brains. Eur J Pharmacol 1996; 314: R3-5
132. Wolkowitz OM, Reus VI, Roberts E, et al. Dehydroepiandros-
121. Ohmori O, Shinkai T, Suzuki T, et al. Polymorphisms of the σ1
terone (DHEA) treatment of depression. Biol Psychiatry 1997;
receptor gene in schizophrenia: an association study. Am J
Med Genet 2000; 96: 118-22
133. Kolb B, Gorny G, Li Y, et al. Amphetamine or cocaine limits
122. Ishiguro H, Ohtsuki T, Toru M, et al. Association between
polymorphisms in the type 1 sigma receptor gene and schizo-
the ability of later experience to promote structural plasticity in
phrenia. Neurosci Lett 1998; 257: 45-8
the neocortex and nucleus accumbens. Proc Natl Acad Sci U S
123. Schwarcz G, Halaris A, Dren A, et al. Open label evaluation of
A 2003; 100: 10523-8
the novel antipsychotic compound BW234U in chronic
134. Robinson TE, Gorny G, Mitton E, et al. Cocaine self-adminis-
schizophrenics. Drug Dev Res 1985; 5: 387-93
tration alters the morphology of dentrites and dentric spines in
124. Guy W, Manov G, Wilson WH, et al. Psychotropic actions of
the nucleus accumbens and neocortex. Synapse 2001; 39: 257-
BW234U in the treatment of inpatient schizophrenics: a dose
range study. Drug Dev Res 1983, 52
135. Manji HK, Drevets WC, Charney DS. The cellular neurobiology
125. Garreau M, Giroux C, L'Heritier C, et al. Pilot studies on the
effect of SL 82.0715 in psychotic syndromes. Clin Neuro-
of depression. Nat Med 2001; 7: 541-7
pharmacol 1992; 15 Suppl. 1: 699A-700A
126. Grunder G, Muller MJ, Andreas J, et al. Occupancy of striatal
D2-like dopamine receptors after treatment with the sigma
Correspondence and offprints: Dr Teruo Hayashi, Cellular
ligand EMD 57445, a putative atypical antipsychotic. Psycho-
Pathobiology Unit, Cellular Neurobiology Research
pharmacology (Berl) 1999; 146: 81-6
Branch, Intramural Research Program, Department of
127. Akiyama K, Kanzaki A, Tsuchida K, et al. Methamphetamine-
induced behavioral sensitization and its implications for re-
Health and Human Services, National Institute on Drug
lapse of schizophrenia. Schizophr Res 1994; 12: 251-7
Abuse, National Institutes of Health, 5500 Nathan Shock
128. Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone
Drive, Baltimore, MD 21209, USA.
levels and reactivity to mental stress in premenstrual dysphoricdisorder. Biol Psychiatry 2001; 49: 788-97
2004 Adis Data Information BV. All rights reserved.
CNS Drugs 2004; 18 (5)
Source: http://homepages.ihug.co.nz/~Smconnell/sigma%20clinical%20and%20preclinical.pdf
VIDEOKIT Serie CVR4KV Videokit anti-vandalo Mono-familiari e Bi-familiariOne Way, Two Way Vandal Resistant Videokit di far installare il presente disposi- tivo esclusivamente da personale Videx Electronics S.p.A. Via del Lavoro, 1 63846 Monte Giberto (FM) - Italy This equipment is installed by a Phone +39 0734 631669 - Fax +39 0734 632475
Volume 10 • Number 5 • 2007 Principles of Good Practice for Budget Impact Analysis:Report of the ISPOR Task Force on Good Research Practices—Budget Impact Analysis Josephine A. Mauskopf, PhD,1 Sean D. Sullivan, PhD,2 Lieven Annemans, PhD, MSc,3 Jaime Caro, MD,4C. Daniel Mullins, PhD,5 Mark Nuijten, PhD, MBA, MD,6 Ewa Orlewska, MD, PhD,7 John Watkins, RPh, MPH,8Paul Trueman, MA, BA9



