Kamagra gibt es auch als Kautabletten, die sich schneller auflösen als normale Pillen. Manche Patienten empfinden das als angenehmer. Wer sich informieren will, findet Hinweise unter kamagra kautabletten.
New perspectives in melatonin uses


Contents lists available at
Pharmacological Research
New perspectives in melatonin uses
A. Carpentieri , G. Díaz de Barboza , V. Areco , M. Peralta López , N. Tolosa de Talamoni
a Laboratorio "Dr. Fernando Ca˜
nas", Cátedra de Bioquímica y Biología Molecular, Facultad de Ciencias Médicas, Universidad Nacional de Córdoba, Córdoba, Argentina
b Cátedra de Química Biológica, Facultad de Odontología, Universidad Nacional de Córdoba, Ciudad Universitaria, Córdoba, Argentina
This review summarizes the metabolism, secretion, regulation and sites of action of melatonin. An
Received 6 December 2011
updated description of the melatonin receptors, including their signal transduction mechanisms, dis-
Accepted 10 January 2012
tribution and characterization of receptor genes, is given. Special emphasis is focused on the clinical
aspects and potential uses of melatonin in the sleep-wake rhythms, in the immune function, in can-
cer therapy, in neuroprotection against oxidative damage and antioxidant activities in different tissues.
Melatonin receptors
Finally, combined effects of melatonin with other drugs are discussed.
Melatonin metabolism
2012 Elsevier Ltd. All rights reserved.
Antioxidant properties
Central nervous system
Melatonin (MEL) (N-acetyl-5-methoxytryptamine) was discov-
Abbreviations: AANAT, N-acetyltransferase; AD, Alzheimer disease; AFMK, N1-
ered about fifty years ago and is a compound synthesized by the
pineal gland in the human brain. It is also produced in retina,
CAT, catalase; CNS, central nervous system; ETC, electron transport chain;
thymus, bone marrow, respiratory epithelium, skin, lens, intes-
GI, gastrointestinal; GPCR, 7-transmembrane G-protein-coupled receptor; GPx,
tine and in other sites Pineal MEL passes freely through
glutathione peroxidase; GR, GSH reductase; GSH, glutathione; GSSG, oxidized
membranes and distributes in all body compartments, whereas
GSH; HIOMT, hydroxyindole-O-methyltransferase; IFN-␣, interferon alpha; IFN-
␥, interferon gamma; IL, interleukin; iNOS, inducible nitric oxide synthase; LOX,
retinal MEL apparently acts locally within the eyes partici-
lipoxygenase; MDM2, murine double minute-2; MEL, melatonin; MEN, menadione;
pates in the regulation of important physiological and pathological
mtDNA, mitochondrial DNA; NAS, N-acetylserotonin; NAT, N-acetyltransferase; NO,
processes. It is considered a hormone that regulates the circa-
nitric oxide; NOS, nitric oxide synthase; PD, Parkinson disease; PLA2, phosholipase
dian day–night rhythm and seasonal biorhythm by the classical
A2; QR2, quinone reductase 2; RNS, reactive nitrogen species; ROS, reactive oxy-
gen species; SCN, suprachiasmatic nucleus; SOD, superoxide dismutase; Th1, Type
chronobiology. MEL has been characterized as an effective synchro-
1 helper T lymphocytes; Th2, Type 2 helper T lymphocytes; TNF␣, tumor necrosis
nizing agent in several physiological and pathological conditions,
factor alpha.
such as in maternal-fetus entrainment in dissociated circa-
∗ Corresponding author at: Cátedra de Bioquímica y Biología Molecular, Facultad
dian rhythms induced by a short light–dark cycle addition,
de Ciencias Médicas, Universidad Nacional de Córdoba, Pabellón Argentina, 2do.
modulation of immune defense responses, body weight and repro-
Piso, Ciudad Universitaria, 5000 Córdoba, Argentina. Tel.: +54 351 4333024;
fax: +54 351 4333072.
duction, tumor growth inhibition and anti-jetlag effects have been
E-mail address: (N. Tolosa de Talamoni).
recognized is also evidence that MEL could act as a potent
1043-6618/$ – see front matter
2012 Elsevier Ltd. All rights reserved.
A. Carpentieri et al. / Pharmacological Research 65 (2012) 437–444
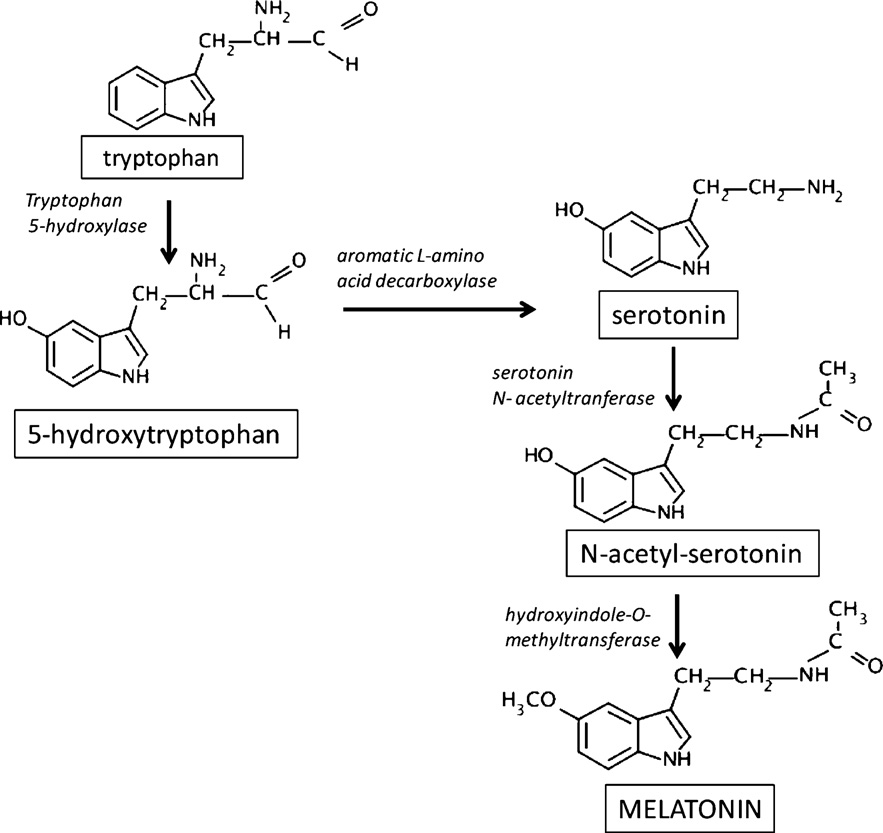
Fig. 1. Metabolic pathway of melatonin synthesis.
direct antioxidant, as a chemotoxicity reducing agent and a putative
cerebrospinal fluid and reaches other body fluids such as bile, cere-
anti-aging substance
brospinal fluid, saliva, semen, ovarian follicular fluid and amniotic
fluid. Small amounts of unmetabolized MEL are excreted in the
1. Structure, metabolism and secretion
The half life of MEL in the serum has been calculated to be
MEL is an indoleamine containing two functional groups, which
in the range 30–50 min The ability to synthesize MEL is
are decisive not only for the receptor binding but also for its
rather constant in a given person, but there is marked variability
amphiphilicity giving to the molecule the capacity to enter any cell
among individuals. There is evidence that MEL levels decrease with
compartment or body fluid. Due to its extensive solubility in lipids,
increasing age in mammals, including humans. The serum level of
MEL easily passes by diffusion from the peripheral circulation to
MEL is very low in the first weeks of postnatal life, without diur-
other fluids or cells. In serum, 70% of the MEL is bound to albumins
nal variation. At six months of life the typical diurnal rhythm of
and the remaining 30% diffuses to the surrounding tissues
secretion appears, reaching the maximum levels between the third
Tryptophan and serotonin are precursors of MEL. Two well char-
and sixth years of life. A marked decrease in MEL secretion has
acterized enzymes participate in its synthesis: N-acetyltransferase
been noted during sexual maturation. At 40–50 years, a noticeable
(AANAT), which converts serotonin to N-acetylserotonin (NAS), and
decrease in daily MEL synthesis has been observed and after the 70
hydroxyindole-O-methyltransferase (HIOMT), which converts NAS
years of age the diurnal rhythm of secretion practically disappears
to MEL (The regulation of MEL synthesis is controlled by
in most individuals seasonal variation in the synthesis of
the light–dark cycle, acting through neural activation of the ante-
MEL in humans seems to exist, the levels being higher in winter
rior hypothalamus via the axons of retinal ganglion cells running
in the optic nerves and forming the retino-hypothalamic tract.
The catabolism of MEL was thought to be almost exclusively
Suprachiasmatic nucleus (SCN) is connected with the pineal gland
done by the hepatic P450 monooxygenases, followed by conju-
through paraventricular nuclei and preganglionic sympathetic neu-
gation of the resulting 6-hydroxy-melatonin to give the main
rons. Norepinephrine released from postganglionic sympathetic
urinary metabolite 6-sulfatoxy-melatonin. This might occur with
fibers at pinealocyte membrane stimulates its adrenoceptors lead-
the circulating hormone. In contrast, in the central nervous sys-
ing to cAMP formation as well as other second messengers, which
tem (CNS) the oxidative pyrrole-ring cleavage predominates and
stimulate the expression and activity of AANAT, the first-rate limit-
no 6-hydroxy-melatonin was detected after MEL injection into the
ing enzyme in MEL production non-mammalian vertebrates,
cisterna magna, which may be important because much more MEL
this enzyme appears to be directly controlled by circadian clock
is released via the pineal recess into the cerebrospinal fluid than
genes in the pineal gland
into the circulation. The primary cleavage product is N1-acetyl-N2-
The secretion of MEL has a typical diurnal rhythm. At night
formyl-5-methoxykynuramine (AFMK). Several different reactions
the synthesis and secretion of MEL are stimulated, reaching a
lead to the same product, AFMK, and this pathway contributes to
peak value (80–150 pg/mL) between midnight and 3 a.m., while its
about one third of the total catabolism. AFMK is converted into
concentration during the day is low (10–20 pg/mL) syn-
N1-acetyl-5-methoxykynuramine (AMK). AFMK and AMK form
thesized in the pineal gland, MEL is secreted into the blood and
metabolites by interaction with reactive oxygen and nitrogen
A. Carpentieri et al. / Pharmacological Research 65 (2012) 437–444
species. Some other metabolites have been detected, but appar-
4. MEL and mitochondria
ently in minor quantities
The lipophilic nature of MEL favors that the indolamine crosses
cell membranes to easily reach subcellular compartments includ-
2. MEL receptors
ing mitochondria. MEL interacts with lipid bilayers and stabilizes
mitochondrial inner membranes, which may improve the elec-
Most actions of MEL are mediated by membrane receptors and
tron transport chain (ETC) activity. At a concentration of 1 nM,
nuclear sites corresponding to orphan members of the nuclear
MEL increases the activity of the complexes I and IV in rat liver
receptor superfamily RZR/ROR. Three subtypes of mammalian MEL
mitochondria, while 10–100 nM MEL stimulates the activity of
receptors have been proposed and cloned. Two of these, MT1 and
those complexes in brain mitochondria. When a dose of cyanide
MT2, are members of the 7-transmembrane G-protein-coupled
decreases the complex IV activity by 50%, MEL counteracts this inhi-
receptor (GPCR) family. These two receptors are classified as unique
bition. In contrast, when the complex IV is totally inactivated by
subtypes based on their molecular structure and chromosomal
cyanide, MEL is unable to counteract this inhibition, independently
localization. Both of them belong to the class A group of rhodopsin-
of its concentration. The previous data suggest that the regulation
like GPCRs. They are formed by 350 and 362 aminoacids and their
by MEL on the activities of complexes I and IV does not only rely
calculated molecular weights are 39 and 40 kDa, respectively. The
on its antioxidant properties. MEL has a high reduction potential,
gene MTRN1A for the MT1 receptor is located at position 4q35-
which suggests that MEL might interact with the components of
1 and the gene MTNR1B for the MT2 receptor at 11q21-22. They
the electron transport chain increasing the electron flow, and con-
share 60% homology. MT1 has two potential glycosylation sites in
sequently, the ATP production
the N-terminal region and MT2 has one potential glycosylation site
As known, reactive oxygen species (ROS) and reactive nitrogen
in the same region. Both receptors involve signaling through inhi-
species (RNS) are synthesized as subproducts of the mitochon-
bition of cAMP formation and protein kinase A activity, and effects
drial electron transport chain, although the main sources of •NO
on phospholipase A2 and C, calcium and potassium channels
are the reactions catalyzed by nNOS (neuronal nitric oxide syn-
MT3, the third receptor, is an enzyme identified as quinone reduc-
thase) and the iNOS (inducible nitric oxide synthase). Moderate
tase 2 (QR2). Little information exists on nuclear MEL receptors.
levels of •NO are considered favorable for mitochondrial function,
Another MEL-related receptor, named GPR50, has also been found
whereas high •NO concentrations produce severe ETC dysfunction,
in different species including humans
1c, the first type of
blocking the respiration in extreme conditions of inflammation
MEL receptor discovered, is a receptor subtype expressed in non
seems to protect proteins of the ETC and mtDNA from
mammalian species is some evidence that MEL receptor
ROS/RNS-induced oxidative damage. The hormone limits the loss
expression exhibits circadian variation
of intramitochondrial GSH, improves the electron transport chain
activity and reduces mtDNA damage. It apparently increases the
3. MEL and antioxidant properties
expression and activity of complex IV and the activity of complex I,
which improves mitochondrial respiration and increases ATP syn-
There is a large body of evidence that MEL is a major scav-
enger of both oxygen and nitrogen-based reactive molecules. MEL
MEL also regulates the GSH redox status in isolated brain and
provokes this effect at both physiological and pharmacological con-
liver mitochondria when it is disrupted by oxidative stress. The
centrations. Several of its metabolites can also detoxify free radicals
indolamine increases the mitochondrial GSH content, decreases
and derivatives The indolamine eliminates the decomposi-
oxidized GSH (GSSG) and hydroperoxide levels and stimulates the
tion products of peroxynitrites, including free hydroxyl radicals and
activity of GPx and GSH reductase (GR), which are two enzymes
nitrogen dioxide radicals and the carbonate radical in the presence
involved in the GSH-GSSG balance et al.
of physiological CO2 concentration induces the synthesis
demonstrated that micromolecular concentrations of MEL prevent
of another intracellular antioxidant, glutathione (GSH), in rabbits
calcium dependent cardiolipin peroxidation in mitochondria, thus
that exhibit diabetes-induced oxidative stress benefit of
protecting against the induction of mitochondrial permeability
antioxidant properties of MEL has been shown in patients with
transition and cytochrome c release.
rheumatoid arthritis (RA) with infertility in
It is of interest that MEL acts as an antiapoptotic agent in
elderly patients with primary essential hypertension How-
mitochondrial ROS/RNS- mediated cell death, but it also has
ever, there is no agreement about the beneficial effects of MEL on
proapoptotic effects in several tumor cell lines such as in MCF-7
RA. Instead, MEL has been reported to be a RA promoter due to
breast cancer cells suggests that MEL has a potential
its capability to act as an immunoenhancing agent and stimula-
use to kill cancer cells preserving normal cells.
tor of proinflammatory cytokine release With regards to
A direct relationship between the indolamine and aging has
the enzymes of the antioxidant system, MEL regulates the expres-
not been proven but certain properties of the hormone indicate
sion of several genes such as those of superoxide dismutase (SOD)
its potential benefit in the elderly. Data obtained in senescence-
and glutathione peroxidase (GPx). The hormone influences the
accelerated mice have demonstrated that chronic administration
enzyme activity and cellular mRNA levels of these proteins under
of MEL does not reduces respiratory function, decreases the neu-
physiological and oxidant conditions MEL concentration
ral and hepatic peroxidation products and the protein carbonyl
to up-regulate the enzyme gene expression corresponds to the
content in brain
physiological night time peak of plasma MEL, whereas higher con-
centration does not affect the gene expression. The antioxidant
effect of low concentrations of MEL seems to be indirect via up-
5. MEL and immune system
regulation of GPx gene expression and antioxidant activity, while
at high concentration the effect is attributed to direct radical scav-
A link between MEL from the pineal gland and the immune sys-
enging actions. It has been proposed that the ability of MEL to
tem in different species, including humans, has been documented
up-regulate the antioxidant enzyme involves both membrane and
immune system is modulated by different environmental
nuclear receptors appears that the stimulation of GPx by MEL
signals being light one of them. Although most of the light received
is the most consistent effect, while the effect on other enzymes is
by the retina goes to the visual cortex, another pathway from the
tissue-specific or conditional.
retina relays to the SCN, which forms part of the hypothalamic
A. Carpentieri et al. / Pharmacological Research 65 (2012) 437–444
region in the brain hypophysis and the pineal gland are
that MEL could be a mediator of acid-induced secretion
also involved in neuroendocrine changes induced by the light. MEL
of the MEL effects on the GI tract seem to be mediated by MT2
is one of the neuroendocrine hormones that are sensitive to changes
receptors the presence of MT1 receptor has been
in circadian rhythm. Since MEL is produced not only by the pineal
also demonstrated in human gallbladder epithelium from patients
gland, but also in the retina and in other parts of the body, the
with cholelithiasis and gallbladder carcinoma et al.
immune system might be affected by MEL originated in different
shown that MEL inhibits serotonin transporter activity in
organs. Besides, human peripheral blood mononuclear cells syn-
Caco-2 cells, apparently without mediation of MT1 or MT2 recep-
thesize important amounts of MEL, which indicates a potential
tors or PKC and cAMP pathways, but the doses used were high. It
intracrine and paracrine role of MEL in immune regulation. Mem-
is not known if this occurs under physiological conditions. MEL has
brane receptors have been found in T and B lymphocytes. MEL
also antioxidant properties in the GI system; its concentration in
also stimulates cytokine production by human peripheral blood
the human liver is suitable for prevention of oxidative damage. In
mononuclear cells through nuclear receptors Although the
our laboratory, MEL has been found to reverse the inhibition of the
mechanism by which MEL enhances the immune response is not
intestinal calcium absorption caused by menadione, apparently by
clear, it is thought that it may increase phagocytosis and antigen
antioxidant mechanisms
presentation. In addition, MEL induces T cell differentiation toward
MEL receptors have been detected in the pancreas and the
the type 1 helper T cells (Th1) phenotype and the activation of the
stimulation of pancreatic enzyme secretion by the hormone in a
immune system by the indolamine has been shown to be mediated
dose-dependent manner has been demonstrated. MEL was also
by the regulation of gene expression of cytokines in the spleen, thy-
implicated in the pancreatic endocrine function. Pinealectomy pro-
mus, lymph nodes and bone marrow killer cell activity
duces hyperinsulinemia and increases the triglyceride content in
and production are apparently increased by MEL administration
the liver of type 2 diabetic rats Endogenous MEL has been
either in humans or mice. Although many studies have implicated
shown to protect against pancreatitis, probably mediated by MT2
MEL as a positive regulator of immune system, other reports have
also suggested that MEL could act as an anti-inflammatory agent
through inhibition of immune responses. The anti-inflammatory
action is considered to be, at least in part, due to the induction
7. MEL and central nervous system
of type 2 helper T lymphocytes (Th2) producing interleukin (IL)-4
and inhibiting Th1 function has pleiotropic effects on dif-
MEL and melatoninergic drugs have hypnotic effects mediated
ferent steps of inflammation. It has a pro-inflammatory role at an
through MT1 and MT2 receptors, especially those in the SCN, which
early phase through activation of pro-inflammatory mediators such
acts on the hypothalamic sleep switch. They favor sleep initiation
as phosholipase A2 (PLA2), lipoxygenase (LOX) and cytokines such
and reset the circadian clock allowing persistent sleep, a require-
as IL-1 and tumor necrosis factor alpha (TNF␣). Contrarily, when
ment in circadian rhythm sleep alterations. The action of MEL on
the inflammatory process proceeds toward the chronic phase, MEL
sleep is mainly of a chronobiological nature. The hormone acts in
has a negative effect. The antagonist role of MEL on chronic inflam-
a dual way, resetting the clock via MT2 receptor and suppressing
mation is due to downregulation of mediators such as PLA2, LOX
neuronal firing via MT1 receptor. Apparently, the soporific action
and cytokines, induction of a survival pathway in leukocytes and
of MEL also involves the thalamus. MEL receptors have been found
blocking of oxidation by its antioxidant properties
in this area and the formation of the spindles is stimulated by
MEL could also play a role in the influence of seasonal changes on
the hormone. Other areas of the brain seem to be involved in
the immune function. In healthy volunteers during autumn/winter
the transmission of MEL-dependent responses. It is uncertain to
season, the production of IL-6, interferon alpha (IFN-␣) and inter-
what extent the thalamus and other brain areas participate in the
feron gamma (IFN-␥) was higher. The seasonal changes in immune
sleep promotion as compared to the hypothalamic via MEL
functions appear to be mediated by alterations in duration of MEL
treatment to patients with severe CNS dysfunction and MEL defi-
secretion. In this sense, changes in mood and behavior such as in
ciency is not sufficient to mitigate sleep difficulties. In addition,
the seasonal affective disorder have been associated with seasonal
CNS destruction causing sleep fragmentation and loss of circadian
changes in cytokines like IL-6, IFN-␣ or the balance between Th1
rhythmicity, allows spindle formation. MEL has a different mode
and Th2 responses
of action as compared to other hypnotics such as benzodiazepins
and z-drugs, which produce a more generalized CNS depression
through GABA receptors. Only very high pharmacological doses
6. MEL and the gastrointestinal (GI) tract
of MEL can produce generalized sedative effects or even narcotic
effects but mediated by other mechanisms, such as antiexcita-
MEL is also produced in high amount in the enteroendocrine
tory suppression of calcium signaling and inhibition of neuronal
cells of GI mucosa. The amount of MEL in the gut is about 400 times
NO synthase. The major obstacle for the use of MEL in primary
larger than the content of MEL in the pineal gland pro-
chronic insomnia is that the half life of circulation is extremely
duction in the GI tract does not follow a circadian rhythm as occurs
short, 20–30 min and even less (eventually it could last up to a max-
in the pineal gland, but it responds to the periodicity of food intake
imum of 45 min). MEL promotes sleep initiation but improves sleep
rich in tryptophan a pinealectomy, serum levels of
maintenance only marginally. Part of this problem has been solved,
MEL do not maintain a rhythm according to the light–dark cycle,
at least in part, by drugs that prolong the release of MEL and the
but values are not too low at daytime because of the contribution
melatoninergic agonists with longer half-life
of MEL by the extra-pineal sources, mainly the GI tract
Neurodegenerative diseases such as Alzheimer disease (AD) and
The main enzymes of MEL synthesis, AANAT and HIOMT, have
Parkinson disease (PD) are age-related disorders that share mito-
also been detected in GI mucosa, supporting the hypothesis that in
chondrial dysfunction, oxidative/nitrosative stress and apoptosis
the gut MEL is synthesized from l-tryptophan present in food
in different areas of the brain. Patients with AD have a reduc-
The function of gut MEL is not clear yet because the intestine is
tion in MEL levels both in blood and cerebrospinal fluid, which
not only source but also sink of MEL, brought by the circulation
is even present in preclinical stages MEL's cognitive bene-
Decreases in motility and increases in mucosal blood flow
fits have been demonstrated either in AD patients or in AD mice.
have been observed It has been also demonstrated that
Low levels of mRNA of SOD-1, GPx and catalase were found in hip-
luminal MEL stimulates duodenal HCO −
secretion, which suggests
pocampus from MEL treated AD mice In PD model animals,
A. Carpentieri et al. / Pharmacological Research 65 (2012) 437–444
MEL normalizes complex I activity from electron transport chain
various organs such as heart, kidney and bone marrow in the course
and oxidative status in mitochondria from substantia nigra and
of chemotherapy and radiotherapy. This protective effect of MEL
striatum and reduces mitochondrial inducible nitric oxide synthase
administration might allow treating patients more effectively using
decreasing nitric oxide radicals and preventing the inhibition of
higher doses of cytostatic drugs and radiation, which by themselves
complex IV by this radical treatment improved the quality
could damage the organs involved it has been shown
of sleep in patients with Parkinson idea that MEL is favor-
that MEL synergistically with vitamin D down-regulates Akt and
able in PD patients or PD animal models is controversial. Willis et al.
MDM2 leading to growth inhibition of breast cancer cells
that the light exposure at the bed time reduces the
In studies in which gastric damage was induced by
severity of PD symptoms, whereas the MEL intracerebroventricu-
indomethacin acetylsalicylic acid MEL prevented
lar implants increased its severity. Further studies about the use of
or reduced the gastric mucosal lesion. In addition, the combination
MEL in the PD treatment are needed.
of MEL with anti-ulcer drugs such as ranitidine and omeprazole
reduces the doses and minimizes the side effects
The pathophysiology of primary headache has been partially
8. MEL and cancer
attributed to desynchrony and dysfunction of the whole or part of
the retino-hypothalamic-pineal axis synchronizing ability
There is abundant evidence indicating that MEL is involved
of MEL together with its antioxidant and anti-inflammatory prop-
in preventing tumor initiation, promotion, and progression. The
erties, make this indolamine effective to decrease the frequency
increased incidence of breast, endometrial and colorectal cancer
or totally suppress headache of different kinds (migraine, cluster
seen in nurses and in other night shift workers suggests a possible
headache, etc.) allowing the patient to reduce the consumption of
link between diminished secretion of MEL and increased exposure
to light during nightime This evidence and the antioxidant
The use of MEL alone or in combination with other effective
and anti or proapoptotic action of MEL and its relationship with
agents has been reported to diminish collateral damage in sev-
the endocrine and immune systems led to examine the effect of
eral psychiatric disorders. Anxiolytic, sedative, anticonvulsant and
the hormone on tumor cells. Recent human and animal studies
anti-hypertensive effects of MEL may be helpful in the treatment
have shown that MEL also has important oncostatic properties by
of depression with inhibitors of monoaminooxidase. A combina-
altering the expression of anti-cancer cytokines IL-2 and IL-12 in
tion of MEL with lithium or valproate was more effective than
human neoplasms of 1 nM MEL to MCF-7 cell culture
either lithium or valproate alone in bipolar syndrome patients. MEL
inhibits proliferation the expression of proapoptotic
has also protective properties against haloperidol adverse effect in
proteins such as p53 and p21 reduces their metastatic
schizophrenia patients should be noted that many of the
potential due to increased expression of E-cadherin and 
beneficial effects of MEL are associated with the improvement of
proteins antiproliferative effects on human breast cancer
sleep disorders that are very frequent in several neuropsychiatric
cells seem to be mediated by MT1 receptor, which suggests that
MEL or its analogs could be used as potential antitumoral agents
The administration of MEL alone is successful in the treatment
of circadian system disorders such as jet lag, shift work, some
The finding of MEL binding sites in human colon tissue suggested
sleeps disturbances, etc. In these cases MEL acts as a synchronizer,
a possible role of MEL in colorectal cancer. Schernhammer et al.
entraining the human activity circadian rhythms according to a
have found increased risk of colorectal cancer in nurses subjected to
phase-response curve
shift work, which was attributed to the suppression of MEL produc-
As shown in MEL is a pleiotropic molecule and its
tion by nocturnal lighting. Farriol et al. demonstrated that
administration to humans and animals at both physiological and
MEL was able to inhibit cell growth in CT-26 cells, a cell line derived
pharmacological concentrations seems to be non-toxic. Since MEL
from a murine colon carcinoma. Although MEL had no effect on cell
has a wide therapeutic range, it can be administered in differ-
growth at low doses, a significant and progressive suppression of
ent doses according to the expected effects. The ability of MEL to
DNA synthesis was found with high doses of the indolamine. The
scavenge ROS is one of the explanations for the reduction of side
authors concluded that MEL exerted its anti-proliferative action
effects produced by some pharmacological agents such as onco-
through a non-hormone dependent mechanism, because no recep-
static, antinflammatory, antiepilepsic drugs these cases, the
tors were involved in the cell line selected. The oncostatic effect
doses of MEL range from 10 to 50 mg, much higher than physiolog-
of MEL on colon cancer was demonstrated to be mediated through
ical level, in order to counteract the high amounts of free radicals
MT2 receptors and through its binding to nuclear RZR/ROR ␣ recep-
Finally, an illustrative list of some recent in vivo
and in vitro studies exemplifying the effects of the combination
9. Combined effects of MEL with other drugs
of MEL with other drugs. As demonstrated, MEL can be useful in
the treatment of a wide variety of diseases improving their effi-
The effect of MEL in the presence of other drugs has been
cacy while reducing the side effects in order to enhance life quality.
studied in different tissues. Several findings support the hypoth-
This broad therapeutic spectrum makes MEL a potential therapeu-
esis that MEL enhances the effect of chemotherapy on colorectal
tic tool in combination with other traditional therapies and opens
carcinoma. Cerea et al. the effect of the simultane-
a promising field of research in the pharmacological area.
ous administration of MEL and the cytotoxic drug CPT-11 in 30
patients with metastatic colorectal carcinoma. It was found that
co-administration of MEL (20 mg/day at bedtime) with CPT-11 was
10. Remarks
more effective in controlling the disease than administration of
CPT-11 alone. It has also been shown that 1 mM MEL potentiates
MEL is a hormone produced in the pineal gland mainly at night
flavone-induced apoptosis in HT-29 human colon cancer cells
time, but is also synthesized by other tissues being the intestine the
by increasing the level of oxidizable substrates that can be incor-
main source during the day. Its lipophilic nature makes MEL easily
porated into mitochondria in the presence of flavones.
cross all membranes. Most of its actions are mediated by membrane
Another relevant aspect related to the application of MEL
and nuclear receptors. It has antioxidant and antiapoptotic prop-
as adjuvant in tumor therapy includes its protective effect on
erties, resulting in the improvement of mitochondrial metabolic
A. Carpentieri et al. / Pharmacological Research 65 (2012) 437–444
Effect of MEL combined with other drugs in the treatment of different diseases. In vivo and in vitro studies.
MEL combined with other drugs or
Suppression of seizures in refractory epilepsy in
Improvement of wake-sleep
Attenuation of aggressive behavior
Improvement of subjective sleep quality
2 mg/day at bed time
Antiparkinsonian drugs: levodopa,
Improvement of subjective sleep quality, sleep
amantadine, selegiline, pramipexol
quantity and daytime sleepiness
Alzheimer disease
Increase of daytime wake time and activity levels
3–6 mg/day, at bed time
Improvement of rest-activity
Increase of apoptosis and inhibition of hepatoma cell
10−5 to 10−8 mol/L
Protection of the gastric mucosa decreasing the doses
Increase of immune response altered by Trypanosoma
5 to 10 mg/kg b.w.
Enhancement of mycobacterial growth inhibition
Gram (−) bacterial infections
Attenuation of nephro and ototoxicity produced by
250 g to 10 mg/kg
a Clinical trials or case reports.
b In vitro studies.
c Animal studies.
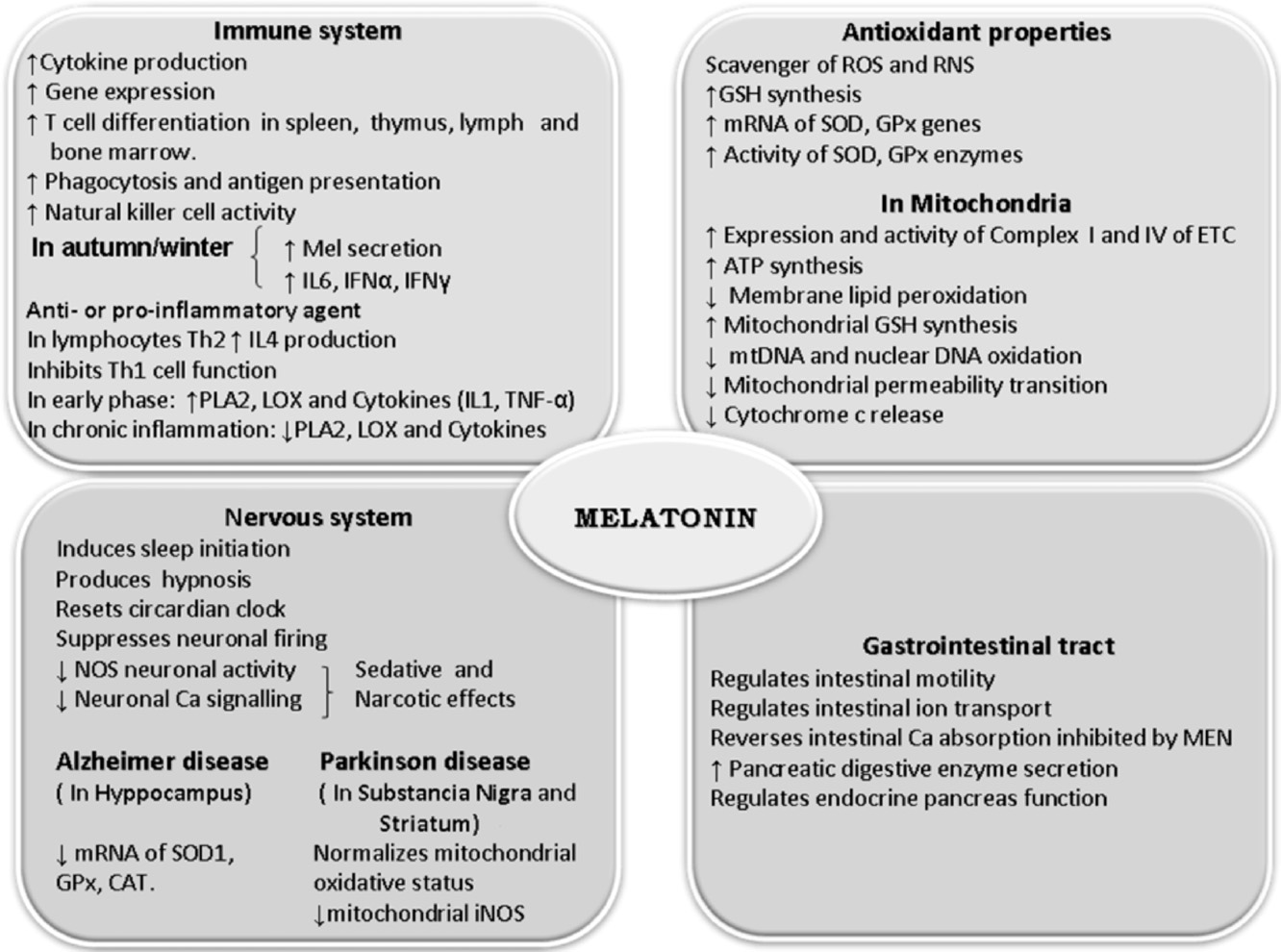
Fig. 2. Effects of melatonin on different tissues and systems. CAT: catalase, ETC: electron transport chain, GPx: glutathione peroxidase, GSH: glutathione, IFN␣: interferon
alpha, IFN␥: interferon gamma, IL: interleukin, iNOS: inducible nitric oxide synthase, LOX: lipoxygenase, MEN: menadione, mtDNA: mitochondrial DNA, NOS: nitric oxide
synthase, PLA2: phosholipase A2, RNS: reactive nitrogen species, ROS: reactive oxygen species, SOD1: superoxide dismutase one, Th1: type 1 helper T lymphocytes, Th2:
type 2 helper T lymphocytes, TNF-␣: tumor necrosis factor alpha.
A. Carpentieri et al. / Pharmacological Research 65 (2012) 437–444
pathways and ATP production. A potential intracrine and paracrine
[18] Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many
role of MEL in immune regulation has been suggested although the
derivatives: a never-ending interaction of melatonin with reactive oxygen and
nitrogen species. J Pineal Res 2007;42:28–42.
molecular mechanisms involved in the immunoregulatory function
[19] Peyrot F, Houee-Levin C, Ducrocq C. Melatonin nitrosation promoted by
remain to be elucidated. The action of MEL on sleep is mainly of a
NO*2 radical; comparison with the peroxynitrite reaction. Free Radic Res
chronobiological nature. One of the major obstacles for the use of
[20] Winiarska K, Fraczyk T, Malinska D, Drozak J, Bryla J. Melatonin attenuates
MEL in primary chronic insomnia is that the half life of circulation
diabetes-induced oxidative stress in rabbits. J Pineal Res 2006;40:168–76.
is extremely short, which has been partially solved by synthesis
[21] Forrest CM, Mackay GM, Stoy N, Stone TW, Darlington LG. Inflammatory status
of drugs that prolong the release of MEL and the melatoninergic
and kynurenine metabolism in rheumatoid arthritis treated with melatonin. Br
agonists with longer half-life. MEL's cognitive benefits have been
J Clin Pharmacol 2007;64:517–26.
[22] Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, et al. Oxidative
demonstrated either in AD patients or in AD mice. Some approaches
stress impairs oocyte quality and melatonin protects oocytes from free radical
indicate that MEL alone or in combination with other drugs could
damage and improves fertilization rate. J Pineal Res 2008;44:280–7.
be used to prevent tumor initiation, promotion, and progression.
[23] Kedziora-Kornatowska K, Szewczyk-Golec K, Czuczejko J, Pawluk H, Van
Marke de Lumen K, Kozakiewicz M, et al. Antioxidative effects of melatonin
Anti-proliferative effects of MEL on breast and colon cancer cells
administration in elderly primary essential hypertension patients. J Pineal Res
have been demonstrated, but the underlying mechanisms are not
clear yet. Another possible use of MEL alone or combined with other
[24] Cutolo M, Maestroni GJ. The melatonin-cytokine connection in rheumatoid
arthritis. Ann Rheum Dis 2005;64:1109–11.
drugs would be for treatment of several psychiatric disorders due to
[25] Maestroni GJ, Otsa K, Cutolo M. Melatonin treatment does not improve rheuma-
its anxiolytic, sedative, anticonvulsant and anti-hypertensive prop-
toid arthritis. Br J Clin Pharmacol 2008;65:797–8.
erties. In conclusion, the spectrum of uses of MEL seems to be wide,
[26] Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, et al. Regu-
lation of antioxidant enzymes: a significant role for melatonin. J Pineal Res
although more investigation is needed in order to know better the
molecular mechanisms and the possible side effects.
[27] Fischer TW, Slominski A, Zmijewski MA, Reiter RJ, Paus R. Melatonin as a major
skin protectant: from free radical scavenging to DNA damage repair. Exp Der-
[28] Leon J, Acu ˜
na-Castroviejo D, Sainz RM, Mayo JC, Tan DX, Reiter RJ. Melatonin
and mitochondrial function. Life Sci 2004;75:765–90.
Dr. Tolosa de Talamoni N. and Carpentieri A. are Members of
[29] Dungel P, Mittermayr R, Haindl S, Osipov A, Wagner C, Redl H, et al. Illu-
mination with blue light reactivates respiratory activity of mitochondria
Career from CONICET. Peralta López M. and Areco V. are recipients
inhibited by nitric oxide, but not by glycerol trinitrate. Arch Biochem Biophys
of Fellowships from CONICET.
Grants: This work was supported by grants from CONICET [PIP
[30] Petrosillo G, Moro N, Ruggiero FM, Paradies G. Melatonin inhibits cardiolipin
peroxidation in mitochondria and prevents the mitochondrial permeability
2010-2012] and SECYT (UNC) (Dr. Nori Tolosa de Talamoni).
transition and cytochrome c release. Free Radic Biol Med 2009;47:969–74.
[31] Proietti S, Cucina A, D'Anselmi F, Dinicola S, Pasqualato A, Lisi E, et al. Mela-
tonin and vitamin D3 synergistically down-regulate Akt and MDM2 leading
to TGF-1-dependent growth inhibition of breast cancer cells. J Pineal Res
[1] Lerner AB, Case JD, Heinzelman RV. Structure of melatonin. J Am Chem Soc
[32] Okatani Y, Wakatsuki A, Reiter RJ. Melatonin protects hepatic mitochon-
drial respiratory chain activity in senescence-accelerated mice. J Pineal Res
[2] Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B,
Hardeland R. Melatonin: nature's most versatile biological signal. FEBS J
[33] Karasek M, Winczyk K. Melatonin in humans. J Physiol Pharmacol
[3] Garbarino-Pico E, Carpentieri AR, Contin MA, Sarmiento MI, Brocco
[34] Roberts JE. Light and immunomodulation. Ann N Y Acad Sci 2000;917:435–45.
MA, Panzetta P, et al. Retinal ganglion cells are autonomous circadian
[35] Garcia-Mauri ˜
no S, Gonzalez-Haba MG, Calvo JR, Goberna R, Guerrero JM.
oscillators synthesizing N-acetylserotonin during the day. J Biol Chem
Involvement of nuclear binding sites for melatonin in the regulation of IL-
2 and IL-6 production by human blood mononuclear cells. J Neuroimmunol
[4] Bellavía SL, Carpentieri AR, Vaqué AM, Macchione AF, Vermouth NT. Pup circa-
dian rhythm entrainment-effect of maternal ganglionectomy or pinealectomy.
[36] Srinivasan V, Maestroni GJ, Cardinali DP, Esquifino AI, Pandi-Perumal SR, Miller
Physiol Behav 2006;89:342–9.
SC. Melatonin, immune function and aging. Immun Ageing 2005;2:17–27.
[5] Carpentieri AR, Anglés-Pujolràs M, Chiesa JJ, Diez-Noguera A, Cambras T. Effect
[37] Shaji AV, Kulkarni SK, Agrewala JN. Regulation of secretion of IL-4 and IgG1
of melatonin and diazepam on the dissociated circadian rhythm in rat. J Pineal
isotype by melatonin-stimulated ovalbumin-specific T cells. Clin Exp Immunol
[6] Waterhouse J, Reilly T, Atkinson G, Edwards B. Jet lag: trends and coping strate-
[38] Radogna F, Diederich M, Ghibelli L. Melatonin: a pleiotropic molecule regulat-
gies. Lancet 2007;369:1117–29.
ing inflammation. Biochem Pharmacol 2010;80:1844–52.
[7] Escames G, López A, García JA, García L, Acu ˜
na-Castroviejo D, García JJ, et al.
[39] Bubenik GA. Thirty four years since the discovery of gastrointestinal melatonin.
The role of mitochondria in brain aging and the effects of melatonin. Curr
J Physiol Pharmacol 2008;59:33–51.
[40] Huether G, Poeggeler B, Reimer A, George A. Effect of Trytophan administration
[8] Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol
on circulating melatonin levels in chicks and rats: evidence for stimula-
tion of melatonin synthesis and release in the gastrointestinal tract. Life Sci
[9] Konturek SJ, Konturek PC, Brzozowska I, Pawlik M, Sliwowski Z, Cze´snikiewicz-
Guzik M, et al. Localization and biological activities of melatonin in intact and
[41] Bubenik GA, Pang SF, Cockshut JR, Smith PS, Grovum LW, Friendship RM, et al.
diseased gastrointestinal tract (GIT). J Physiol Pharmacol 2007;58:381–405.
Circardian variations of potent arterial and venous blood levels of melatonin in
[10] Dziegiel P, Podhorska-Okolow M, Zabel M. Melatonin: adjuvant therapy of
pigs and its relationship to food intake and sleep. J Pineal Res 2000;28:9–15.
malignant tumors. Med Sci Monit 2008;14:RA64–70.
[42] Stefulj J, Hörtner M, Ghosh M, Schauenstein K, Rinner I, Wölfler A, et al. Gene
[11] Lane EA, Moss HB. Pharmacokinetics of melatonin in man: first pass hepatic
expression of the key enzymes of melatonin synthesis in extrapineal tissues of
metabolism. J Clin Endocrinol Metab 1985;61:1214–6.
the rat. J Pineal Res 2001;30:243–7.
[12] Vijayalaxmi Reiter RJ, Tan D, Herman TS, Thomas Jr CR. Melatonin as a radio-
[43] Poeggeler B, Cornélissen G, Huether G, Hardeland R, Józsa R, Zeman M, et al.
protective agent: a review. Int J Radiat Oncol Biol Phys 2004;59:639–53.
Chronomics affirm extending scope of lead in phase of duodenal vs. pineal
[13] Hardeland R. New approaches in the management of insomnia: weighing the
circadian melatonin rhythms. Biomed Pharmacother 2005;59:S220–4.
advantages of prolonged-release melatonin and synthetic melatoninergic ago-
[44] Lu WZ, Song GH, Gwee KA, Ho KY. The effects of melatonin on colonic transit
nists. Neuropsychiatr Dis Treat 2009;5:341–54.
time in normal controls and IBS patients. Dig Dis Sci 2009;54:1087–93.
[14] Mathes AM. Hepatoprotective actions of melatonin: possible mediation by
[45] Sjöblom M, Flemström G. Melatonin in the duodenal lumen is a potent stimu-
melatonin receptors. World J Gastroenterol 2010;16:6087–97.
lant of mucosal bicarbonate secretion. J Pineal Res 2003;34:288–93.
[15] Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a
[46] Aust S, Thalhammer T, Humpeler S, Jäger W, Klimpfinger M, Tucek G, et al. The
mammalian melatonin receptor that mediates reproductive and circadian
melatonin receptor subtype MT1 is expressed in human gallbladder epithelia.
responses. Neuron 1994;13:1177–85.
J Pineal Res 2004;36:43–8.
[16] Ebisawa T, Karne S, Lerner MR, Reppert SM. Expression cloning of a high-affinity
[47] Matheus N, Mendoza C, Iceta R, Mesonero JE, Alcalde AI. Melatonin inhibits
melatonin receptor from Xenopus dermal melanophores. Proc Natl Acad Sci
serotonin transporter activity in intestinal epithelial cells. J Pineal Res
[17] Park YJ, Park JG, Hiyakawa N, Lee YD, Kim SJ, Takemura A. Diurnal and circa-
[48] Tolosa de Talamoni NG, Marchionatti AM, Areco VA, Perez A, CentenoVA, Car-
dian regulation of a melatonin receptor, MT1, in the golden rabbitfish, Siganus
pentieri AR. Melatonin restores the intestinal calcium absorption altered by
guttatus. Gen Comp Endocrinol 2007;150:253–62.
oxidative stress. Bone 2011;48:S147–8.
A. Carpentieri et al. / Pharmacological Research 65 (2012) 437–444
[49] Nishida S, Sato R, Murai I, Nakagawa S. Effect of pinealectomy on plasma levels
[70] Maldonado MD, Pérez-San-Gregorio MA, Reiter RJ. The role of melatonin in the
of insulin and leptin and on hepatic lipids in type 2 diabetic rats. J Pineal Res
immuno-neuro-psychology of mental disorders. Recent Pat CNS Drug Discov
[50] Jaworek J, Konturek SJ, Leja-Szpak A, Nawrot K, Bonior J, Tomaszewska R,
[71] Lewy AJ, Ahmed S, Jackson JM, Sack RL. Melatonin shifts human circadian
et al. Role of endogenous melatonin and its MT2 receptor in the mod-
rhythms according to a phase-response curve. Chronobiol Int 1992;9:380–92.
ulation of caerulein-induced pancreatitis in the rat. J Physiol Pharmacol
[72] Reiter RJ, Tan DX, Sainz RM, Mayo JC. Melatonin: reducing the toxicity and
increasing the efficacy of drugs. J Pharm Pharmacol 2002;54:1299–321.
[51] Tapias V, Escames G, López LC, López A, Camacho E, Carrión MD, et al. Melatonin
[73] Sánchez-Barceló EJ, Mediavilla MD, Tan DX, Reiter RJ. Clinical uses of melatonin:
and its brain metabolite N(1)-acetyl-5-methoxykynuramine prevent mito-
evaluation of human trials. Curr Med Chem 2010;17:2070–95.
chondrial nitric oxide synthase induction in parkinsonian mice. J Neurosci Res
[74] Molina-Carballo A, Mu ˜
noz-Hoyos A, Reiter RJ, Sánchez-Forte M, Moreno-
Madrid F, Rufo-Campos M, et al. Utility of high doses of melatonin as adjunctive
[52] Dowling GA, Mastick J, Colling E, Carter JH, Singer CM, Aminoff MJ. Melatonin
anticonvulsant therapy in a child with severe myoclonic epilepsy: two years'
for sleep disturbances in Parkinson's disease. Sleep Med 2005;6:459–66.
experience. J Pineal Res 1997;23:97–105.
[53] Willis GL, Armstrong SM. A therapeutic role for melatonin antagonism in exper-
[75] Peled N, Shorer Z, Peled E, Pillar G. Melatonin effect on seizures in children with
imental models of Parkinson's disease. Physiol Behav 1999;66:785–95.
severe neurologic deficit disorders. Epilepsia 2001;42:1208–10.
[54] Willis GL, Turner EJ. Primary and secondary features of Parkinson's disease
[76] Coppola G, Iervolino G, Mastrosimone M, La Torre G, Ruiu F, Pascotto A.
improve with strategic exposure to bright light: a case series study. Chronobiol
Melatonin in wake-sleep disorders in children, adolescents and young adults
with mental retardation with or without epilepsy: a double-blind, cross-over,
[55] Reiter RJ, Tan DX, Korkmaz A, Erren TC, Piekarski C, Tamura H, et al. Light at
placebo-controlled trial. Brain Dev 2004;26:373–6.
night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit
[77] Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van
Rev Oncog 2007;13:303–28.
Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive
[56] Blask DE, Hill SM. Effects of melatonin on cancer: studies on MCF-7 human
function in elderly residents of group care facilities: a randomized controlled
breast cancer cells in culture. J Neural Transm Suppl 1986;21:433–49.
trial. JAMA 2008;299:2642–55.
[57] Mediavilla MD, Cos S, Sánchez-Barceló EJ. Melatonin increases p53 and
[78] Dolberg OT, Hirschmann S, Grunhaus L. Melatonin for the treatment of sleep
p21WAF1 expression in MCF-7 human breast cancer cells in vitro. Life Sci
disturbances in major depressive disorder. Am J Psychiatry 1998;155:1119–21.
[79] Mayo JC, Sainz RM, Tan DX, Antolín I, Rodríguez C, Reiter RJ. Melatonin and
[58] Cos S, Fernández R, Güézmes A, Sánchez-Barceló EJ. Influence of melatonin on
Parkinson's disease. Endocrine 2005;27:169–78.
invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer
[80] Medeiros CA, Carvalhedo de Bruin PF, Lopes LA, Magalhães MC, de Lour-
des Seabra M, de Bruin VM. Effect of exogenous melatonin on sleep and
[59] Ram PT, Dai J, Yuan L, Dong C, Kiefer TL, Lai L, et al. Involvement of the mt1
motor dysfunction in Parkinson's disease. A randomized, double blind, placebo-
melatonin receptor in human breast cancer. Cancer Lett 2002;179:141–50.
controlled study. J Neurol 2007;254:459–64.
[60] Schernhammer ES, Razavi P, Li TY, Qureshi AA, Han J. Rotating night shifts
[81] Chiueh CC, Andoh T, Lai AR, Lai E, Krishna G. Neuroprotective strategies in
and risk of skin cancer in the nurses' health study. J Natl Cancer Inst
Parkinson's disease: protection against progressive nigral damage induced by
free radicals. Neurotox Res 2000;2(2–3):293–310.
[61] Farriol M, Venereo Y, Orta X, Castellanos JM, Segovia-Silvestre T. In vitro effects
[82] Brusco LI, Márquez M, Cardinali DP. Monozygotic twins with Alzheimer's dis-
of melatonin on cell proliferation in a colon adenocarcinoma line. J Appl Toxicol
ease treated with melatonin: case report. J Pineal Res 1998;25:260–3.
[83] Dowling GA, Burr RL, Van Someren EJ, Hubbard EM, Luxenberg JS, Mastick J, et al.
[62] Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N,
Melatonin and bright-light treatment for rest-activity disruption in institution-
et al. Physiological effects of melatonin: role of melatonin receptors and signal
alized patients with Alzheimer's disease. J Am Geriatr Soc 2008;56:239–46.
transduction pathways. Prog Neurobiol 2008;85:335–53.
[84] Fan LL, Sun GP, Wei W, Wang ZG, Ge L, Fu WZ, et al. Melatonin and doxorubicin
[63] Cerea G, Vaghi M, Ardizzoia A, Villa S, Bucovec R, Mengo S, et al. Biomodulation
synergistically induce cell apoptosis in human hepatoma cell lines. World J
of cancer chemotherapy for metastatic colorectal cancer: a randomized study of
weekly low-dose irinotecan alone versus irinotecan plus the oncostatic pineal
[85] Brazão V, Filipin MdelV, Santello FH, Caetano LC, Abrahão AA, Toldo MP, et al.
hormone melatonin in metastatic colorectal cancer patients progressing on
Melatonin and zinc treatment: distinctive modulation of cytokine production in
5-fluorouracil-containing combinations. Anticancer Res 2003;23:1951–4.
chronic experimental Trypanosoma cruzi infection. Cytokine 2011;56:627–32.
[64] Wenzel U, Nickel A, Daniel H. Melatonin potentiates flavone-induced apoptosis
[86] Oliveira LG, Kuehn CC, Santos CD, Toldo MP, do Prado Jr JC. Enhanced protec-
in human colon cancer cells by increasing the level of glycolytic end products.
tion by melatonin and meloxicam combination in experimental infection by
Int J Cancer 2005;116:236–42.
Trypanosoma cruzi. Parasite Immunol 2010;32:245–51.
[65] Othman AI, El-Missiry MA, Amer MA. The protective action of melatonin on
[87] Kuehn CC, Rodrigues Oliveira LG, Santos CD, Ferreira DS, Alonso Toldo MP,
indomethacin-induced gastric and testicular oxidative stress in rats. Redox Rep
de Albuquerque S, et al. Melatonin and dehydroepiandrosterone combination:
does this treatment exert a synergistic effect during experimental Trypanosoma
[66] Konturek PC, Celinski K, Slomka M, Cichoz-Lach H, Burnat G, Naegel A, et al.
cruzi infection? J Pineal Res 2009;47:253–9.
Melatonin and its precursor l-tryptophan prevent acute gastric mucosal dam-
[88] Wiid I, Hoal-van Helden E, Hon D, Lombard C, van Helden P. Potentiation of iso-
age induced by aspirin in humans. J Physiol Pharmacol 2008;59:67–75.
niazid activity against Mycobacterium tuberculosis by melatonin. Antimicrob
[67] Bandyopadhyay D, Bandyopadhyay A, Das PK, Reiter RJ. Melatonin protects
Agents Chemother 1999;43:975–7.
against gastric ulceration and increases the efficacy of ranitidine and omepra-
[89] Sener G, Sehirli AO, Altunbas HZ, Ersoy Y, Paskaloglu K, Arbak S, et al. Mela-
zole in reducing gastric damage. J Pineal Res 2002;33:1–7.
tonin protects against gentamicin-induced nephrotoxicity in rats. J Pineal Res
[68] Deshmukh VD. Retino-hypothalamic-pineal hypothesis in the pathophysiology
of primary headaches. Med Hypotheses 2006;66:1146–51.
[90] Lopez-Gonzalez MA, Guerrero JM, Torronteras R, Osuna C, Delgado F. Ototoxic-
[69] Peres MF. Melatonin, the pineal gland and their implications for headache
ity caused by aminoglycosides is ameliorated by melatonin without interfering
disorders. Cephalalgia 2005;25:403–11.
with the antibiotic capacity of the drugs. J Pineal Res 2000;28:26–33.
Source: http://www.valeant.com.mx/cif/repositorio/archivos/literatura/20130213_16501297_literatura_ccg.pdf
l'affaire de tous Centraliens no632 [mars/avril 2014] Le secteur de la santé, qui est la première source d'emploi en France, est en train de faire face à une mutation quantique que nous vivons en temps réel sans en sentir encore tous les effets. Ce dossier a pour objectif de vous ac- d'invalidité dans le monde (OMS, 2 002). compagner dans un voyage où vous L'industrie pharmaceutique a contribué découvrirez les éléments de cette signifi cativement à la lutte contre les mala-
Journal of Case Reports in Practice (JCRP) 2014; 2(2): 48-50 CASE REPORT Persistent pruritic skin rashes masquerading sulfasalazine sensitivity in a newly diagnosed Adult-Onset Still's Disease (AOSD) ohreh Akhoundi Meybodi 1 and SinA owliA 1Department of Medicine, Shahid Sadoughi University of medical sciences, Yazd, Iran




